Abstract
Gibberellins (GAs) are a class of plant hormones involved in the regulation of flower development in Arabidopsis. The GA-deficient ga1-3 mutant shows retarded growth of all floral organs, especially abortive stamen development that results in complete male sterility. Until now, it has not been clear how GA regulates the late-stage development of floral organs after the establishment of their identities within floral meristems. Various combinations of null mutations of DELLA proteins can gradually rescue floral defects in ga1-3. In particular, the synergistic effect of rga-t2 and rgl2-1 can substantially restore flower development in ga1-3. We find that the transcript levels of floral homeotic genes APETALA3 (AP3), PISTILLATA (PI), and AGAMOUS (AG) are immediately upregulated in young flowers of ga1-3 upon GA treatment. Using a steroid-inducible activation of RGA, we further demonstrated that these floral homeotic genes are transcriptionally repressed by RGA activity in young flowers whereas the expression of LEAFY (LFY) and APETALA1 (AP1) is not substantially affected. In addition, we observed the partial rescue of floral defects in ga1-3 by overexpression of AG. Our results indicate that GA promotes the expression of floral homeotic genes by antagonizing the effects of DELLA proteins, thereby allowing continued flower development.
Flower development starts with the specification of floral meristem identity, and of floral organ identity within nascent floral meristems flanking an inflorescence meristem. During this process, LEAFY (LFY) stands out as a major regulator in both integrating upstream floral inductive signals and controlling three classes of downstream floral homeotic genes, called A, B, and C function genes, respectively. These classes of downstream genes control floral organ identity in discrete domains (1–3). Although it has been clear that combinatorial activation of A, B, and C function genes governs the identity of different floral organs (3–5), little is known about how a young floral bud with established floral organ identities continues to develop into a mature flower. It is possible that floral homeotic genes play consistent roles in initiation and further promotion of floral organ growth because the region-specific expression of these genes is present throughout the whole process of flower development (6–11). Indeed, the C function gene AGAMOUS (AG) has been suggested to function not only in the early specification of stamen and carpel identity, but also in the late patterning of carpel structures (12). It is noteworthy that, as a major promoter of floral homeotic genes, LFY is not expressed in flowers after stage 5 (13). If floral homeotic genes are the regulators required for both floral organ identity and their continued development, the promotion of floral homeotic genes at later stages of flower development should be regulated by mechanisms other than LFY-dependent ones. The gibberellin (GA)-deficient ga1-3 mutant develops flowers with retarded growth of all floral organs despite their normal identities (14, 15), which provides a useful experimental system to distinguish between the different mechanisms involved in the establishment of floral organ identity and at least some aspects of the later development of floral organs.
Gibberellins are one class of tetracyclic diterpenoid phytohormones affecting many aspects of plant growth and development, including seed germination, root growth, stem elongation, leaf expansion, floral induction, and flower development (16, 17). Recent advances have shown that GA regulates various plant developmental programs by suppressing a group of DELLA protein nuclear repressors (18–24). There are a total of five DELLA proteins (GAI, RGA, RGL1, RGL2, and RGL3) encoded in the Arabidopsis genome. All of these proteins contain a conserved N-terminal DELLA domain, which is possibly involved in the inactivation of these proteins by GA signals (18, 25). GAI and RGA are negative regulators of GA responses in the control of stem elongation, flowering time, and root growth. Removing both gene functions causes a synergistic suppression of the corresponding defects in ga1-3 mutants (20, 21, 24). Similarly, RGL2 is a major repressor of seed germination because rgl2 null mutations can significantly promote the germination of ga1-3 seeds, which require GA for normal germination (22). Although it has been suggested that GA overcomes the function of DELLA repressors by inducing degradation of these proteins by means of a ubiquitin/proteasome-dependent pathway (25–28), the mechanisms by which these proteins control downstream developmental processes have not yet been clarified.
In this study, we find that GA regulates flower development by opposing the function of several DELLA repressors and thereby partly promoting the expression of the floral homeotic genes APETALA3 (AP3), PISTILLATA (PI), and AG. Absence of RGA and RGL2 function is almost sufficient to restore normal flower development in ga1-3 (29), indicating that both genes are major repressors of GA responses in this specific process. By using a steroid-inducible system for the activation of RGA, we present evidence showing that AP3, PI, and AG are targets of transcriptional repression by RGA. Moreover, overexpression of AG causes partial rescue of abortive stamen development in ga1-3. Our results thus suggest that continuous maintenance of floral homeotic gene expression is important for normal flower development and that DELLA proteins, especially RGA, play critical roles in linking the GA-signaling pathway with homeotic gene activity in flower development.
