Abstract
Background
The coupling of biotic and abiotic stresses leads to high yield losses in rainfed rice (Oryza sativa L.) growing areas. While several studies target these stresses independently, breeding strategies to combat multiple stresses seldom exist. This study reports an integrated strategy that combines QTL mapping and phenotypic selection to develop rice lines with high grain yield (GY) under drought stress and non-stress conditions, and tolerance of rice blast.
Methodology
A blast-tolerant BC2F3-derived population was developed from the cross of tropical japonica cultivar Moroberekan (blast- and drought-tolerant) and high-yielding indica variety Swarna (blast- and drought-susceptible) through phenotypic selection for blast tolerance at the BC2F2 generation. The population was studied for segregation distortion patterns and QTLs for GY under drought were identified along with study of epistatic interactions for the trait.
Results
Segregation distortion, in favour of Moroberekan, was observed at 50 of the 59 loci. Majority of these marker loci co-localized with known QTLs for blast tolerance or NBS-LRR disease resistance genes. Despite the presence of segregation distortion, high variation for DTF, PH and GY was observed and several QTLs were identified under drought stress and non-stress conditions for the three traits. Epistatic interactions were also detected for GY which explained a large proportion of phenotypic variance observed in the population.
Conclusions
This strategy allowed us to identify QTLs for GY along with rapid development of high-yielding purelines tolerant to blast and drought with considerably reduced efforts. Apart from this, it also allowed us to study the effects of the selection cycle for blast tolerance. The developed lines were screened at IRRI and in the target environment, and drought and blast tolerant lines with high yield were identified. With tolerance to two major stresses and high yield potential, these lines may provide yield stability in rainfed rice areas.
Introduction
Developing crop varieties with higher yield potential is a challenging task in modern-day plant breeding. The Green Revolution led to a historic breakthrough in increasing the yield potential of crops, such as rice and wheat, through the development of semi-dwarf varieties that has fed the increasing population till now. However, it is predicted that a 60% increase in agricultural production needs to be achieved by 2050 to feed the ever-growing population [1].
Unlike other cereals, rice is cultivated across a much wider range of ecosystems, including irrigated, rainfed upland, rainfed lowland, flooded, and deepwater ecosystems across tropical and temperate climate conditions. Despite this diversity of cultivation systems and environments, the major focus of research in increasing rice yield potential has been for the irrigated ecosystem in the post-Green Revolution period. However, no major breakthrough in breaking the yield potential barrier has been achieved until now.
The low-yielding rainfed lowland rice ecosystem has great potential for yield improvement. This ecosystem occupies about 38% of the total rice area, but contributes only 21% to total rice production [2]. In Asia alone, 34 million ha of rainfed lowland rice and 8 million ha of rainfed upland rice [3] experience drought stress of varying intensity almost every year. These drought events are often coupled with increased incidence of other biotic stresses, such as blast, brown spot, and bacterial blight, leading to a further decline in rice yield. Rice yields in these ecosystems remain low at 1.0–2.5 t ha−1 [4].
Despite the importance of drought as a major factor in yield reduction in rainfed ecosystems, few efforts have been made to develop high-yielding drought-tolerant cultivars. As a result, a large proportion of these rainfed areas are covered with high-yielding varieties such as Swarna, Samba Mahsuri, IR64, and MTU1010 that were specifically developed for irrigated ecosystems and suffer heavy yield losses under drought. On the other hand, several drought-tolerant cultivars, such as N22 and Moroberekan, have evolved in these rainfed ecosystems through natural or artificial selection over the years. These cultivars carry genes for tolerance of a wide range of biotic and abiotic stresses, but lack farmers' and consumers' preference because of their low yield potential and poor grain quality. In many cases, a single cultivar shows tolerance of a combination of biotic and abiotic stresses. This provides a unique opportunity for rice breeders to develop high-yielding varieties with tolerance of multiple abiotic and biotic stresses.
The lack of effective selection criteria and low heritability of grain yield (GY) under drought have been reported as the two major reasons for the slow progress in breeding drought-tolerant varieties [5]. With increased precision in large-scale phenotyping, many studies have shown moderate to high heritability for GY under drought [6]–[8]. These studies used direct selection for GY in progenies derived from high-yielding drought-susceptible varieties and drought-tolerant donors as a successful strategy to improve yield under drought. This strategy has also proven beneficial to combining high yield potential with drought tolerance and has resulted in the development and release of several high-yielding rice varieties with good yield under drought in countries such as India, Nepal, Bangladesh, Indonesia, and the Philippines [9]. Along with the release of varieties through conventional breeding approaches, several large-effect QTLs for GY under drought have also been identified [2], [10]–[15]. Similarly, several studies have also reported independent and epistatic QTLs for GY and other traits of agronomic importance [16]–[19]. However, only a few studies have reported QTLs leading to a yield advantage under both drought stress and non-stress environments, taking grain yield as the primary selection criterion. Occurrence of drought is normally coupled with increased incidence of diseases such as rice blast, brown spot, and bacterial blight. However, very few studies have been undertaken to understand the genetics of these abiotic and biotic stresses simultaneously in a mapping population.
In our study, an advanced backcross (AB) QTL approach was used on a large backcross population developed from a cross between tropical japonica, drought- and rice blast-tolerant donor Moroberakan with highly popular recipient indica rice variety Swarna. The objectives were to: (1) develop a strategy to combine breeding approaches for tolerance of blast and drought with QTL mapping, (2) understand the effect of a selection cycle for blast tolerance during population development on segregation distortion and population structure, (3) identify QTLs for GY under drought stress and non-stress conditions, and (4) develop high-yielding blast- and drought-tolerant breeding lines from the mapping population and evaluate them in the target environment.
Materials and Methods
Experiments for this study were conducted at the Experiment Station of the International Rice Research Institute (IRRI), Los Baños, Laguna, Philippines, during the dry seasons (DS) of 2011, 2012, and 2013 and the wet season (WS) of 2012. IRRI is located at 14°13′ N and 121°15′ E, at an elevation of 21 m above mean sea level. The study also presents results from two experiments conducted at the IRRI South Asia breeding hub (IRRI-SA) located at the International Crop Research Institute for Semi-Arid Tropics (ICRISAT), Patancheru, Hyderabad (AP), India in WS2013. ICRISAT is located at 17°31′ N and 78°15′ E at an elevation of 516 m above sea level.
Plant material
A BC2F3-derived population developed from the cross of Moroberekan and Swarna was used in this study. Moroberekan, the tolerant donor, is an upland adapted tropical japonica [20] cultivar from New Guinea. It is a long-duration line with sturdy plant type, deep roots, and tolerance of drought and rice blast. However, this variety has poor yield potential due to its low tillering ability and lower number of grains per panicle. On the other hand, Swarna (MTU7029), the drought-susceptible recipient parent used in this study, is a lowland-adapted high-yielding indica [2], [21] variety derived from the cross Vashishtha × Mahsuri. It is a long-duration semi-dwarf variety with high tillering ability and grain yield but is highly susceptible to diseases such as rice blast and bacterial blight. This variety is grown on a large area in rainfed and irrigated ecologies across India, Nepal, and Bangladesh and is regarded as a mega-variety of rice.
