Abstract
To assess the effects of the orphan nuclear Estrogen receptor-related receptor gamma (ERRγ) deficiency on skeletal development and bone turnover, we utilized an ERRγ global knockout mouse line. While we observed no gross morphological anomalies or difference in skeletal length in newborn mice, by 8 weeks of age ERRγ +/− males but not females exhibited increased trabecular bone, which was further increased by 14 weeks. The increase in trabecular bone was due to an increase in active osteoblasts on the bone surface, without detectable alterations in osteoclast number or activity. Consistent with the histomorphometric results, we observed an increase in gene expression of the bone formation markers alkaline phosphatase (Alp) and bone sialoprotein (Bsp) in bone and increase in serum ALP, but no change in the osteoclast regulators receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) or the resorption marker carboxy-terminal collagen crosslinks (CTX). More colony forming units-alkaline phosphatase and -osteoblast (CFU-ALP, CFU-O respectively) but not CFU-fibroblast (CFU-F) formed in ERRγ +/− versus ERRγ +/+ stromal cell cultures, suggesting that ERRγ negatively regulates osteoblast differentiation and matrix mineralization but not mesenchymal precursor number. By co-immunoprecipitation experiments, we found that ERRγ and RUNX2 interact in an ERRγ DNA binding domain (DBD)-dependent manner. Treatment of post-confluent differentiating bone marrow stromal cell cultures with Runx2 antisense oligonucleotides resulted in a reduction of CFU-ALP/CFU-O in ERRγ +/− but not ERRγ +/+ mice compared to their corresponding sense controls. Our data indicate that ERRγ is not required for skeletal development but is a sex-dependent negative regulator of postnatal bone formation, acting in a RUNX2- and apparently differentiation stage-dependent manner.
Introduction
The Estrogen receptor-related receptors (ERR) are orphan nuclear receptors comprising three family members: ERRα, ERRβ and ERRγ (NR3B1, NR3B2 and NR3B3 respectively) [1]. They are similar in structure to the classic Estrogen receptors, ERα and ERβ, with high amino acid identity in their DNA binding domain (DBD; e.g., over 60% in human ERRα and ERα), but lower (e.g., less than 35%) identity in the ligand binding domain (LBD); the low sequence identity in the LBD is consistent with the observation that the ERRs do not bind Estrogen [2], [3]. Mouse knockout studies have revealed that ERRα and ERRγ are important regulators of energy metabolism [4]–[7]. ERRγ in particular is a key regulator of mitochondrial genes, and its absence results in perinatal lethality, as a consequence of a failure to transition from carbohydrate dependence to fatty acid oxidation [6].
The role of ERRs in bone formation and turnover is also being investigated. ERRα is expressed in osteoblasts throughout the skeleton and was shown to be a positive regulator of osteoblast proliferation and differentiation in vitro [8]. There have been conflicting data published on ERRα activity on bone in vivo, with data supporting both a negative regulatory [9], [10] and a positive regulatory [11] role. These latter differences may reflect inherent differences in different knockout mouse strains, differences in activities in female [9], [10] versus male [11] mice, and age-related cell autonomous versus non-autonomous roles in bone cells (osteoblasts, osteoclasts) versus other lineages in the bone environment (e.g., adipocytes) [7].
There are fewer studies addressing the role of ERRγ in bone. It has been reported that an ESRRγ polymorphism is associated with bone mineral density in females of European ancestry [12]. Nuclear receptor gene expression profiling has shown ERRγ to be expressed early in the differentiation time course of mouse calvaria cells [13] and bone marrow-derived stromal cells [14] in vitro, with a steady decrease in expression as differentiation progresses. ERRγ expression was reported to be increased in mouse calvaria cells in culture upon stimulation by bone morphogenetic protein 2 (BMP2) and, through protein interaction with RUNX2, to prevent normal cofactor interaction, resulting in repression of transactivation of its target genes, bone sialoprotein (Bsp) and osteocalcin (Ocn) [15]. ERRγ was also recently reported to regulate BMP-stimulated osteogenesis in vitro through up-regulation of the microRNA miR-433, which targeted the 3′-UTR region of Runx2, decreasing its protein expression [16]. However, the endogenous role of ERRγ during early bone development and postnatal bone turnover has not yet been characterized.