Materials and Methods
Plant Materials. All Arabidopsis mutants used in this study are in the Landsberg erecta (Ler) background unless stated otherwise. They were grown at 22°C in continuous light. To break dormancy, all seeds with ga1-3 background were imbibed in 100 μM GA at 4°C for 7 days, and then rinsed thoroughly with water before sowing. Mutant lines ga1-3, rgl1-1, rgl2-1, gai-t6, rga-t2, ga1-3 rgl1-1, ga1-3 rgl2-1, ga1-3 gai-t6, ga1-3 rga-t2, and ga1-3 gai-t6 rga-t2 have been described (22). The other mutant lines in this study were created by cross-pollination between the above relevant mutants, and their genotypes were verified as reported (21, 22).
To create ga1-3 rga-t2 35S::RGA-GR, ga1-3 rga-t2 was treated weekly with 100 μM GA and transformed with the binary vector harboring the 35S::RGA-GR cassette. Transgenic plants containing 35S::RGA-GR were screened by Basta selection and further tested for phenotypic effects by dexamethasone treatment. We isolated one transgenic line, which contains only one transgene insertion and shows the phenotype closely resembling ga1-3 after dexamethasone treatment, to cross with ga1-3 rgl2-1 rga-t2 to create ga1-3 rgl2-1 rga-t2 35S::RGA-GR.
To create 35S::AG-GR, Arabidopsis ecotype Landsberg erecta (Ler) was transformed with the binary vector harboring the 35S::AG-GR cassette (T.I. and E.M.M., unpublished results). We selected a transgenic line, which contains only one transgene insertion and shows the phenotype closely resembling 35S::AG after dexamethasone treatment, to cross with ga1-3 to generate ga1-3 35S::AG-GR.
Dexamethasone treatment and sample collection were as described (30).
Plasmid Constructs. We constructed a derivative pGreen0229TI vector by cloning the cauliflower mosaic virus 35S coat protein gene (35S) promoter with tandem enhancers and transcriptional terminator into the KpnI and XhoI sites of pGreen0229 (31). The hormone-binding domain of the rat glucocorticoid receptor (GR) was amplified from pRI-ΔGR (32) by the primers GR1 (5′-TCCCCCGGGGGATCCTGAAGCTCGAA-3′) and GR2 (5′-GCTCTAGAGCTCAGTCATTTTTGATGA-3′). The amplified GR fragment was cut with BamHI and XbaI and cloned into the corresponding sites of the pGreen0229TI to generate pGreen0229TI:GR. The entire RGA cDNA was amplified by RT-PCR with the primers RGA-G1 (5′-AACTGCAGAATCGAAACTCATAGCTGAA-3′) and RGA-G2 (5′-AAGGATCCCCGTGCGCCGCCGTCGAGAGTTTC-3′). The resulting fragment was digested by PstI and BamHI and subsequently cloned into the corresponding sites of pGreen0229TI:GR to create a 35S::RGA-GR cassette.
Analysis of Gene Expression. To investigate gene expression in young flowers, we selected inflorescence apices containing floral buds younger than stage 10. Total RNA was extracted by RNeasy Plant Mini Kit (Qiagen, Valencia, CA) and reverse-transcribed by using the ThermoScript RT-PCR system (Invitrogen). Under our RT-PCR conditions, we performed 22–25 cycles of amplification to make sure that quantification for all genes examined was within a linear range. The amplified PCR products were detected as described (33). RT-PCR was repeated three times by using samples collected separately.