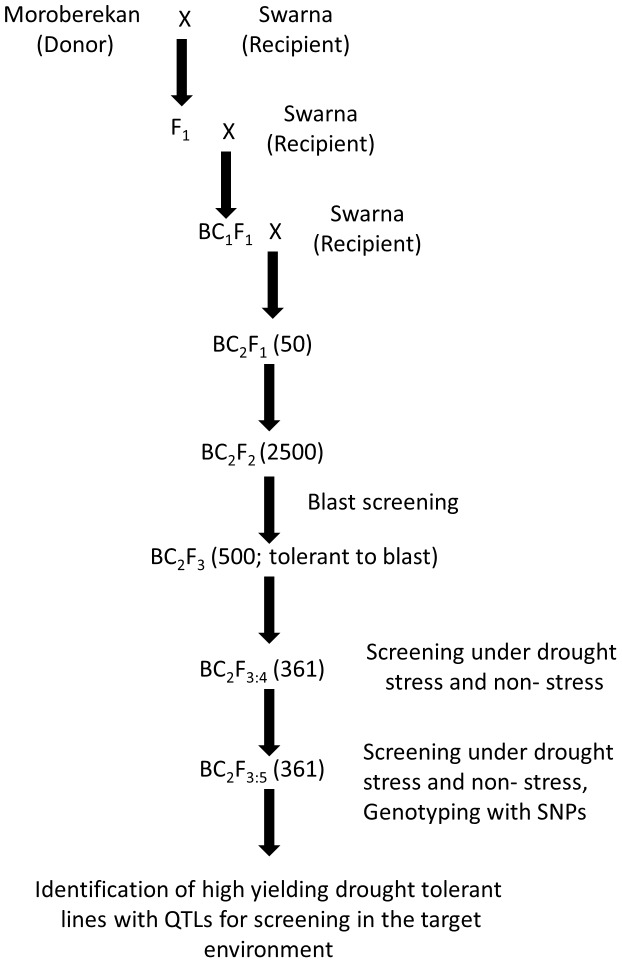
Figure 1 presents the strategy used to develop the mapping population. The BC2F1 plants developed through the cross of the two parents were advanced through the single seed descent (SSD) method to develop a large BC2F2 population. This population was screened for tolerance of rice blast. Blast-tolerant plants identified through this screening were advanced to the BC2F3 generation through SSD. Seeds from BC2F3 single plants were used to develop a BC2F3∶4 mapping population to screen for GY under drought stress and non-stress conditions. Furthermore, single plant selections were made from the mapping population under drought stress. These selections were advanced, purified, and screened at IRRI, Philippines and IRRI-SA, India to identify lines that perform the best in both environments.
Figure 1. Population development and phenotypic screening strategy used to develop a blast-tolerant BC2F3-derived mapping population for mapping QTLs for high grain yield under drought stress and non-stress conditions.
Stress ecosystems
Field experiments for mapping QTLs were conducted in upland and lowland ecosystems. Throughout the study, the term “upland” refers to field experiments conducted under dry direct-seeded, unpuddled, aerobic conditions, whereas “lowland” refers to field experiments conducted under flooded, puddled, transplanted, and anaerobic conditions. Experiments were conducted under both drought stress and non-stress conditions. In this study, fully irrigated experiments with no drought stress imposed throughout the season are referred to as non-stress experiments; whereas, those experiments on which drought stress was imposed during the reproductive stage (starting at 40 and 50 days after seeding, respectively, under upland and lowland conditions) are referred to as drought stress experiments. In order to further classify the stress experiments into mild, moderate, or severe stress, the percentage reduction in yield as compared to non-stress conditions was used as a criterion [6].
Phenotypic evaluation and data collection
Details of all the experiments, including population size, experimental design, number of replications, and plot size are presented in Table 1. A population of 361 BC2F3-derived lines was screened under lowland stress and non-stress conditions in DS2011 and DS2012. A subset of 100 lines from this population was screened under upland stress conditions in WS2012, along with parents Moroberekan and Swarna. Breeding lines developed through plant selection from this population were screened under lowland stress and non-stress conditions in DS2013 at IRRI and WS2013 at IRRI-SA. All experiments were conducted as replicated field trials in α lattice design in continuous plots. The experimental setup has proved effective for screening large rice mapping populations for grain yield under drought using smaller plot sizes. The method has been used in several QTL identification studies, which have led to the identification of large and consistent QTLs now being used in marker-assisted breeding (MAB) programs [2], [10]–[15]. Field and crop management were carried out using the protocol presented in File S1.
Table 1. Details of experiments conducted for identification of QTLs and screening of drought-tolerant lines identified from the mapping population at IRRI and in IRRI-SA.
| Experiment | Season | Ecosystem | Population size | Location | Experimental design | Replications | Plot size |
| 1 | DS2011 | LSS | 361 | IRRI | 38×10 AL | 2 | 1.0 |
| 2 | DS2012 | LMS | 361 | IRRI | 38×10 AL | 2 | 2.0 |
| 3 | DS2011 | LNS | 361 | IRRI | 38×10 AL | 2 | 1.0 |
| 4 | DS2012 | LNS | 361 | IRRI | 38×10 AL | 2 | 2.0 |
| 5 | WS2012 | USS | 99 | IRRI | 12×10 AL | 2 | 1.0 |
| 6 | DS2013 | LSS | 77 | IRRI | 10×10 AL | 2 | 2.0 |
| 7 | DS2013 | LNS | 77 | IRRI | 10×10 AL | 2 | 2.0 |
| 8 | WS2013 | LMiS | 95 | IRRI-SA | 8×13 AL | 2 | 2.4 |
| 9 | WS2013 | LNS | 95 | IRRI-SA | 8×13 AL | 2 | 3.2 |
DS = dry season, WS = wet season, LSS = lowland severe stress, LMS = lowland moderate stress, LNS = lowland non-stress, UMS = upland moderate stress.
In all experiments, data on days to flowering (DTF), plant height (PH) at maturity, and grain yield (GY) were recorded. DTF was recorded as the number of days from seeding to the day that 50% of the plants had flowering tillers. The PH of three plants from each plot were measured at maturity from the ground to the tip of the tallest tiller, and then averaged to get the mean PH for analysis. GY from each plot was harvested at physiological maturity, dried to a moisture content of 14%, and then weighed [11]. This data set was then used to calculate the quantity used in QTL analysis, namely GY of the genotypes in kg ha−1.
Generation of genotypic data
Fresh leaves for all lines were collected and freeze-dried. DNA was extracted from freeze-dried leaf samples by a modified CTAB method in deep-well plates. The DNA was then quantified and purified and lines were genotyped using KASPar SNP assays. These SNPs were selected as subsets from the set of 1,536 and 44K SNP chips [22], [23] converted to SNP assays and made available through the integrated breeding platform (https://www.integratedbreeding.net/snp-marker-conversion). A total of 2,015 SNP markers were screened for polymorphism between the two parents. Out of the 2,015 SNPs screened for polymorphism, 591 polymorphic SNP loci were identified. A total of 200 polymorphic markers spaced uniformly across the genome were used to generate the genotypic data of the population. To ensure data quality, lines having percentages higher than expected of heterozygote alleles (>40%), Moroberekan alleles (>40%), or missing data (>30%) were filtered before analysis. Furthermore, markers were removed if the frequency of genotypes deriving from Moroberekan (homozygote and heterozygote) was <2%, as they were essentially monomorphic.
Statistical analysis
ANOVA and means
Data from all experiments for computation of means and standard error of difference (SED) were analyzed using CROPSTAT version 7.2.3. Mixed model analysis of data from individual years was carried out using the model
where yijk is the measurement recorded in a plot, μ is the overall mean, gi is the effect of the ith genotype, rj is the effect of the jth replicate, bk(rj) is the effect of the kth block within the jth replicate, and eijk is the error. Genotypic effects were considered fixed and the replicates and block effects were random.
A combined analysis was conducted for lowland experiments to obtain line means across years (2011 and 2012) under stress and non-stress conditions. The model used was
where yijkl is the measurement recorded in a plot, μ is the overall mean, gi is the effect of the ith genotype, ml is the effect of the lth year, rj(ml) is the effect of the jth replicate within the lth year, bk[rj(ml)] is the effect of the kth block within the jth replicate of the lth year, giml is the effect of the interaction between the ith genotype and lth year, and eijkl is the error. The effects of replicate within year and block within replicate of year were considered random, whereas the other effects were considered fixed. Due to the high variability for DTF in the population, adjusted mean GY were also calculated by taking DTF as covariate using the above models for QTL mapping. This was done to estimate the mean GY of lines free from the variation caused due to their difference in flowering time (File S3).
Heritability
Broad-sense heritability (H) of the traits for single years was calculated as shown below:
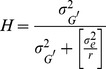 |
where 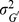 is the genetic variance,
is the genetic variance, 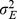 is the plot residual variance and r is the number of replications. The formula used to calculate H across years (2011 and 2012) was
is the plot residual variance and r is the number of replications. The formula used to calculate H across years (2011 and 2012) was
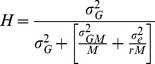 |
[24], where 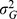 is the genetic variance,
is the genetic variance, 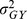 is the genotype × year variance,
is the genotype × year variance, 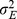 is the plot residual variance, and r and M are the number of replicates and years, respectively. Variance components were calculated using the REML algorithm of PROC VARCOMP of SAS V.9.1 [25]. A class analysis was conducted to understand the effect of DTF and PH on GY. Lines were divided into five DTF and GY classes and heat maps were constructed showing GY response in all possible combinations of these classes under drought stress and non-stress conditions. ‘Levelplot’ in R was used to construct the heat maps.
is the plot residual variance, and r and M are the number of replicates and years, respectively. Variance components were calculated using the REML algorithm of PROC VARCOMP of SAS V.9.1 [25]. A class analysis was conducted to understand the effect of DTF and PH on GY. Lines were divided into five DTF and GY classes and heat maps were constructed showing GY response in all possible combinations of these classes under drought stress and non-stress conditions. ‘Levelplot’ in R was used to construct the heat maps.