We report here that ERRγ is not required for skeletal development but is a sex-dependent negative regulator of postnatal bone formation. Further, we show that ERRγ acts in a RUNX2-and apparently differentiation stage-dependant manner in vitro.
Materials and Methods
Ethics Statement
All experimental procedures were performed in accordance with protocols approved by the Canadian Council on Animal Care and the University of Toronto Faculty of Medicine and Pharmacy Animal Care Committee.
Mouse gene nomenclature
We followed the mouse nomenclature guide as stated on the Mouse Genome Informatics web page (http://www.informatics.jax.org/mgihome/nomen/short_gene.shtml). Thus, genes are written with first letter capitalized followed by small letters, all italicized, while proteins are written all capitalized and non-italics.
Animals
The ERRγ knockout mouse line was obtained from the Mutant Mouse Regional Resource Center (MMRRC). The ERRγ null mouse strain originated from Deltagen (San Carlos, CA) by insertion of a lacZ-neomycin cassette into ERRγ such that the endogenous gene promoter drives expression of beta-galactosidase and nucleotides from base 586 to 610 of exon 2 were deleted. DNA was isolated from either yolk sacs (embryonic mice) or tail clips (postnatal mice) and were genotyped using the following primers:
Breeding was performed by crossing heterozygous male with female C57BL/6J mice. For all experiments, littermates were used as controls. Animals were sacrificed by cervical dislocation.
Whole mount skeletal staining
E15 - P0 animals were dissected, eviscerated and fixed in 95% ethanol overnight or up to two weeks, and then processed for whole mount skeletal staining as previously described [17]. When samples were fully cleared, skeletons were dissected and photographed in a Petri dish containing 100% glycerol, using a Nikon Coolpix P5100 digital camera affixed to a dissecting scope. The images were then quantified in Image J by taking linear measurements of individual skeletal components.
Microcomputed tomography (µCT)
8, 14, and 52-week old mouse femurs were dissected and stored in 70% ethanol. µCT imaging was performed on a GE Xplore Locus SP imager. A manual trace beginning from just below to 2 mm below the growth plate of the distal femur was used to analyze the cancellous bone. The cortical bone region of interest was defined as a 2 mm long region beginning 2 mm below the growth plate. For P0 mice the whole femur was analyzed. Quantification was performed by an observer blinded to genotype.
Histology and histomorphometry
14-week old male femurs were dissected and fixed in 4% paraformaldehyde (PFA) overnight at 4°C, dehydrated and stored in 70% ethanol prior to methylmethacrylate (MMA) embedding and sectioning. The distal femur was used to evaluate all histomorphometric parameters. 5 µm sections were double stained for Von Kossa/toluidine blue to evaluate osteoblast properties, or tartrate-resistant acid phosphatase (TRAP)-stained according to manufacture's instructions (387A TRAP kit, Sigma-Alderich) to evaluate osteoclast properties. These measurements were performed on 3 separate sections from each animal, and the average number or surface was calculated. To evaluate dynamic properties, mice were injected intraperitoneally with 30 µg/g body weight of calcein (C0875, Sigma-Alderich) 10 and 3 days prior to dissection. Mineral apposition rate (MAR) was calculated by measuring the distance between 2 calcein labels and dividing by 7 days. Four different regions within the trabecular area were used for calculations. BFR was calculated by multiplication of MAR by the ratio of mineralizing surface (MS) (calcein positive) to the bone surface (BS). Five images were taken at different regions within a section, and 3 different sections were used per animal, and the average was calculated. All quantification was performed by observers blinded to genotype.