Primers designed for RT-PCR were as follows: AP1-P1 (5′-GCACCTGAGTCCGACGTC-3′) and AP1-P2 (5′-GCGGCGAAGCAGCCAAGG-3′) for APETALA1 (AP1); AP2-P1 (5′-TAGCCACCGGATCGTCCGCGGGTAAA-3′) and AP2-P2 (5′-GTTGTTGTTGGTTCATCCTGAGCCGCAT-3′) for APETALA2 (AP2); AP3-P1 (5′-AGCTGCGTCGTCTTGAGGAT-3′) and AP3-P2 (5′-GGTTTTAGCAACACCATGCCT-3′) for AP3; PI-P1 (5′-CTTACAACTGGAGCTCAGGCA-3′) and PI-P2 (5′-GCTCGAGATTAAGACACACAG-3′) for PI; AG-P1 (5′-GCTCAGGA ACT TGGA AGGCAG-3′) and AG-P2 (5′-TCACTCCAGGCCATTTCCTTC-3′) for AG; LFY-P1 (5′-TGAAGGACGAGGAGCTT-3′) and LFY-P2 (5′-TTGCCACGTGCCACTTC-3′) for LFY; and TUB2-P1 (5′-ATCCGTGAAGAGTACCCAGAT-3′) and TUB2-P2 (5′-TCACCTTCTTCATCCGCAGTT-3′) for β-tubulin (TUB2). Some other primers were according to the following references: WUSCHEL (WUS) (34), SUPERMAN (SUP) (35), and SEPALLATA 3 (SEP3) (36).
In Situ Hybridization. Nonradioactive in situ hybridization was performed according to a published protocol (37). Synthesis of antisense probes has been described (38). Sections of both WT and ga1-3 plants were placed on the same slide, which was hybridized and detected under the same conditions. The comparable panels for different probes in in situ figures were recorded from the same slide.
Results and Discussion
DELLA proteins in Arabidopsis include GAI, RGA, RGL1, RGL2, and RGL3, which contain a conserved DELLA domain at their N termini (18, 25). The stability of these proteins is thought to be reduced in the presence of GA (25–28). It has been suggested that DELLA proteins play repressive roles in various aspects of plant growth and development (18–24). Flowers in GA-deficient mutants ga1-3 possess undeveloped floral organs in all four whorls. In particular, stamen development, including filament elongation and pollen maturation, is abortive. Recently reported work (29) and our observations (Fig. 6, which is published as supporting information on the PNAS web site) show that various combinations of null mutations of DELLA proteins gai-t6, rga-t2, rgl1-1, and rgl2-1 (22) can rescue floral phenotypes of ga1-3 to different degrees (Table 1). These results suggest that RGA and RGL2 play major functions in repressing the continued growth of floral organs, and that the sequence of the importance of DELLA proteins involved in flower development is RGA, RGL2, RGL1, and GAI.
Table 1. Classification of mutants in terms of their flower phenotype.
| Degree of rescue of gal-3 | Genotype* | Flower phenotype |
|---|---|---|
| 0 | gal-3† | Retarded growth of petals, stamens and pistils; male sterility with lack of mature pollen |
| 1 | gal-3 rgl1-1† | Partial rescue of gal-3 with elongated pistils |
| gal-3 rgl2-1 | ||
| gal-3 gai-t6 | ||
| 2 | gal-3 rga-t2† | Partial rescue of gal-3 with elongated petals, filaments, and pistils |
| gal-3 rgl1-1 rgl2-1 | ||
| gal-3 rgl1-1 gai-t6 | ||
| gal-3 rgl2-1 gai-t6 | ||
| gal-3 rgl1-1 rgl2-1 gai-t6 | ||
| 3 | ga1-3 rgl1-1 rga-t2† | Partial rescue of gal-3 with more elongated petals, filaments, and pistils |
| gal-3 gai-t6 rga-t2 | ||
| ga1-3 rgl1-1 gai-t6 rga-t2 | ||
| 4‡ | gal-3 rgl2-1 rga-t2† | Significant rescue of gal-3 with normal petals, pistils, and much developed stamens; partial infertility of early arising flowers |
| gal-3 rgl2-1 gai-t6 rga-t2 | ||
| 5 | gal-3 rgl1-1 rgl2-1 rga-t2† | Almost total rescue of gal-3 with normal petals, stamens and pistils; normal fertility |
| gal-3 rgl1-1 rgl2-1 gai-t6 rga-t2 |
All of the plants are of the same Landsberg erecta background
Representative mutants displaying different degrees of rescue of gal-3 are shown in Fig. 6
Mutants in this degree generate two kinds of flowers: flowers arising at apical positions in a main inflorescence are fertile with significant rescue of gal-3 whereas flowers arising at basal positions are still sterile with the degree 3 phenotype