QTL mapping
Genetic analysis of the population included estimation of segregation distortion, composite interval mapping (CIM), and estimation of epistatic effects. The level of segregation distortion was assessed using the function ‘geno.table’ in the R package qtl [26]. In order to understand the co-location of markers showing significant distortion to blast QTLs and known genes for disease resistance in rice, a physical map of SNP markers (used in this study), QTLs for blast resistance (reported in Moroberekan-derived mapping populations), and known disease resistance genes in rice was constructed. Information about disease resistance genes as well as those within the identified QTLs and their locations was obtained from the MSU Rice Genome Annotation Project, whereas QTL information was obtained from www.gramene.org and used along with the SNP markers from this study to construct a map using GGT 2 [27]. To map QTLs for GY, DTF, and PH, the genome was scanned for QTLs at a step size of 5 cM, using composite interval mapping (‘cim’) in R/qtl [26] with 5 cofactors. The genome-wide significance level was determined based on 1,000 permutations. A two-dimensional scan was also performed to identify epistatic QTLs using ‘scantwo’ in R/qtl with 1,000 permutations.
Results
Population development and segregation patterns
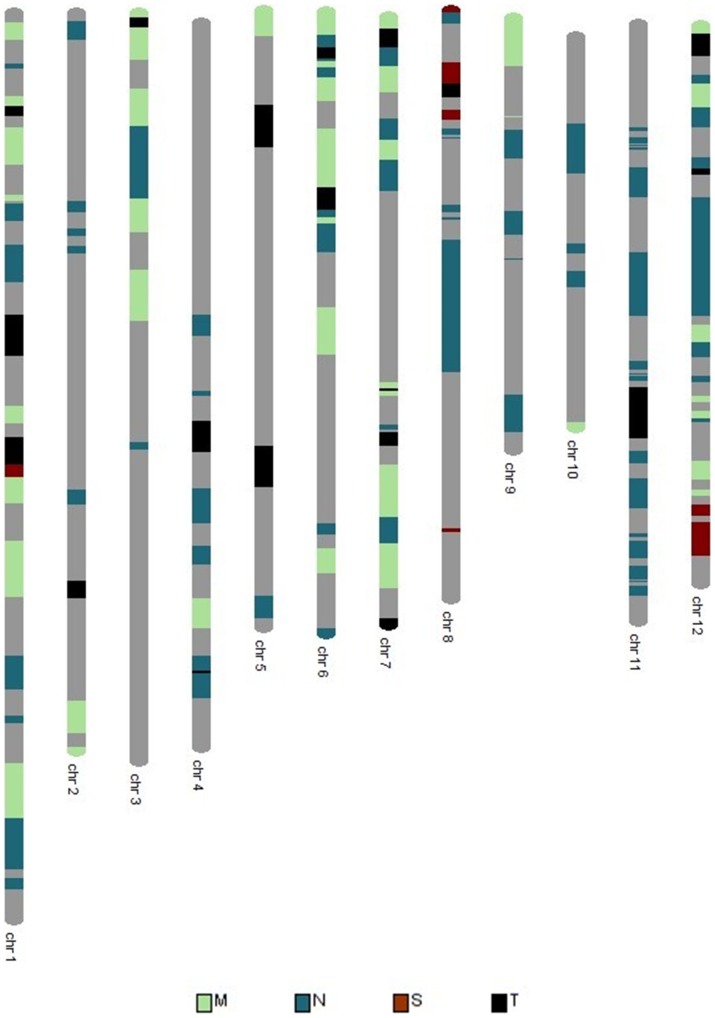
Our study reports a population development strategy using donors tolerant of more than one biotic and/or abiotic stress for simultaneous QTL mapping and pureline development in elite genetic background (Fig. 1). In this study, tropical japonica cultivar Moroberekan was used as the donor parent for tolerance of drought and rice blast, whereas indica variety Swarna was used as the recipient. Our strategy involved developing a large BC2F2 population and screening this population for blast tolerance. Only blast-tolerant plants were advanced further to develop the BC2F3-derived mapping population, which was used to detect QTLs for grain yield under drought and to develop purelines by plant selection in the mapping population. Since selection pressure was applied for blast tolerance, segregation distortion was expected within the mapping population. The genotypic data generated from BC2F3-derived lines were used to conduct the test for segregation distortion. A total of 50 and 9 marker loci showed segregation distortion in favour of the donor parent Moroberekan and recipient parent Swarna, respectively (Table S1). The largest segregation distortions were seen on chromosomes 3, 4, and 7 (Table S1). Segregation distortion in favour of Swarna was seen at chromosomes 1, 8, and 12. The physical map of SNP markers from this study, known disease-resistance genes, and meta QTLs for blast resistance along the rice genome, highlighted NBS-LRR disease resistance genes close to the markers showing significant segregation distortion in favour of Moroberekan on chromosomes 3, 6, and 7 (Fig. 2). Since the majority of blast resistance genes code for NBS-LRR type proteins, the presence of these genes close to the markers showing distortion may be expected as a result of phenotypic selection for blast tolerance.
Figure 2. Physical map of markers showing segregation distortion, known genes for disease resistance in rice, and QTLs reported for blast resistance in the Moroberekan/CO39 population.
M = markers showing distortion in favour of Moroberekan, S = markers showing distortion in favour of Swarna, N = genes coding for NBS-LRR proteins, and T = QTLs reported for blast resistance in the Moroberekan/CO39 population.
Phenotypic variation
Table 2 presents the results of ANOVA, means, heritability, and percentage yield reduction (under drought stress) in the experiments conducted at IRRI and IRRI-South Asia (IRRI-SA) under upland and lowland conditions. The full population was screened in Experiments 1-4 under stress and non-stress conditions. Yield reduction in Experiments 1 and 2 varied with stress levels in the respective seasons from severe for Experiment 1 to moderate in Experiment 2. Experiments 6 and 7 followed similar trends as Experiments 1 and 2 had in terms of yield. Both parents had similar yield under severe stress, however, Swarna (P2), being the high-yielding parent, had higher yield under moderate stress conditions in the lowland. The high yield of Swarna under moderate stress was also due to the poor adaptation of Moroberekan, an upland cultivar to lowland conditions. The population means remained higher than those for parents in both stress experiments, suggesting the role of high yield and drought tolerance in deciding the yield under drought.
Table 2. Means ± SED (M), broad-sense heritability (H) and P values for grain yield, days to flowering, and plant height and percentage yield reduction (YR) of trial mean grain yield under stress and non-stress conditions.
| Grain yield (kg ha−1) | Days to flowering | Plant height (cm) | ||||||||||||||
| Experiment | P1 | P2 | M | H | P | P1 | P2 | M | H | P | P1 | P2 | M | H | P | YR |
| 1 | 88 | 87 | 677±563 | 0.60 | **** | 109 | 110 | 99±4 | 0.85 | **** | 72 | 56 | 68±9 | 0.8 | **** | 93 |
| 2 | 954 | 2326 | 2115±688 | 0.61 | **** | 100 | 102 | 92±5 | 0.83 | **** | 102 | 76 | 82±8 | 0.87 | **** | 45 |
| Combined | 384 | 1211 | 1390±626 | 0.55 | **** | 104 | 106 | 95±5 | 0.87 | **** | 87 | 66 | 75±9 | 0.70 | **** | 66 |
| 3 | 2234 | 4552 | 4370±868 | 0.78 | **** | 97 | 98 | 91±5 | 0.72 | **** | 126 | 91 | 102±8 | 0.84 | **** | |
| 4 | 1248 | 4012 | 3859±1049 | 0.54 | **** | 93 | 93 | 88±7 | 0.78 | **** | 118 | 92 | 99±10 | 0.69 | **** | |
| Combined | 1701 | 4282 | 4118±676 | 0.71 | **** | 95 | 95 | 90±5 | 0.72 | **** | 122 | 90 | 101±6 | 0.80 | **** | |
| 5 | 691 | 89 | 827±357 | 0.92 | **** | 95 | 112 | 100±5 | 0.88 | **** | 107 | 75 | 89±7 | 0.85 | **** | 80 |
| 6 | 0 | 0 | 1274±433 | 0.93 | **** | 105 | 105 | 87±2 | 0.98 | **** | 84 | 58 | 74±6 | 0.86 | **** | 83 |
| 7 | 2893 | 6857 | 7380±1628 | 0.67 | **** | 94 | 95 | 86±2 | 0.95 | **** | 128 | 91 | 100±7 | 0.82 | **** | |
| 8 | NA | NA | 4757±856 | 0.83 | **** | NA | NA | 94±2 | 0.85 | **** | NA | NA | 97±6 | 0.59 | *** | 40 |
| 9 | NA | NA | 7895±1027 | 0.31 | **** | NA | NA | 93±3 | 0.92 | **** | NA | NA | 116±7 | 0.61 | **** | |
P1 = Moroberekan, P2 = Swarna, M = population mean, H = heritability, **** = significant at 0.01% P level.