Immunohistochemical detection of Ki67 and TUNEL assay
To immunodetect Ki67, a common proliferation marker [18], sections were de-plasticized, and rehydrated in ethanol washes, followed by antigen retrieval in citrate buffer (10 mM citric acid, 0.05% Tween-20, pH 6) in a 65°C water bath overnight. The slides were blocked in normal goat serum (Invitrogen) for 30 minutes at room temperature, washed, incubated with rabbit polyclonal anti-Ki67 antibody (diluted 1∶25 in blocking buffer) for 1 hour at room temperature, washed, then incubated with biotinylated goat anti-rabbit secondary antibody, and visualized using the Vectastain Elite ABC kit (Vector Labs, Burlingame, California), and counterstained with methylene blue.
To perform TUNEL assay, femoral sections were processed as described above, before using the FragEL DNA fragmentation detection kit (Calbiochem), as per the manufacturers instructions, and counterstained with methyl green. In each case, femoral sections were imaged, and Ki67 (or TUNEL) positive and negative cells on the trabecular bone surface were quantified using Bioquant Osteo 2012; only cells with the morphological characteristics of osteoblasts were quantified. All quantification was performed by observers blinded to genotype.
Serum biochemistry
Whole blood was collected through the saphenous vein, and the plasma was separated from whole blood by centrifugation and stored at −80°C until biochemical analysis (Vita-Tech, Ontario, Canada). OPG and RANKL in serum were assayed using a Quantikine M Murine OPG ELISA kit and a Quantikine M Murine TRANCE/RANKL ELISA kit (No. MOP00 and No. MTR00, R&D Systems, Minneapolis, MN), respectively following the manufacturer’s directions. CTX-1 levels in serum was determined from fasted mice using Serum CrossLaps ELISA (RatLaps EIA No. AC-06F1, Immunodiagnostic Systems, Fountain Hills, AZ). Serum testosterone levels were quantified by Cornell University Animal Health Diagnostic Center (Ithaca, NY).
Gene expression analysis
The trabecular bone from 14-week old male femur and tibiae were dissected, had marrow removed, and were manually ground with a mortar and pestle under liquid nitrogen. Stromal cell cultures were rinsed twice with phosphate buffered saline. In either case, total RNA was extracted using TRIzol (Invitrogen), and reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen), according to the manufacturer’s directions. All primers were designed with intron inclusion in corresponding genomic DNA, and are common to all potential transcript variants (Table 1). The reactions were performed in triplicate on a 96-well plate in a BioRad MyIQ iCycler, for 50 cycles with an annealing temperature of 59°C. The amplification data was uploaded into the PCR miner program (http://www.ewindup.info/miner/version2/) to obtain the Ct and reaction efficiency values. The relative expression levels of the target gene were normalized to expression of ribosomal protein L32 as internal control; we confirmed that the expression of L32 remained constant throughout cultures under the conditions used, including in knockdown experiments.
Table 1. Primer sequences used in gene expression analysis.
| Gene | Upstream sequence | Downstream sequence |
| L32 | CACAATGTCAAGGAGCTGGAAGT | TCTACAATGGCTTTTCGGTTCT |
| Bmp2 | CTCAGCGAATTTGAGTTGAGGC | GGCTTCTAGTTGATGGAACGTG |
| Runx2 | TGTTCTCTGATCGCCTCAGTG | CCTGGGATCTGTAATCTGACTCT |
| Osx | ATGGCGTCCTCTCTGCTTG | TGAAAGGTCAGCGTATGGCTT |
| Alp | CCAACTCTTTTGTGCCAGAGA | GGCTACATTGGTGTTGAGCTTTT |
| Bsp | CAGGGAGGCAGTGACTCTTC | AGTGTGGAAAGTGTGGCGTT |
| Ocn | CTGACCTCACAGATCCCAAGC | TGGTCTGATAGCTCGTCACAAG |
| Nfatc1 | CAGCTGTTCCTTCAGCCAAT | GGAGGTGATCTCGATTCTCG |
| Ctsk | ACCCATATGTGGGCCAGGATGA | GAGATGGGTCCTACCCGCGC |
Isolation of Bone Marrow Cells, Runx2 antisense assay, and CFU assay
Bone marrow cells were isolated from dissected tibiae and femora, using a modification of a previously published method [19]. Cells were plated in α-MEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (1 IU penicillin, 1 µg/mL streptomycin, 50 µg/mL gentamicin, 250 ng/mL fungizone) (standard medium) at 3×106 nucleated cells/35-mm dish. After 4 days, the medium was changed to differentiation medium (standard medium with 50 µg/mL ascorbic acid and 10 mM β-glycerophosphate). For Runx2 antisense assay, differentiation medium was supplemented with 10 µM Runx2 oligonucleotides from d5–d19 of culture. Runx2 oligonucleotide sequences were as follows, as previously described [20]: Runx2AS A*G*T*G*TGG TAG TGA GTG GT*G*G*C; Runx2S G*C*C*ACC ACT CAC TAC CA*C*A*C (*denotes phosphorothioate modification) (Integrated DNA Technologies, Coralville, Iowa). At day 19, cultures were stained for ALP activity and mineralization (Von Kossa), counted, then re-stained with methylene blue.