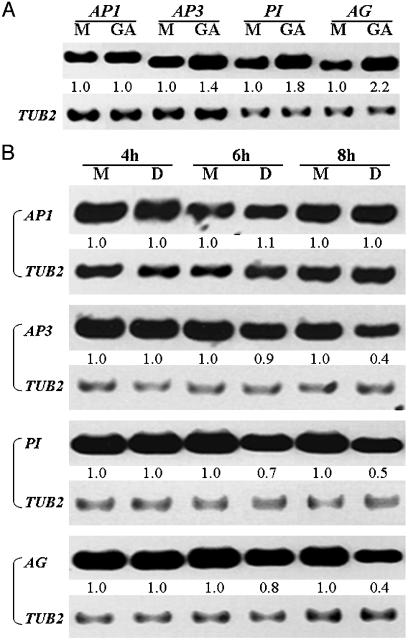
GA Promotes Flower Development Partly by Up-Regulating Floral Homeotic Genes. To identify the downstream genes regulated by GA signaling, we examined the expression of a set of genes involved in floral patterning (12) upon GA treatment, which included the floral meristem identity gene LFY, floral homeotic genes AP1, AP2, AP3, PI, and AG, and floral organ identity and growth regulators WUS, SUP, and SEP3. Our results showed that the expression of B and C function genes AP3, PI, and AG was up-regulated in inflorescence apices of ga1-3, ≈2 h after GA treatment (Fig. 1A) whereas expression of the other genes was not substantially changed under the conditions tested (data not shown). Although the selected inflorescence apices for RT-PCR contained floral buds from stages 1 to 10, RNA of old floral buds after stage 5 would be expected to constitute the greatest part of the RNA isolated from the batch of floral buds (T.I. and E.M.M., unpublished data). Thus, up-regulation of these floral homeotic genes after GA treatment mostly reflected a change in their transcript levels in older floral buds after stage 5, after the time when the floral meristems had already established floral organ identity (39). Because GA treatment is sufficient to restore the normal growth of floral organs in ga1-3 flowers, we suggest that the promotion of floral homeotic gene expression by GA signaling may be important for the continued development of floral buds to late-stage flowers, which have already established floral organ identity.
Fig. 1.
Expression of floral homeotic genes. (A) Expression of AP1, AP3, PI, and AG in inflorescence apices of ga1-3 mutants mock-treated with 0.1% ethanol (M), or treated with 100 μM GA (GA). Expression analyses were done after 2 h of treatment. (B) Time-course expression of AP1, AP3, PI, and AG in inflorescence apices of ga1-3 rgl2-1 rga-t2 35S::RGA-GR plants mock-treated with 0.03% ethanol and 0.015% Silwet L-77 (M) or treated with 10 μM dexamethasone and 0.015% Silwet L-77 (D). The β-tubulin gene (TUB2) was amplified as a quantitative control. The numbers below each lane indicate the relative expression of each gene studied, calculated by first normalizing each expression signal against the signal for TUB2 and then against the value of a corresponding mock-treated sample, which is always set as 1.0.
To further investigate the potential involvement of GA signals in the promotion of floral homeotic gene expression, we performed in situ hybridization with relevant probes, to ga1-3 and WT inflorescences. The B function gene AP3 and C function gene AG were expressed at lower levels in ga1-3 than in WT plants (Figs. 2 and 3) although their expression domains were not changed. Such reduction of expression levels was observed through the whole process of flower development in ga1-3 but was particularly evident by stage 8 (Figs. 2 B and E and 3 B and F). On the contrary, the expression of LFY, AP2 (data not shown), and AP1 (Fig. 7, which is published as supporting information on the PNAS web site) was not noticeably changed in ga1-3, which is consistent with the RT-PCR result (Fig. 1 A). As compared with their expression in ga1-3, expression of AP3 and AG was also higher in ga1-3 rgl2-1 rga-t2 (data not shown), which showed significant rescue of floral defects of ga1-3, indicating that GA may up-regulate the expression of target genes in flower development by overcoming the effects of DELLA proteins, especially RGA and RGL2.
Fig. 2.
In situ localization of AP3 expression in WT plants (A–C) and ga1-3 mutants (D–F). (A and D) An inflorescence apex with stage-2 and stage-4 flowers. (B and E) A stage-8 flower. (C and F) A stage-10 flower. (Bars = 100 μm.)
Fig. 3.