The advantage of Moroberekan (P1) over Swarna was seen more clearly under upland conditions (Experiment 5), in which a large difference was recorded in the yield of both parents. Mild stress levels were recorded in Experiment 8 as this experiment was conducted in the wet season under naturally occurring drought stress. Moderate to high heritability estimates were recorded for yield in all experiments.
In all DS experiments, both parents had similar DTF under stress and non-stress conditions, but their progeny showed a wide range of DTF values. While Swarna is a well-known long-duration variety, there was a possibility of Moroberekan contributing to the earliness of the progeny. While, this earliness was not seen in the cultivar itself in DS experiments, this difference became clear in the WS experiment (5) when Moroberekan flowered much earlier than Swarna. Similar observations were also recorded in seed multiplication trials under lowland conditions in WS2013 (data not presented).
Trait correlation and effect
Genetic correlations between DTF, PH, and GY under lowland stress and non-stress conditions (DS2011 and DS2012) are presented in Table 3. These four experiments were conducted using the whole population, and hence, were used to understand trait correlations. Clear correlation patterns between DTF and GY were seen in all experiments. The two traits were negatively correlated in both stress and non-stress experiments. Regardless of stress severity levels, earliness of lines related to higher yield under stress environments. Also, under non-stress conditions, a similar pattern was observed, indicating that the reduced growth period did not lead to reduced yield potential. The degree of correlation however was much higher in stress experiments than in non-stress experiments. In contrast to DTF, no clear correlation patterns between PH and GY were observed in both stress and non-stress conditions.
Table 3. Genetic correlation between grain yield (GY), days to flowering (DTF), and plant height (PH) under lowland drought stress and non-stress conditions.
| DTF(LSS) | PH(LSS) | GY(LSS) | DTF(LMS) | PH(LMS) | GY(LMS) | DTF (LNS I) | PH (LNS I) | GY(LNS I) | DTF (LNS II) | PH(LNS II) | GY (LNS II) | |
| DTF(LSS) | 1 | |||||||||||
| PH(LSS) | −0.597*** | 1 | ||||||||||
| GY(LSS) | −0.813*** | 0.551*** | 1 | |||||||||
| DTF(LMS) | 0.936*** | −0.495*** | −0.716*** | 1 | ||||||||
| PH(LMS) | −0.042 | 0.727*** | −0.018 | −0.040 | 1 | |||||||
| GY(LMS) | −0.519*** | 0.168*** | 0.711*** | −0.679*** | −0.145** | 1 | ||||||
| DTF (LNS I) | 0.998*** | −0.699*** | −0.794*** | 0.869*** | −0.166*** | −0.568*** | 1 | |||||
| PH (LNS I) | −0.238*** | 0.831*** | 0.185*** | −0.170*** | 0.969*** | −0.063 | −0.297*** | 1 | ||||
| GY(LNS I) | −0.297*** | 0.348*** | 0.308*** | −0.318*** | 0.264*** | 0.579*** | −0.403*** | 0.317*** | 1 | |||
| DTF (LNS II) | 0.877*** | −0.426*** | −0.672*** | 0.845*** | −0.137* | −0.664*** | 0.810*** | −0.166** | −0.226*** | 1 | ||
| PH(LNS II) | 0.114* | 0.668*** | −0.204*** | 0.095 | 0.801*** | −0.238*** | −0.086 | 0.894*** | 0.123* | 0.037 | 1 | |
| GY (LNS II) | −0.401*** | 0.245*** | 0.471*** | −0.457*** | 0.084 | 0.949**** | −0.482*** | 0.058 | 0.861*** | −0.368*** | −0.095 | 1 |
LSS = lowland severe stress, LMS = lowland moderate stress, LNS = lowland non-stress. *, **, ***, **** = significant at 5%, 1%, 0.1% 0.01% P levels, respectively.
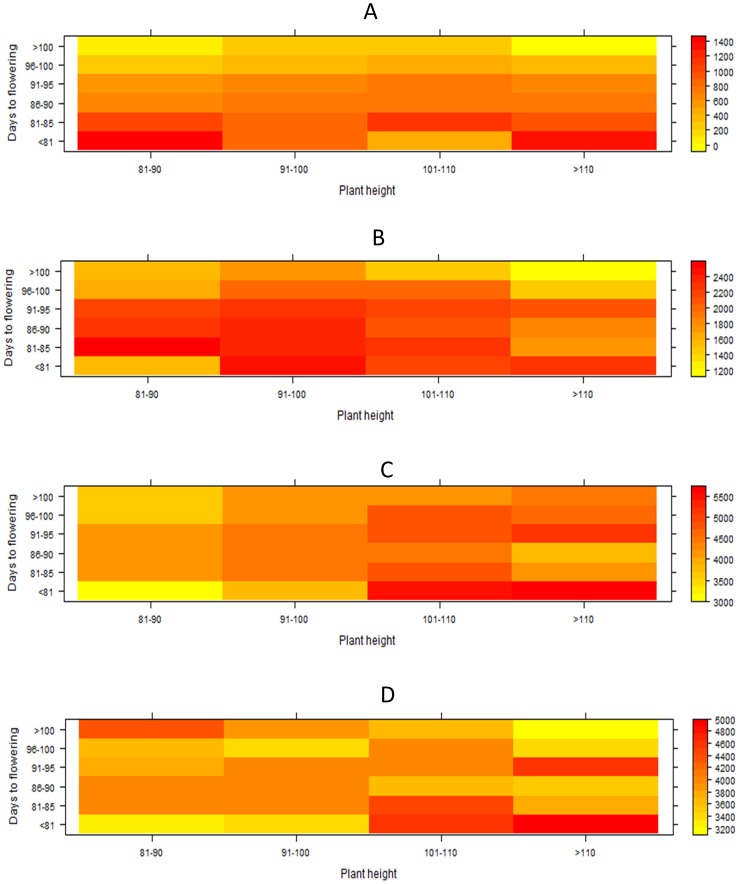
In order to further understand the relationships between DTF, PH, and GY, a class analysis was conducted to measure GY for varying combinations of DTF and PH levels (Fig. 3). At any level of PH, greater differences were seen in GY for varying levels of DTF than vice versa. This agrees with the higher correlation observed between DTF and GY than between PH and GY. In general, lines with medium maturity (DTF = 81–95 days) were the most consistent at all levels of plant height across all four experiments. However, lines with plant height from 101–110 cm showed the most consistent performance across the four experiments as compared to those beyond these levels.
Figure 3. Heat maps showing the effect of DTF and PH on yield performance of lines under (A) lowland severe stress (LSS), (B) lowland moderate stress (LMS), (C) lowland non-stress (LNS I) and (D) LNS II conditions.
Increasing intensity of colors from yellow to orange indicates increasing yield levels while white color shows the unavailable PH class.