Stable cell line constructs
The plasmids pcDINmERRγ2, pcDINmERRγ2ΔAF2 and pcDINmERRγ2C148G have been described [21]. For the generation of stable overexpressing MC3T3-E1 clone 26 cell lines, 20 µg of plasmid was linearized with SspI and precipitated with ethanol and then air dried. The plasmid pellet was resuspended in 200 µl of α-MEM and then combined with 200 µl α-MEM with 40 µl Lipofectamine 2000 (Invitrogen) before being added onto the cells at 60% confluence in a 10 cm dish. The following day medium was changed to allow the cells to recover. On day two after transfection, regular medium was supplemented with 180 µg/ml (final) of active G418. One non-transfected plate of cells was used as a control. After 2–3 weeks of selection more than 10 colonies could easily be identified on the plate for any of the transfected plasmids and the G418 was reduced to 120 µg/ml until the plate grew confluent. Cells were split for expansion and freezing. When thawing out the vials for experiments, the cells were plated in 180 µg/ml of G418 for initial expansion to ensure high levels of expression, but subsequent passaging was in medium containing 120 µg/ml of G418. High levels of expression were verified by RT-qPCR and Western blot.
Co-immunoprecipitation Analysis
Co-IP assay was performed essentially as previously reported [15]. Briefly, stable overexpressing MC3T3-E1 cell lines were lysed in Co-IP buffer, and lysates were precleared with 50 µl of pansorbin cells (Calbiochem) for 2 h, which were removed by centrifugation. A total of 2 µg of rabbit polyclonal antibody against RUNX2 (Santa Cruz Biotechnology), or normal rabbit IgG (negative control) were added to the precleared lysates, and incubated at 4°C overnight with rotation. After washing cells in Co-IP buffer, the samples were centrifuged, and SDS sample buffer was added, and boiled. The immunoprecipitated complexes were separated by SDS-PAGE, transferred to polyvinylidene difluoride, and immunoblotted with a rabbit polyclonal antibody against ERRγ (Santa Cruz Biotechnology).
Western blotting
Whole cell extracts were lysed in RIPA buffer (50 mM Tris HCl pH 8, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulphate) with added protease inhibitors. Protein samples were quantified using the Bio-Rad DC Protein Assay kit, following the manufacturer’s instructions. Thirty µg of each sample was run in a 10% SDS-PAGE gel, transferred to polyvinylidene fluoride (PVDF) membrane, followed by blocking in 5% milk-TBS-T for 30 minutes at room temperature. Immunodetection was carried out using a rabbit polyclonal anti-ERRγ antibody (H38x, Santa Cruz Biotechnology Inc.) diluted 1∶5000 in blocking buffer, or rabbit polyclonal anti-RUNX2 antibody (Santa Cruz Biotechnology) diluted to 1∶1000, or rabbit anti-β-ACTIN antibody diluted to 1∶2000 (Sigma). This was followed by a one hour incubation with a goat anti-rabbit IgG, conjugated to horse radish peroxidase (HRP; Santa Cruz Biotechnology), diluted 1∶5000–1∶8000 in blocking buffer. The HRP was visualized using WEST-one Western Blot Detection, as per the manufacturer’s instructions. Densitometry was performed using Image Lab software (Bio-Rad). The proteins of interest were normalized to the β-ACTIN band to assess proportionate protein levels.