In situ localization of AG expression in WT plants (A–D) and ga1-3 mutants (E–H). (A and E) An inflorescence apex with a stage-4 flower. (B and F) A stage-6 flower. (C and G) A stage-9 flower. (D and H) A stage-12 flower. (Bars = 100 μm.)
Our data showed that GA can specifically and continuously promote the expression of B and C function genes during flower development, but not the LFY, AP1, and AP2 genes. The significance of this finding lies in two aspects. First, compared with the promotion of LFY expression by GA in the control of flowering time (40), LFY expression in emerging floral meristems is independent of GA signaling. Flower phenotypes in ga1-3 suggest that, without GA, the normal expression of LFY in young floral meristems is sufficient to promote the transcript levels of floral homeotic genes to establish normal floral organ identity, but not enough to secure the continued development of floral organs. Although we cannot exclude the possibility that GA signaling may coordinate with LFY in early development of floral meristems, our results suggest that GA can promote expression of floral homeotic genes independently of LFY activity in late-stage flowers, where LFY expression is absent. Second, it has been reported that GA signals can greatly promote petal development in ap1-1 and ap2-1 (41), indicating the possible presence of an A function-independent pathway in floral organogenesis that can be induced by GA signal transduction. This finding may be explained by the observation that GA can up-regulate B and C function genes, but not A function genes.
Inducible Activation of RGA. Among the identified DELLA proteins, RGA plays a more prominent role than GAI, RGL1, and RGL2 in mediating GA signaling during flower development (29). RGA contains a putative nuclear localization signal, and an RGA fusion with green fluorescent protein is localized in the nucleus of onion epidermal cells, indicating that RGA functions in the nucleus, perhaps as a transcriptional regulator (42). To further elucidate the mechanistic links between GA signaling and the activity of downstream genes in flower development, we created a steroid-inducible version of RGA in transgenic plants containing the RGA protein fused to the hormone-binding domain of a rat GR under the control of a 35S promoter. Posttranslational activation of RGA can be achieved in plants transgenic for this construct by dexamethasone treatment, which releases the fusion protein bound in the cytoplasm by means of the rat protein domain to the nucleus (43).
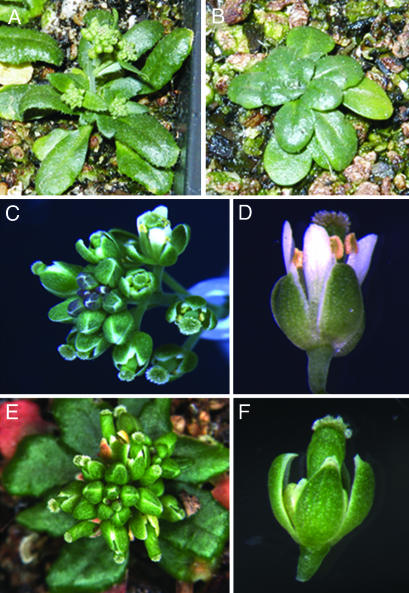
The loss-of-function rga mutation can partially rescue a wide range of phenotypic defects in ga1-3 plants, such as stem elongation, flowering time, and flower development (ref. 42 and Fig. 6C). To closely examine the effects of RGA activity, we transformed ga1-3 rga-t2 double mutants with 35S::RGA-GR. The rationale is that, if the RGA-GR protein is biologically functional, activation of RGA by dexamethasone should revert the rescued phenotypes of ga1-3 rga-t2 to those of ga1-3. We subsequently isolated one ga1-3 rga-t2 35S::RGA-GR transgenic line, which showed phenotypic reversion from ga1-3 rga-t2 to ga1-3 after weekly treatment with dexamethasone (Fig. 4 A and B). This result indicates that the RGA-GR fusion protein has similar biological functions as WT RGA and allows control of RGA activity in a glucocorticoid-dependent manner.
Fig. 4.
A biologically active RGA-GR fusion. (A and B) Phenotypes of ga1-3 rga-t2 35S::RGA-GR. Mock-treated plants (A) had the same phenotypes as ga1-3 rga-t2 whereas plants treated with 10 μM dexamethasone (B) developed as ga1-3. (C–F) Phenotypes of ga1-3 rgl2-1 rga-t2 35S::RGA-GR. Inflorescences (C) and flowers (D) of mock-treated plants had the same phenotypes as ga1-3 rgl2-1 rga-t2 whereas inflorescences (E) and flowers (F) of dexamethasone-treated plants mimicked the phenotypes of ga1-3 rgl2-1. All plants were treated continuously once a week.