Genetic variation
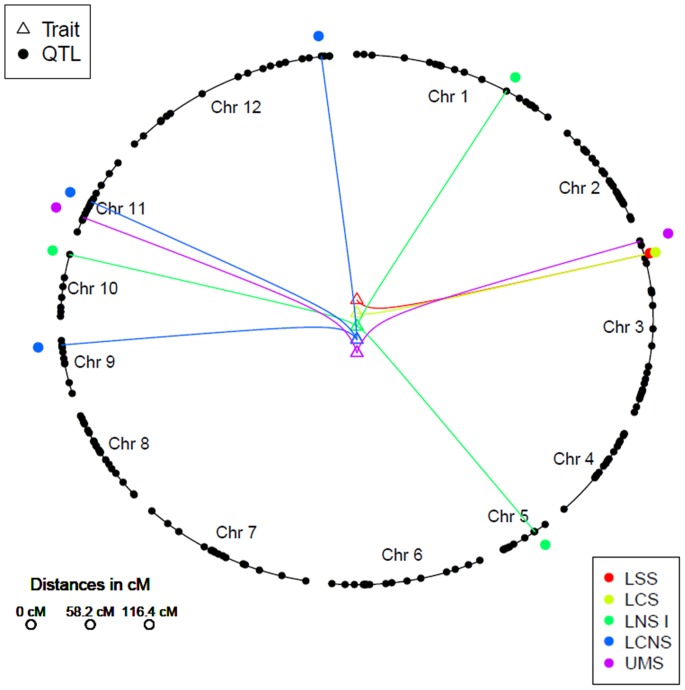
Analysis of GY identified several QTLs for each trait significant within and across environments (Fig 4.,Table 4). The most consistent QTL identified for GY was qDTY3.2, which had an effect under drought stress across both upland and lowland conditions. The QTL explained a phenotypic variance effect of 16.0% and 19.0% under lowland severe stress (LSS) and upland moderate stress (UMS), respectively (Table 4). Apart from this, qDTY11.1 was identified was identified under UMS condition. The QTL explained a phenotypic variance of 25.0%. To the best of our knowledge, this is the first time qDTY11.1 is being reported for GY under upland drought stress.
Figure 4. Circular genome plot showing the positions of QTLs identified for grain yield (adjusted for flowering time) under drought stress and non-stress conditions.
LSS = lowland severe stress, LNS = lowland non-stress, LCS = lowland combined stress, LCNS = lowland combined non-stress, UMS = upland moderate stress.
Table 4. QTLs for grain yield adjusted for flowering time (GY), plant height (PH), and flowering time (DTF) identified under severe and/or moderate reproductive-stage drought stress and non-stress conditions in DS2011 and DS2012.
| Trait | QTL | Ecosystem | Peak marker | Chromosome | cM | R2 | A | P |
| GY | qDTY3.2 | LSS | Id3000757 | 3 | 15.0 | 16.0 | 281.6 | <0.001 |
| qDTY3.2 | LCS | Id3000757 | 3 | 15.0 | 14.0 | 211.3 | <0.001 | |
| qDTY1.1 | LNS I | id1021344 | 1 | 154.0 | 5.5 | 552.5 | 0.009 | |
| qDTY5.1 | LNS I | wd5002636 | 5 | 13.5 | 17.0 | 1743.0 | 0.009 | |
| qDTY10.2 | LNS I | id10006250 | 10 | 69.3 | 4.9 | −350.0 | 0.047 | |
| qDTY9.2 | LCNS | id9007259 | 9 | 54.1 | 4.7 | −353.4 | 0.012 | |
| qDTY11.2 | LCNS | id11009456 | 11 | 36.7 | 10.0 | 1223.0 | 0.025 | |
| qDTY12.2 | LCNS | ud12000118 | 12 | 212.0 | 4.8 | 620.7 | 0.050 | |
| qDTY3.2 | UMS | id3000019 | 3 | 0.0 | 19.0 | 417.6 | 0.014 | |
| qDTY11.1 | UMS | id11002801 | 11 | 17.4 | 25.0 | 1967.0 | 0.001 | |
| PH | qDTH1.1 | LSS | Id1021344 | 1 | 154.0 | 4.2 | 5.1 | 0.003 |
| qDTH2.1 | LMS | Id2006643 | 2 | 70.9 | 6.3 | 5.6 | <0.001 | |
| qDTH8.1 | LMS | Id8007896 | 8 | 95.0 | 4.9 | 4.2 | <0.001 | |
| qDTH10.1 | LMS | Id10004477 | 10 | 43.0 | 4.4 | 2.0 | <0.001 | |
| qDTH1.1 | LCS | Id1021344 | 1 | 150.0 | 31.0 | 9.5 | <0.001 | |
| qDTH1.1 | LNS I | Id1021344 | 1 | 154.0 | 9.1 | 9.1 | <0.001 | |
| qDTH2.1 | LNS I | Id2007213 | 2 | 75.0 | 4.5 | 6.5 | 0.008 | |
| qDTH10.1 | LNS I | Id10004477 | 10 | 43.0 | 3.6 | 4.4 | 0.002 | |
| qDTH2.1 | LNS II | Id2007213 | 2 | 74.3 | 9.1 | 6.6 | 0.008 | |
| qDTH3.1 | LNS II | Id3002556 | 3 | 58.2 | 4.9 | −3.3 | 0.031 | |
| qDTH1.1 | LCNS | Id1021344 | 1 | 154.0 | 9.1 | 7.6 | <0.001 | |
| qDTH2.1 | LCNS | Id2007213 | 2 | 74.3 | 7.4 | 6.5 | <0.001 | |
| qDTH7.1 | LCNS | Id7005631 | 7 | 107.0 | 2.8 | 4.6 | 0.024 | |
| DTF | qDTF3.1 | LSS | Id3000757 | 3 | 20.0 | 30 | −5.0 | <0.001 |
| qDTF7.1 | LSS | Id7003271 | 7 | 91.0 | 8.4 | −5.6 | <0.001 | |
| qDTF10.1 | LSS | Id10006250 | 10 | 69.3 | 3.4 | 1.9 | 0.040 | |
| qDTF11.1 | LSS | Id11000855 | 11 | 5.0 | 4.9 | −2.5 | 0.028 | |
| qDTF3.1 | LMS | Id3000757 | 3 | 20.0 | 29.0 | −4.9 | <0.001 | |
| qDTF8.1 | LMS | Id8004692 | 8 | 60.4 | 13.0 | −6.7 | <0.001 | |
| qDTF3.1 | LCS | Id3000757 | 3 | 20.0 | 34.0 | −5.2 | <0.001 | |
| qDTF8.1 | LCS | Id8004692 | 8 | 60.4 | 13.0 | −6.6 | <0.001 | |
| qDTF1.1 | LNS I | Id1014783 | 1 | 130.0 | 7.9 | −2.7 | 0.031 | |
| qDTF3.1 | LNS I | Id3000946 | 3 | 25.0 | 17.0 | −3.4 | <0.001 | |
| qDTF3.1 | LNS II | Id3000019 | 3 | 0.0 | 14.0 | −2.6 | <0.001 | |
| qDTF1.1 | LCNS | Id1014783 | 1 | 125.0 | 3.9 | −1.5 | 0.023 | |
| qDTF3.1 | LCNS | Id3000946 | 3 | 25.0 | 21.0 | −3.3 | <0.001 | |
| qDTF6.1 | LCNS | Id6010576 | 6 | 118.0 | 7.8 | −4.0 | 0.044 | |
| qDTF2.2 | UMS | Id2008112 | 2 | 90.0 | 11.0 | −7.0 | <0.001 | |
| qDTF3.1 | UMS | Id3000019 | 3 | 0.0 | 19.0 | −4.2 | <0.001 |
qDTY, qDTH, and qDTF are QTLs for GY, PH, and DTF, respectively. Numbers before decimals denote the chromosome number, whereas those after are the order of their detection. A = additive effect of the peak marker, R2 = percentage of phenotypic variance explained by the QTL, LSS = lowland severe stress, LMS = lowland moderate stress, LCS = lowland combined stress, LNS = lowland non-stress, LCNS = lowland combined non-stress, USS = upland moderate stress.
In this study, large QTLs for DTF were identified, which reflect the negative correlations between DTF and GY under drought. A QTL for DTF (qDTF3.1) was also identified at the qDTY3.2 locus in this study. Several other QTLs for DTF were also observed at chromosomes 2, 6, 7, 8, 10 and 11 although qDTF3.1 remained to be the most consistent QTL across drought stress and non-stress conditions (Table 4). Similarly, for PH, several QTLs were identified across the genome with qDTH1.1 as the largest and most consistent QTL for the trait (Table 4). Majority of the GY QTLs under stress and non-stress were contributed by Moroberekan; however, two QTLs (qDTY9.2 and qDTY10.2) for yield under non-stress were also contributed by Swarna. Similarly, for PH and DTF, majority of the QTLs were contributed by the donor parent Moroberekan, but one QTL for each trait (qDTH3.1 for PH and qDTF10.1 for DTF) was also contributed by the recipient Swarna.