Statistical Analysis
All data were analyzed using Graphpad Prism 4.0 software, or Microsoft Excel 2003 software. In most cases, datasets were compared using student's t-Test. When three datasets were analysed, ANOVA was used to determine significance. All the graphs are plotted as the mean ± standard deviation and the p values listed are for the comparison to the WT values. Graphs were constructed using Microsoft Excel 2003 software.
Results
ERRγ −/− mice die perinatally, but display no skeletal abnormalities
ERRγ null mice (ERRγ −/−) died perinatally, with no pups observed beyond P1. This is consistent with the observation that mice of a different ERRγ −/− mouse strain, made using a similar gene targeting strategy, also die perinatally due to mitochondrial abnormalities and impaired oxidative metabolism [6]. ERRγ +/− mice were healthy and viable, and were used for subsequent breeding.
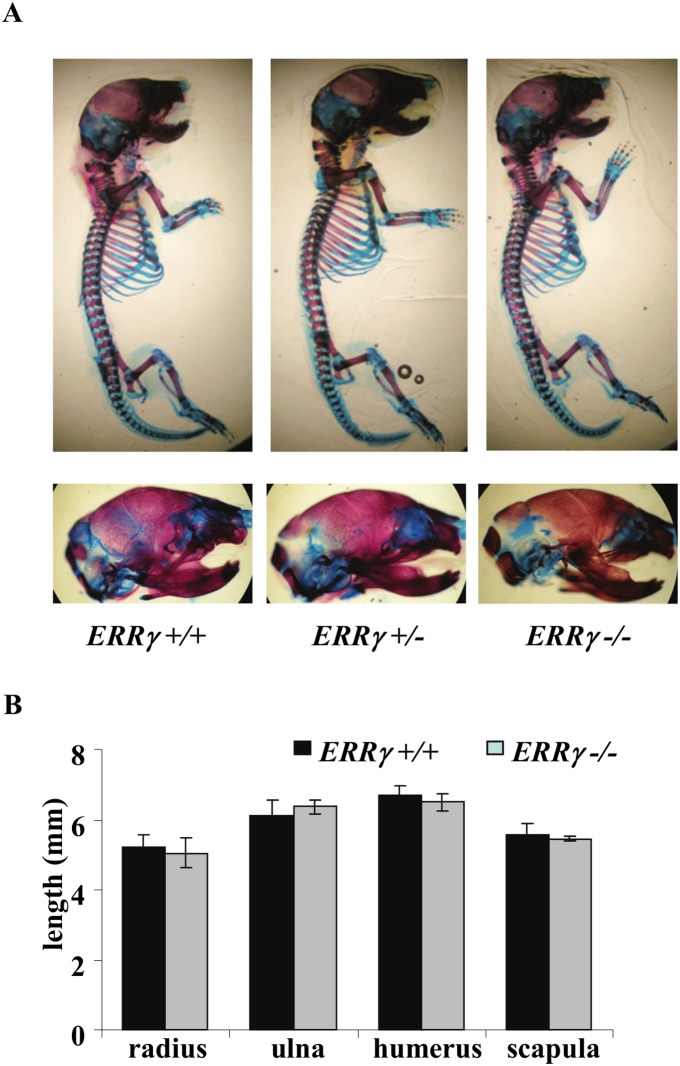
No significant differences in forelimb length were observed between E15-P0 in ERRγ +/+, ERRγ +/− and ERRγ −/− whole mount skeletal preparations (Figure 1A, B, P0 shown). There was also no significant difference observed in any of the growth plate zones in sections of P0 humeri (data not shown). MicroCT (µCT) analyses confirmed no significant difference in bone mineral density, content, or bone volume fraction between genotypes in P0 mice (data not shown). Taken together, the data indicate that ERRγ, similarly to ERs and ERRα [9], [10], [22], [23], either plays no apparent role or plays a redundant role in embryonic/early postnatal endochondral bone growth and mineralization.