Published work (29) and our genetic analysis indicate that RGA is a key regulator of GA signaling involved in the control of continued development of floral organs, as reflected in the major phenotypic difference between ga1-3 rgl2-1 rga-t2 and ga1-3 rgl2-1. The former genotype showed significant rescue of floral defects as compared with the latter (Table 1). As RGL2 is the second most important DELLA protein after RGA in the control of flower development; lack of RGL2 activity in ga1-3 would potentially remove a majority of the redundant repressive effects with RGA. Thus, in the ga1-3 rgl2-1 rga-t2 background, the steroid-inducible activation of RGA could reveal major genes responding to RGA activity. We crossed ga1-3 rga-t2 35S::RGA-GR with ga1-3 rgl2-1 rga-t2 to generate ga1-3 rgl2-1 rga-t2 35S::RGA-GR. As expected, mock-treated ga1-3 rgl2-1 rga-t2 35S::RGA-GR (Fig. 4 C and D) showed the flower phenotypes of ga1-3 rgl2-1 rga-t2 plants (Fig. 6E) whereas dexamethasone-treated plants (Fig. 4 E and F) displayed the phenotypes of ga1-3 rgl2-1 (Table 1).
Floral Homeotic Genes Function Downstream of GA Signaling. Using the established steroid-inducible activation of RGA, we further studied whether the expression of floral homeotic genes is repressed by RGA activity. Dexamethasone treatment of inflorescence apices of ga1-3 rgl2-1 rga-t2 35S::RGA-GR for <6 h caused very little change in AP3, PI, and AG RNA levels, but 8 h of treatment resulted in a 2-fold reduction of transcript levels of these three genes (Fig. 1B), which was consistent with the up-regulation of the expression of these floral homeotic genes by GA treatment. Thus, B and C function genes are transcriptionally repressed by RGA, which is the major mediator of GA signaling involved in flower development.
However, our results demonstrated that the expression of AP3, PI, and AG did not respond to RGA activity within 4 h of treatment by dexamethasone (Fig. 1B). Furthermore, a combined treatment of dexamethasone and cycloheximide, an inhibitor of translation, for 6 h did not reveal any alteration of expression of these genes (data not shown). These results imply that RGA may control the expression of these floral homeotic genes in an indirect way. Also, whereas RGA may specifically regulate the B and C function genes, it does not seem to regulate AP1 and LFY because their expression did not respond either to GA treatment or to RGA activity (Fig. 1 and data not shown).
To further confirm that floral homeotic genes act downstream of GA signaling in later stages of flower development, we generated ga1-3 35::AG-GR, where a biologically active AG-GR fusion protein can be induced by dexamethasone (T.I. and E.M.M., unpublished results). If down-regulation of AG expression is partially responsible for ga1-3 floral phenotypes, provision of additional AG activity by dexamethasone should at least restore some phenotypic defects. This suggestion was confirmed by the following observations. Dexamethasone treatment of ga1-3 35::AG-GR provided functional AG activity, causing the phenotypic rescue of ga1-3 flowers with elongated stamens and pistils (Fig. 5B). At a later stage, stamen development was at least partially rescued (Fig. 5D), which eventually resulted in partial fertility (Fig. 5F). However, mock-treated plants still developed as ga1-3, with retarded growth of all floral organs and infertility (Fig. 5 A, C, and E). These observations suggest that the promotion of AG is necessary for continued development of reproductive organs, and that AG is a target of GA signaling in flower development.
Fig. 5.
Partial rescue of flower phenotype in ga1-3 by a biologically active AG-GR fusion. (A, C, and E) Mock-treated ga1-3 35S::AG-GR plants. (B, D, and F) Dexamethasone-treated ga1-3 35S::AG-GR plants. Plants were treated twice within a 1-day interval. Seven days after the first treatment, mock-treated plants had similar inflorescences (A) to ga1-3 whereas the inflorescences of dexamethasone-treated plants (B) developed flowers with elongated stamens and pistils. After one month, the most advanced flowers of mock-treated plants (C) contained withering stamens, and later they were completely sterile (E). The most advanced flowers of dexamethasone-treated plants (D) contained developing stamens, and later they were partially fertile (F). Dexamethasone treatment itself had no effects on the development of ga1-3 mutants.