While trangressive segregants were observed in the population for all three traits, the contribution of both parents to higher performance of progeny lines and the presence of epistatic effect have become clear. In order to further explain the phenotypic variance present for grain yield under drought stress and non-stress conditions, tests for epistatic interactions were done. Epistatic interactions for GY under non-stress were observed between several loci under non-stress conditions. However, eQTL10.1 consistently showed epistatic interactions with other loci across the genome (Table 5). Other consistant loci include eQTL1.1 and eQTL8.1 at chromosomes 1 and 8 respectively (Table 5). The high and consistent epistatic interactions of these loci suggest their importance in explaining the genetic basis of GY in lines under non-stress conditions. In this study, lines with high yield under both drought stress and non-stress conditions were identified (Table S2). Despite being more early-maturing than the recipient parent Swarna, these lines were able to maintain their yield potential equal to or more than Swarna under non-stress conditions.
Table 5. Epistatic QTLs affecting grain yield (kg ha-1) adjusted for flowering time under drought stress and non-stress conditions in DS2011 and DS2012.
| Experiment | QTL 1 | Chr (1) | cM (1) | Marker (1) | QTL 2 | Chr 2 | cM (2) | Marker (2) | P | R2 |
| LNS I | eQTL8.1 | 8 | 75 | id8006997 | eQTL10.1 | 10 | 55 | id10004477 | 0.001 | 11.9 |
| LNS II | eQTL1.1 | 1 | 200 | id1010697 | eQTL10.1 | 10 | 45 | id10004477 | 0.015 | 13.6 |
| LNS II | eQTL1.1 | 1 | 200 | id1010697 | eQTL12.1 | 12 | 175 | id12008700 | 0.037 | 14.7 |
| LCNS | eQTL4.1 | 4 | 55 | id4001882 | eQTL9.1 | 9 | 40 | id9002357 | 0.039 | 10.8 |
| LCNS | eQTL7.1 | 7 | 80 | id7002907 | eQTL10.1 | 10 | 50 | id10004477 | 0.021 | 8.8 |
| LCNS | eQTL8.1 | 8 | 75 | id8006997 | eQTL10.1 | 10 | 55 | id10004477 | <0.001 | 12.1 |
| LCNS | eQTL10.1 | 10 | 50 | id10004477 | eQTL8.1 | 11 | 25 | id11006588 | 0.024 | 8.9 |
LNS = lowland non-stress, LCNS = lowland combined non-stress, A = additive effect, R2 = phenotypic variance explained.
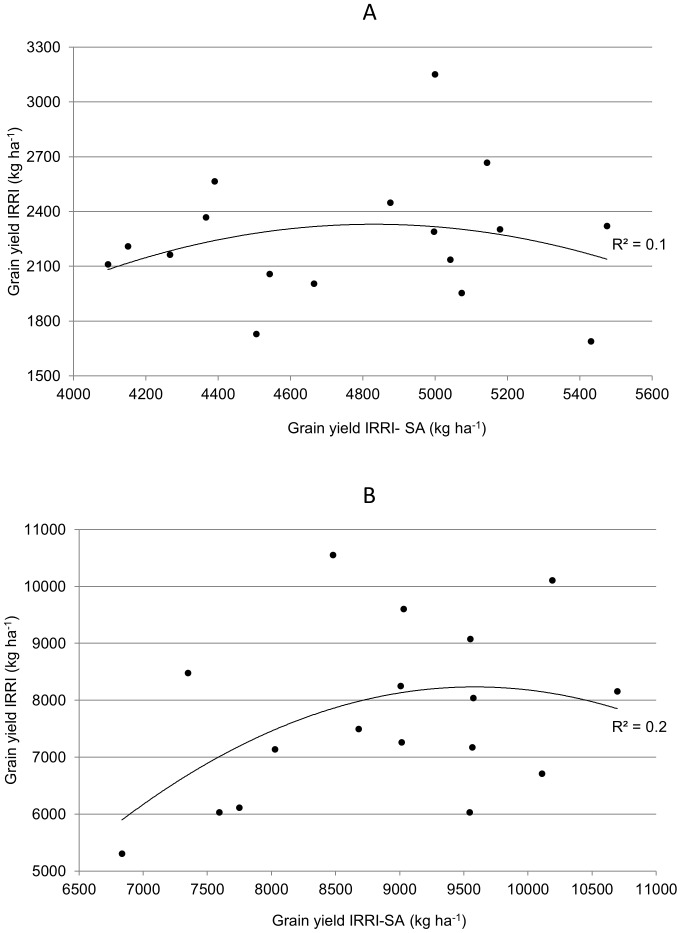
Lines identified from the mapping population were evaluated in the target environment (IRRI-SA) for GY under naturally occurring drought stress and non-stress conditions to understand the consistency of performance of the lines. Figure 5 presents the scatter plots between the GY of selected lines at IRRI and IRRI-SA. Although the stress levels were very mild, the high-yielding drought-tolerant lines selected in DS2013 at IRRI were able to hold similar yield patterns at IRRI-SA. The R2 values were non-significant, but no negative correlations were observed (Fig. 5). More significant correlations may have been apparent under higher severity of stress in the target environment. Lines finally identified from these experiments will undergo another testing for tolerance to blast to check for any loss of tolerance and grain quality testing to shortlist a final set of lines for deployment in the target environment.
Figure 5. Correlation of GY performance of selected lines under drought stress and non-stress conditions in IRRI and IRRI-SA.
Gene content of qDTY3.2 and qDTY11.1
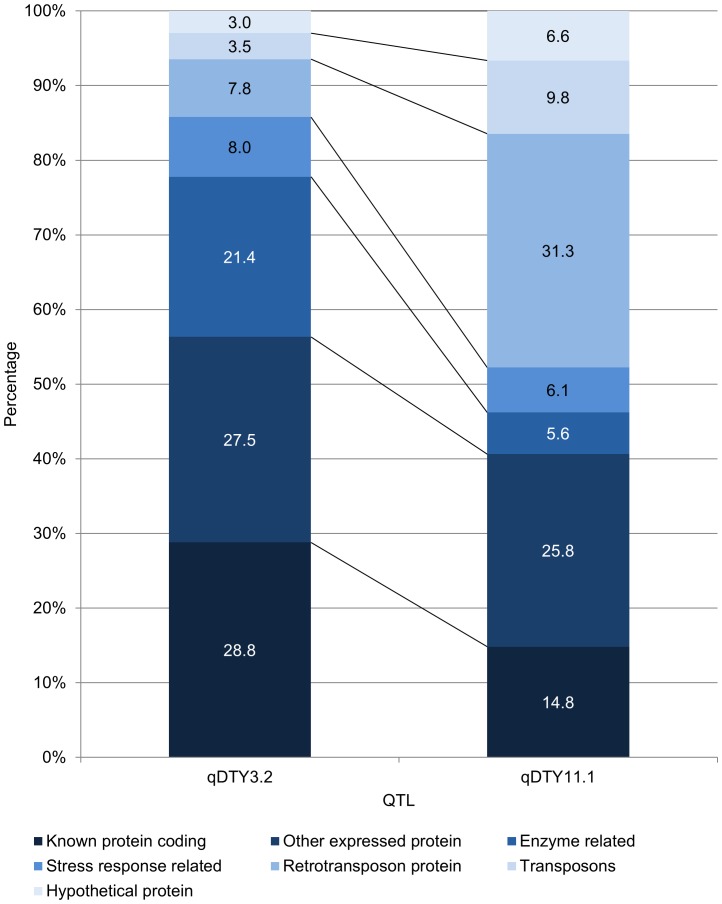
Gene content of qDTY3.2 and qDTY11.1 was classified into 7 classes and percentage of genes falling into each class was calculated (Fig 6.). A large proportion of the genes in bot QTLs were genes coding for known proteins other than those known to be directly related to stress response. Apart from this, large proportion of genes were also related to other expressed proteins in both QTLs. qDTY3.2 however showed a much higher percentage of genes related to enzyme synthesis and function while qDTY11.1 showed a much higher percentage of retrotransposon proteins as compared to qDTY3.2. 8% of the genes within qDTY3.2 6% of genes for qDTY11.1 were genes known to be directly associated to stress response or those which could be crucial for maintenance of plant function under stress. These included genes coding for heat shock proteins, no apical meristem proteins, membrane, cell cycle related proteins and zinc finger proteins (File S2). qDTY3.2 also showed the presence of flowering related genes such as AP2 domain containing proteins and MADS box family genes while qDTY11.1 showed the presence of a large number of nucleotide- binding site leucine- rich repeats (NBS-LRR) genes which are known to be major candidates for blast resistance.