Figure 1. Neither ERRγ ablation nor haploinsufficiency has a detectable effect on embryonic bone development and growth.
(A) Whole mount alizarin red/alcian blue staining revealed no observable morphological differences in newborn mouse ERRγ +/+, ERRγ +/− and ERRγ −/− skeletons (upper panel) or in the craniofacial regions (lower panel). (B) Skeletal elements that make up the mouse forelimb of each genotype were measured and found to have no significant differences. (N = 5 ERRγ +/+; N = 3 ERRγ −/−). Values are expressed as mean ± SD.
Adult ERRγ +/− male mice have increased trabecular bone, increased osteoblast number and surface, but no change in osteoclast number or surface
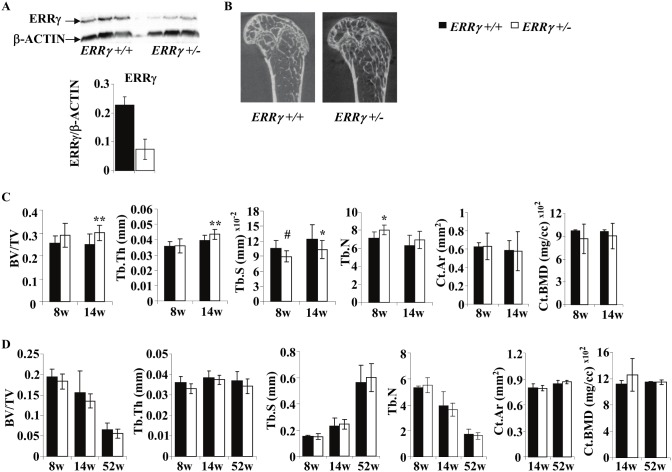
To address whether ERRγ plays a role in adult bone, we first quantified ERRγ by immunoblotting of whole cell lysates of femoral trabecular bone of 14-week old mice; ERRγ protein was reduced approximately 67% in ERRγ +/− mice compared to ERRγ +/+ (Figure 2A). µCT analyses of 8- and 14-week old male mice (representative image shown in Figure 2B) was performed. Trabecular bone volume (BV/TV) and thickness (Tb.Th) were increased (21% and 10.8%, respectively) at 14 weeks, while trabecular separation (Tb.S) showed a trend (p = 0.069) towards a decrease at 8 weeks, and a significant decrease (16.5%) at 14 weeks in ERRγ +/− versus ERRγ +/+ mice (Figure 2C). Trabecular number (Tb.N) was significantly increased (12.4%) at 8, but not 14 weeks of age. The differences observed were specific to trabecular bone, as there were no changes detectable in cortical bone parameters, including cortical area (Ct.Ar) and cortical BMD (Ct.BMD) (Figure 2C), or when periosteal and endosteal perimeters were quantified (data not shown). Further, no significant differences were observed in any of these parameters in female ERRγ +/− mice (Figure 2D).
Figure 2. Trabecular bone formation is increased in 14-week old male distal femurs.