Taken together, the work presented here suggests that GA promotes normal development of floral organs partly by up-regulating the expression of floral homeotic genes AP3, PI, and AG. GA achieves this effect by suppressing the function of two DELLA proteins, RGA and RGL2. It has been shown recently that GA regulates cell elongation in filament development and cellular differentiation in anthers leading from microspore to mature pollen grains (29). Our results indicate that GA may perform these functions by regulating the late functions of floral homeotic genes. Indeed, continuous AG activity seems to be necessary for promoting the growth of WT stamens with sporogenous cells, elongated filaments, and dehiscent 4-loculed anthers (T.I. and E.M.M., unpublished results). Thus, GA signaling in flower development is possibly coordinated by a regulatory network involving DELLA proteins and floral homeotic genes.
The absence of typical DNA-binding domains in DELLA proteins indicates that these transcriptional regulators may form complexes with other transcription factors to control the expression of downstream genes (18, 22, 23, 42). Because DELLA proteins function in a wide range of plant developmental programs, their involvement in flower development may be mediated by additional flower-specific regulators. It will be interesting to clarify whether floral homeotic genes are simply the downstream targets of DELLA proteins, or, alternatively, whether they may also interact with DELLA proteins as region-specific cofactors.
Supplementary Material
Acknowledgments
We thank Dr. Nick Harberd and Dr. Tai-Ping Sun for helpful comments and critical reading of the manuscript. This work was funded by National Institutes of Health Grant GM45697 (to E.M.M). H.Y. was supported in part by an overseas postdoctoral fellowship from the National University of Singapore.
Abbreviations: GA, Gibberellin; GR, glucocorticoid receptor; LFY, LEAFY; AG, AGAMOUS; PI, PISTILLATA; AP1, APETALA1; AP2, APETALA2; AP3, APETALA3.
References
- 1.Blázquez, M. A. & Weigel, D. (2000) Nature 404, 889-892. [DOI] [PubMed] [Google Scholar]
- 2.Parcy, F., Nilsson, O., Busch, M. A., Lee, I. & Weigel, D. (1998) Nature 395, 561-566. [DOI] [PubMed] [Google Scholar]
- 3.Weigel, D. & Meyerowitz, E. M. (1994) Cell 78, 203-209. [DOI] [PubMed] [Google Scholar]
- 4.Bowman, J. L., Smyth, D. R. & Meyerowitz, E. M. (1991) Development 112, 1-20. [DOI] [PubMed] [Google Scholar]
- 5.Coen, E. S. & Meyerowitz, E. M. (1991) Nature 353, 31-37. [DOI] [PubMed] [Google Scholar]
- 6.Mandel, M. A., Gustafson-Brown, C., Savidge, B & Yanofsky, M. F. (1992) Nature 360, 273-277. [DOI] [PubMed] [Google Scholar]
- 7.Jack, T., Brockman, L. L. & Meyerowitz, E. M. (1992) Cell 68, 683-697. [DOI] [PubMed] [Google Scholar]
- 8.Goto, K. & Meyerowitz, E. M. (1994) Genes Dev. 8, 1548-1560. [DOI] [PubMed] [Google Scholar]
- 9.Yanofsky, M. F., Ma, H., Bowman, J. L., Drews, G., Feldmann, K. & Meyerowitz, E. M. (1990) Nature 346, 35-39. [DOI] [PubMed] [Google Scholar]
- 10.Bowman, J. L., Drews, G. N. & Meyerowitz, E. M. (1991) Plant Cell 3, 749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drews, G. N., Bowman, J. L. & Meyerowitz, E. M. (1991) Cell 65, 991-1002. [DOI] [PubMed] [Google Scholar]
- 12.Lohmann, J. U. & Weigel, D. (2002) Dev. Cell 2, 135-142. [DOI] [PubMed] [Google Scholar]
- 13.Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. (1992) Cell 69, 843-859. [DOI] [PubMed] [Google Scholar]
- 14.Wilson, R., Heckman, J. W. & Somerville, C. (1999) Plant Physiol. 100, 403-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto, N. & Pharis, R. P. (1999) Can. J. Bot. 77, 944-954. [Google Scholar]