Figure 6. Comparison of gene content of qDTY3.2 and qDTY11.1 based on percentage of genes belonging to seven gene classes within the two QTLs.
Discussion
Our study reports an integrated strategy to develop drought- and blast-tolerant lines along with simultaneous identification of QTLs for GY under drought. The study also attempts to explain the genetics behind the selection cycle for blast tolerance and its effect on drought tolerance. Several previous studies have used different kinds of mapping populations and strategies to identify QTLs for tolerance of rice blast and grain yield under drought. However, to the best of our knowledge, there has been no attempt to identify QTLs for high grain yield under drought stress on a mapping population tolerant of rice blast while simultaneously developing high-yielding, drought-tolerant breeding lines. Ballini et al. [28] conducted a meta-analysis to pinpoint the location of major genes reported for blast tolerance in rice. Similarly, Swamy et al. [29] conducted a meta-analysis on QTLs for grain yield under drought and reported several meta-QTLs controlling the trait across the rice genome. Both studies provide an extensive coverage of literature, indicating the large number of studies targeting these traits independently. However, reports of the two traits targeted in the same study seldom exist. In our study, the test for segregation distortion in the population showed most of the distortion to be toward the blast-tolerant parent Moroberekan, showing that it was the major contributor for blast tolerance in the population. Interestingly, distortion in favour of Swarna was also observed at some loci. While some of these marker loci co-locate with NBS-LRR genes, it is likely that these regions play a role in imparting tolerance to blast. The breeding strategy not only allowed us to select for but also to detect loci with small and large effects contributed by both parents. With observations of increased incidence of diseases such as blast in drought conditions, it becomes important to develop lines tolerant to both traits to have more stable yields in rainfed environments. Identifying QTLs independently affecting two different traits and pyramiding them together is a two-step process that is normally used to accomplish this task. The alternative mapping strategy presented in Figure 1 allows the simultaneous achievement of both these goals, particularly for traits where QTLs/genes conferring tolerance are unknown. We observed that, through systematic early-generation screening of large populations, lines with higher tolerance of one of the two targeted traits (blast in this study) may be obtained. These lines can then be used as mapping populations for QTL identification for the second target trait (high GY under drought stress and non-stress in this study), thus enabling the identification of QTLs and development of high-yielding lines with tolerance of multiple stresses in a reduced time span. Despite the segregation distortion due to screening for blast tolerance, high genetic variation could be seen for DTF, PH, and GY under drought and non-stress conditions. Clear pattern of genetic correlations between the three traits were observed and specific DTF and PH classes showed GY advantage under different stress and non-stress conditions (Table 3, Fig. 3).
Advanced backcross (AB) lines were particularly suitable in such cases where a traditional cultivar is used as a donor for an elite recipient background. Moroberekan has long been known as an important donor for tolerance of drought. In addition, being a tropical japonica accession, it is likely to carry genes that may provide opportunity to increase yield potential under normal situations. Despite these advantages, it has not been successfully used in the breeding program because of its poor combining ability and probable adverse linkage drag. In this study, the earlier segregating generations, such as F1- and BC1-derived populations, were not found suitable for the study because of the high percentage of lines with poor performance and poor plant type. However, the BC2-derived lines showed a very high recovery of recipient parent Swarna. In our evaluation, several lines with 10–20 days earlier duration than Swarna, but that had higher yield than Swarna under both irrigated control and drought conditions, were identified (Table S2). DS screening at IRRI has proven highly beneficial in uniform screening of large segregating populations to identify high-yielding drought-tolerant lines. Moderate to high correlation has been reported previously between yield in DS screening at IRRI and WS screening in India [30]. Similar patterns were also seen for the lines selected in this study (Figure 5).
qDTY3.2 was the most consistent QTL identified for GY under drought in this study (Table 4, Figure 4). This QTL has been reported in several previous studies for showing independent or epistatic effects on GY under upland and lowland conditions [11], [13], [31], [32]. The QTL region coincides with the HD9 locus, which controls DTF in rice. While qDTF3.1 was found to collocate with this locus in the present study, it is likely that the Moroberekan allele at this locus causes earliness, leading to yield advantage under drought.
The role of drought escape mechanism coming into play due to this locus cannot be denied. However, the large variety of genes directly or indirectly related to stress response at this locus (File S2) shows that this locus could affect other traits related to stress response apart from flowering. Several existing reports also suggest the effect of this locus on a wide range of traits. Yadav et al. [13] reported the effect of this locus on grain yield under drought while Bernier et al. [10] reported its effect on plant height and biomass. Similarly, the region has been reported to affect traits including chlorophyll content, root volume under drought, low-temperature germination, and spikelet fertility [32]–[34]. The wide range of QTLs detected at this locus for traits related to yield and adaptation to abiotic stresses indicates the existence of higher genetic diversity on this chromosomal region. These results are suggestive of either multiple-linked QTLs or pleiotropic effects of one or more than one QTL on a wide range of traits.
The high genic diversity along with the effect of this region on flowering time may be the reason of its effect on yield under drought under wider range of environments as compared to qDTY11.1 which showed effect under upland conditions only.
Similar to qDTY3.2, qDTY11.1 also showed the presence of a wide range of stress response related genes (File S2). However, NBS-LRR genes were by far the most dominating group within this region, while these genes are well known candidates for major blast resistance genes [28]; it is likely that the QTL may affect tolerance to blast along with affecting yield under upland drought.
Epistatic interactions are one of the major factors that determine the phenotypic expression of a mapping population [35]. It has also been seen in a large number of QTL mapping studies conducted in the past that, despite nearly complete coverage of the chromosomes, the identified QTLs do not explain the entire phenotypic variance observed for a trait [35]. This potentially indicates the presence of epistatic interactions contributing to phenotypic variation in a segregating population along with other factors contributing the same. The presence of transgressive segregants in mapping populations is also suggestive of epistasis. In this study, transgressive segregants for all three traits were observed. The study of epistatic interactions showed the presence of loci having significant interactions with several other loci across the genome. These loci could be very important in understanding the genetic basis of yield under drought and non-stress conditions. Understanding the genetic composition of these loci and their effect on plant function may allow us to use these loci for maximum advantage in breeding programs.
Our study involved a two-way approach to develop lines tolerant of blast and drought with high yield potential. Some of these lines show plant type and grain type similar to Swarna with added tolerance of drought and blast. These lines may act as a replacement of the variety where Swarna is highly preferred. On the other hand, lines with different plant and grain types can be tested as prospective new high-yielding cultivars. In addition, lines with the identified QTLs are also being used in other marker-assisted breeding programs as improved donors. The study has allowed us to demonstrate the combination of conventional breeding approaches with QTL identification and to understand the effect of selection pressure on QTL mapping. This strategy can be very effective in cases where traits are likely to be controlled by multiple genes/QTLs across the genome. In such cases, phenotypic selection for one trait is less likely to affect the other, leaving sufficient variation in the population for QTL mapping.
Supporting Information
Markers showing significant segregation distortion across the genome with the direction of distortion.
(PDF)
Grain yield performance of lines selected under severe stress and non-stress conditions in dry-season screening at IRRI, and under naturally occurring wet-season drought and non-stress conditions at IRRI-SA.
(PDF)
Standard evaluation system used for large scale screening of rice lines to determine their tolerance reaction rice blast (A), field management of lowland experiments with details of crop cultivation, fertilizer management, and management of insect pests and weeds (B).
(PDF)
List of genes with possible function related to biotic and abiotic stress response within qDTY3.1 , qDTY11.1 .
(XLSX)
Phenotypic and genotypic data used for QTL mapping including trait values, marker names, positions and genotypes for individual lines in the mapping population.
(XLSX)
Acknowledgments
The authors are thankful for the support received from Dr. H. Leung, Dr. M. Thomson, during the process of genotyping and development of the manuscript, Dr Nickolai Alexandrov, Dr Casiana Vera Cruz, Dr Bo Zhou and Ms Rose Imee Zhella Morantte for the support received during the process of manuscript development and Teodoro Cuevas, Marcial Panting, Jocelyn O. Guevarra, Danilo De Ocampo, Carmela Malabanan, Ariel Formarejo, and Ruth Erica Carpio for the technical support received in the completion of this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was funded by Global Rice Science Partnership (GRiSP), Generation Challenge Program (GCP), Federal ministry for economic cooperation and development (BMZ), Germany, and the Bill and Melinda Gates Foundation (BMGF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alexandratos N, Bruinsma J (2012) World agriculture towards 2030/2050. ESA Working Paper No. 12–03.