(A) Quantification of Western blots revealed that ERRγ protein expression was reduced in whole cell lysates of trabecular bones of 14-week old ERRγ +/− mice compared to ERRγ +/+ mice. (B) Representative µCT images of 14-week old ERRγ +/+ and ERRγ +/− male distal femurs. (C) Quantitative analysis revealed a significant increase in trabecular bone volume fraction (BV/TV) and thickness (Tb.Th), and a decrease in separation (Tb.S) at 14 weeks. Trabecular number (Tb.N) was significantly increased at 8 weeks, but not significantly at 14 weeks (upper panels). There were no significant differences in cortical bone area (Ct.Ar) or bone mineral density (BMD) (lower panel). (D) Analysis on 8, 14 and 52-week old female mice revealed no differences in any bone parameters assessed. Values are expressed as mean ± SD (Male: 8w: N = 7 ERRγ +/+; N = 5 ERRγ +/−; 14w: N = 10 ERRγ +/+; N = 14 ERRγ +/−; Female: 8w: N = 3 ERRγ +/+; N = 5 ERRγ +/−; 14w: N = 10 ERRγ +/+; N = 7 ERRγ +/−; 52w: N = 6 ERRγ +/+; N = 5 ERRγ +/−) * = p<0.05; ** = p<0.01; # = 0.069.
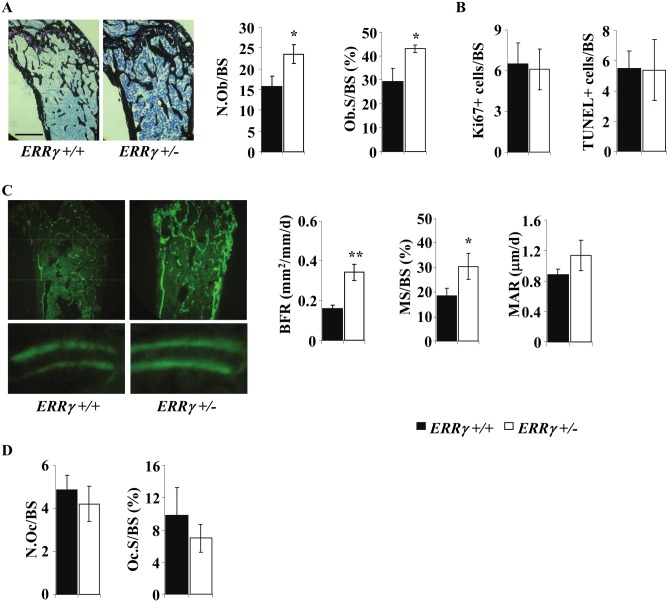
Histomorphometric analyses indicated that osteoblast number per bone surface (N.Ob/BS) and osteoblast surface per bone surface (Ob.S/BS) were increased in 14-week ERRγ +/− compared to ERRγ +/+ male femurs (Figure 3A). Ki67, an established marker of proliferating cells, and TUNEL staining indicated that neither changes in proliferation nor in apoptosis could account for this increase (Figure 3B). Consistent with the increased N.Ob/BS and Ob.S/BS, bone formation rate (BFR) and mineralizing surface per bone surface (MS/BS) were significantly increased, with no significant difference in mineral apposition rate (MAR) (Figure 3C). No significant difference was seen in either osteoclast number per bone surface (N.Oc/BS) or osteoclast surface per bone surface (Oc.S/BS) in ERRγ +/− versus ERRγ +/+ trabecular bone (Figure 3D).
Figure 3. ERRγ regulates osteoblast number and differentiation, but does not affect osteoclast number or activity.
(A) von Kossa/toluidine blue double stain was used to quantify osteoblast number and osteoblast surface per bone surface; both are significantly increased in 14 week ERRγ +/− compared to ERRγ +/+ male femurs. Scale = 50 µm (N = 3 ERRγ +/+; N = 3 ERRγ +/−) (B) Adjacent femoral sections were immunostained for proliferating (Ki67) and apoptotic (TUNEL) osteoblasts; no significant differences were seen between genotypes. (N = 5 ERRγ +/+; N = 4 ERRγ +/−) (C) Double calcein labels were used to quantify bone formation rate (BFR), mineralizing surface per bone surface (MS/BS) and mineral apposition rate (MAR); both BFR and MS/BS were significantly increased, while MAR was not significantly increased in ERRγ +/− compared to ERRγ +/+ mice. (N = 3 ERRγ +/+; N = 3 ERRγ +/−) (D) There was no significant difference observed between genotypes in either TRAP-positive osteoclast number (Oc.N/BS) or osteoclast surface per bone surface (Oc.S/BS) (N = 5 ERRγ +/+; N = 4 ERRγ +/−). All values are expressed as mean ± SD. * = p<0.05; ** = p<0.01.