- 16.Langridge, J. (1957) Nature 180, 36-37. [Google Scholar]
- 17.Ross, J. J., Murfet, I. C. & Reid, J. B. (1997) Physiol. Plant 100, 550-560. [Google Scholar]
- 18.Peng, J., Carol, P., Richards, D. E., King, K. E., Cowling, R. J., Murphy, G. P. & Harberd, N. P. (1997) Genes Dev. 11, 3194-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng, J., Richards, D. E., Hartley, N. M., Murphy, G. P., Devos, K. M., Flintham, J. E., Beales, J., Fish, L. J., Worland, A. J., Pelica, F., et al. (1999) Nature 400, 256-261. [DOI] [PubMed] [Google Scholar]
- 20.King, K. E., Moritz, T. & Harberd, N. P. (2001) Genetics 159, 767-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dill, A. & Sun, T.-P. (2001) Genetics 159, 777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S., Cheng, H., King, K. E., Wang, W., He, Y., Hussain, A., Lo, J., Harberd, N. P. & Peng, J. (2002) Genes Dev. 16, 646-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen, C.-K. & Chang, C. (2002) Plant Cell 14, 87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu, X. & Harberd, N. P. (2003) Nature 421, 740-743. [DOI] [PubMed] [Google Scholar]
- 25.Dill, A., Jung, H.-S. & Sun, T.-P. (2001) Proc. Natl. Acad. Sci. USA 98, 14162-14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu, X., Richards, D. E., Ait-Ali, T., Hynes, L. W., Ougham, H., Peng, J. & Harberd, N. P. (2002) Plant Cell 14, 3191-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubler, F., Chandler, P. M., White, R. G., Llewellyn, D. J. & Jacobsen, J. V. (2002) Plant Physiol. 129, 191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M. & Matsuoka, M. (2002) Plant Cell 14, 57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng, H., Qin L., Lee, S., Fu, X., Richards, D. E., Cao D., Luo, D., Harberd, N. P. & Peng, J. (2004) Development 131, 1055-1064. [DOI] [PubMed] [Google Scholar]
- 30.Wagner, D., Sablowski, R. W. & Meyerowitz, E. M. (1999) Science 285, 582-584. [DOI] [PubMed] [Google Scholar]
- 31.Hellens, R., Edwards, E., Leyland, N., Bean, S. & Mullineaux, P. (2000) Plant Mol. Biol. 42, 819-832. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd, A. M., Schena, M., Walbot, V. & Davis, R. W. (1994) Science 266, 436-439. [DOI] [PubMed] [Google Scholar]
- 33.Yu, H., Xu, Y., Tan, E. L. & Kumar, P. P. (2002) Proc. Natl. Acad. Sci. USA 99, 16336-16341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer, K. F., Schoof, H., Haecker, A., Lenhard, M., Jürgens, G. & Laux, T. (1998) Cell 95, 805-815. [DOI] [PubMed] [Google Scholar]
- 35.Sakai, H., Medrano, L. J. & Meyerowtiz, E. M. (1995) Nature 378, 199-203. [DOI] [PubMed] [Google Scholar]
- 36.Pelaz, S., Gustafson-Brown, C., Kohalmi, S. E., Crosby, W. L. & Yanofsky, M. F. (2001) Plant J. 26, 385-394. [DOI] [PubMed] [Google Scholar]
- 37.Long, J. A. & Barton, M. K. (1998) Development 125, 3027-3035. [DOI] [PubMed] [Google Scholar]
- 38.Yu, H., Ito, T., Wellmer, F. & Meyerowitz, E. M. (2004) Nat. Genet. 36, 157-161. [DOI] [PubMed] [Google Scholar]
- 39.Smyth, D. R., Bowman, J. L. & Meyerowitz, E. M. (1990) Plant Cell 2, 755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blázquez, M. A., Green, R., Nilsson, O., Sussman, M. R. & Weigel, D. (1998) Plant Cell 10, 791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamuro, J. K., Szeto, W., Lotys-Prass, C. & Jofuku, K. D. (1997) Plant Cell 9, 37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstone, A. L., Ciampaglio, C. N. & Sun, T.-P. (1998) Plant Cell 10, 155-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalman, F. C., Scherrer, L. C., Taylor, L. P., Akil, H. & Pratt, W. B. (1991) J. Biol. Chem. 266, 3482-3490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.