- 2. Vikram P, Mallikarjuna Swamy BP, Dixit S, Ahmed HU, Sta Cruz MT, et al. (2011) qDTY1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet 12: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huke RE, Huke EH (1997) Rice area by type of culture: South, Southeast, and East Asia. IRRI, Los Baños, Philippines.
- 4.Serraj R, Bennett J, Hardy B (2008) Drought frontiers in rice: crop improvement for increased rainfed production. Singapore: World Scientific Publishing and Los Baños (Philippines): International Rice Research Institute.
- 5. Ouk M, Basnayake J, Tsubo M, Fukai S, Fischer KS, et al. (2006) Use of drought response index for identification of drought tolerant genotypes in rainfed lowland rice. Field Crops Res 99: 48–58. [Google Scholar]
- 6. Kumar A, Bernier J, Verulkar S, Lafitte HR, Atlin GN (2008) Breeding for drought tolerance: direct selection for yield, response to selection and use of drought-tolerant donors in upland and lowland-adapted populations. Field Crops Res 107: 221–231. [Google Scholar]
- 7. Venuprasad R, Lafitte HR, Atlin GN (2007) Response to direct selection for grain yield under drought stress in rice. Crop Sci. 47: 285–293. [Google Scholar]
- 8. Venuprasad R, Sta Cruz MT, Amante M, Magbanua R, Kumar A, et al. (2008) Response to two cycles of divergent selection for grain yield under drought stress in four rice breeding populations. Field Crops Res 107: 232–244. [Google Scholar]
- 9. Kumar A, Verulkar SB, Mandal NP, Variar M, Shukla VD, et al. (2012) High-yielding, drought-tolerant, stable rice genotypes for the shallow rainfed lowland drought-prone ecosystem. Field Crops Res 133: 37–47. [Google Scholar]
- 10. Bernier J, Kumar A, Venuprasad R, Spaner D, Atlin GN (2007) A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Sci 47: 507–516. [Google Scholar]
- 11. Venuprasad R, Dalid CO, Del Valle M, Zhao D, Espiritu M, et al. 2009 Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk-segregant analysis. Theor Appl Genet 120: 177–190. [DOI] [PubMed] [Google Scholar]
- 12. Ghimire KH, Quiatchon LA, Vikram P, Mallikarjuna Swamy BP, Dixit S, et al. (2012) Identification and mapping of a QTL (qDTY1.1) with a consistent effect on grain yield under drought. Field Crops Res 131: 88–96. [Google Scholar]
- 13. Yadav RB, Dixit S, Raman A, Mishra KK, Vikram P, et al. (2013) A QTL for high grain yield under lowland drought in the background of popular rice variety Sabitri from Nepal. Field Crops Res 144: 281–287. [Google Scholar]
- 14. Mishra KK, Vikram P, Yadaw RB, Swamy BPM, Dixit S, et al. (2013) qDTY12 .1: a locus with a consistent effect on grain yield under drought in rice. BMC Genet 14: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixit S, Singh A, Sta Cruz MT, Maturan PT, Amante M, et al. (2014) Multiple major QTLs lead to stable yield performance of rice cultivars across variable drought intensities. BMC Genet 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. You A, Lu X, Jin H, Ren X, Liu K, et al. (2006) Identification of quantitative trait loci across recombinant inbred lines and testcross populations for traits of agronomic importance in rice. Genetics 172: 1287–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamagishi J, Miyamoto N, Hirotsu S, Laza RC, Nemoto K (2004) QTLs for branching, floret formation, and pre-flowering floret abortion of rice panicle in a temperate japonica × tropical japonica cross. Theor Appl Genet 109: 1555–1561. [DOI] [PubMed] [Google Scholar]
- 18. Xing YZ, Tan YF, Hua JP, Sun XL, Xu CG, et al. (2002) Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor Appl Genet 105: 248–257. [DOI] [PubMed] [Google Scholar]
- 19. Thomson MJ, Tai TH, McClung AM, Lai X-H, Hinga ME, et al. (2003) Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between Oryza rufipogon and the Oryza sativa cultivar Jefferson. Theor Appl Genet 107: 479–493. [DOI] [PubMed] [Google Scholar]
- 20. McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, et al. 2009 Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Nat Acad Sci USA 106(30): 12273–12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neeraja CN, Maghirang-Rodriguez R, Pamplona A, Heuer S, Collard B, et al. (2007) A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor Appl Genet 115: 767–776. [DOI] [PubMed] [Google Scholar]
- 22.Zhao K, Wright M, Kimball J, Eizenga G, McClung A, et al.. (2010) Genomic diversity and introgression in O. sativa reveal the impact of domestication and breeding on the rice genome. PLOS One 5 (5): : e10780. [DOI] [PMC free article] [PubMed]
- 23.Zhao K, Tung C-W, Eizenga GC, Wright MH, Ali ML, et al.. (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Comm 2: 467/DOI: 10.1038/ncomms1467. [DOI] [PMC free article] [PubMed]
- 24.Cooper M, DeLacy IH, Basford KE (1996) Relationship among analytical methods used to analyse genotypic adaptation in multi-environment trials, pp-193–224. In M. Cooper and G.L. Hammer editors Plant adaptation and crop improvement University Press, Cambridge, UK.
- 25.SAS Institute Inc (2004) SAS online doc 9.1.3. SAS Institute Inc., Cary, NC, USA.
- 26. Broman KW, Wu H, Sen S. Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- 27. Berloo RV (2008) GGT 2.0: versatile software for visualization and analysis of genetic data. J Heredity 99(2): 232–236. [DOI] [PubMed] [Google Scholar]
- 28. Ballini E, Morel J-B, Droc G, Price A, Courtois B, et al. (2008) A genome-wide meta-analysis of rice blast resistance genes and quantitative trait loci provides new insights into partial and complete resistance. Mol. Plant Microbe Interact 21(7): 859–868. [DOI] [PubMed] [Google Scholar]
- 29. Swamy BPM, Vikram P, Dixit S, Ahmed HU, Kumar A (2011) Meta-analysis of grain yield QTL identified during agricultural drought in grasses showed consensus. BMC Genomics 12: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verulkar SB, Mandal NP, Dwivedi JL, Singh BN, Sinha PK, et al. (2010) “Breeding resilient and productive rice genotypes adapted to drought-prone rainfed ecosystems of India”. Field Crops Res 117: 197–208. [Google Scholar]
- 31. Dixit S, Mallikarjuna Swamy BP, Vikram P, Bernier J, Sta Cruz MT, et al. (2012) Increased drought tolerance and wider adaptability of qDTY12.1 conferred by its interaction with qDTY2.3 and qDTY3.2″. Mol Breed. 30: 1767–1779. [Google Scholar]
- 32. Ding X, Li X, Xiong L (2011) Evaluation of near-isogenic lines for drought resistance QTL and fine mapping of a locus affecting flag leaf width, spikelet number, and root volume in rice Theor Appl Genet. 123: 815–826. [DOI] [PubMed] [Google Scholar]
- 33. Ishimaru K, Yano M, Aoki N, Ono K, Hirose T, et al. (2001) Toward the mapping of physiological and agronomic characters on a rice function map: QTL analysis and comparison between QTLs and expressed sequence tags. Theor Appl Genet 102: 793–800. [Google Scholar]
- 34. Fujino K, Sekiguchi H, Sato T, Kiuchi H, Nonoue Y, et al. (2004) Mapping of quantitative trait loci controlling low-temperature germinability in rice (Oryza sativa L.). Theor Appl Genet 108: 794–799. [DOI] [PubMed] [Google Scholar]
- 35. Li Z, Pinson SRM, Park WD, Paterson AH, Stansel JW (1997) Epistasis for three grain yield components in rice (Oryza sativa L.). Genetics 145: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Markers showing significant segregation distortion across the genome with the direction of distortion.
(PDF)
Grain yield performance of lines selected under severe stress and non-stress conditions in dry-season screening at IRRI, and under naturally occurring wet-season drought and non-stress conditions at IRRI-SA.
(PDF)
Standard evaluation system used for large scale screening of rice lines to determine their tolerance reaction rice blast (A), field management of lowland experiments with details of crop cultivation, fertilizer management, and management of insect pests and weeds (B).
(PDF)
List of genes with possible function related to biotic and abiotic stress response within qDTY3.1 , qDTY11.1 .
(XLSX)
Phenotypic and genotypic data used for QTL mapping including trait values, marker names, positions and genotypes for individual lines in the mapping population.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.