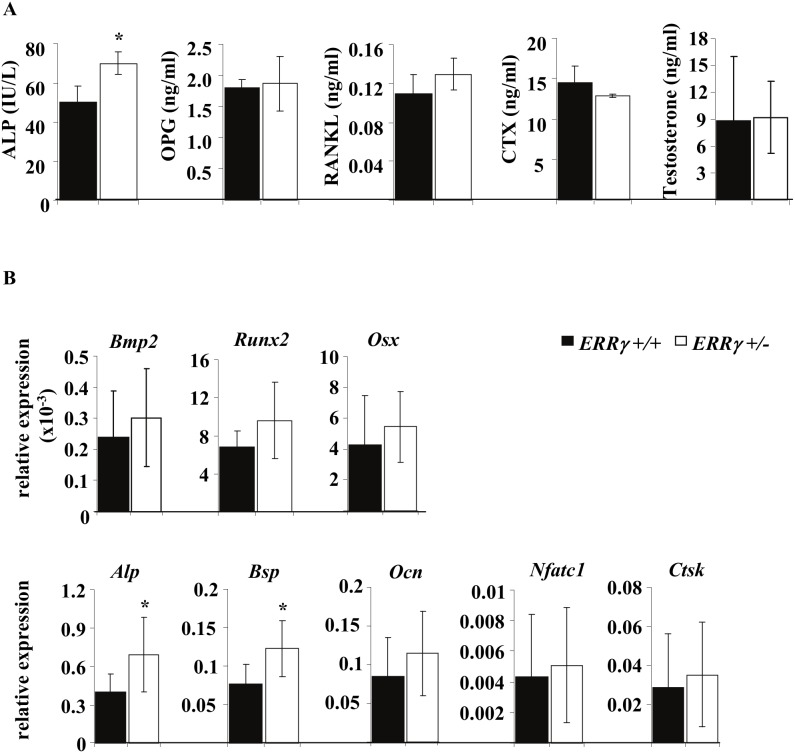
Consistent with the morphometric analyses, a statistically significant increase in serum alkaline phosphatise (ALP) levels, a marker of bone formation, was observed in ERRγ +/− compared to ERRγ +/+ mice, while no significant differences were seen between genotypes in serum RANKL, OPG or CTX (Figure 4A). Because adult male ERα knockout mice also exhibit high trabecular bone volume and high serum levels of testosterone, which account for the bone phenotype [24], [25], we assessed serum testosterone levels; no significant difference in testosterone levels between genotypes were detectable (Figure 4A).
Figure 4. ERRγ regulates markers of osteoblast differentiation and mineralization.
(A) Serum analysis of bone turnover markers revealed a significant increase in ALP, an indication of increased bone formation, with no changes in the bone resorption marker CTX or osteoclastogenic factors (OPG, RANKL) or serum testosterone. (N = 4 ERRγ +/+; N = 4 ERRγ +/−) (B) Gene expression analysis of trabecular bone from 14-week old mice revealed an increase in formation markers and BMP target genes Alp and Bsp, but no difference in Bmp2, Runx2, Osx, Ocn, and no difference in the osteoclast markers Nfatc1 and Ctsk. (N = 8 ERRγ +/+; N = 6 ERRγ +/−). All values are expressed as mean ± SD. * = p<0.05.
Taken together, the data indicate that the high trabecular bone phenotype observed in ERRγ +/− male mice was due to an increased number of active osteoblasts but no change in osteoclast number, activity (Figure 3D) or osteoclast marker (Nfatc1, Ctsk) gene expression (Figure 4B), suggesting that ERRγ regulates bone formation but not bone resorption in male but not female mice.
Increased osteoblast differentiation in ERRγ +/− mice
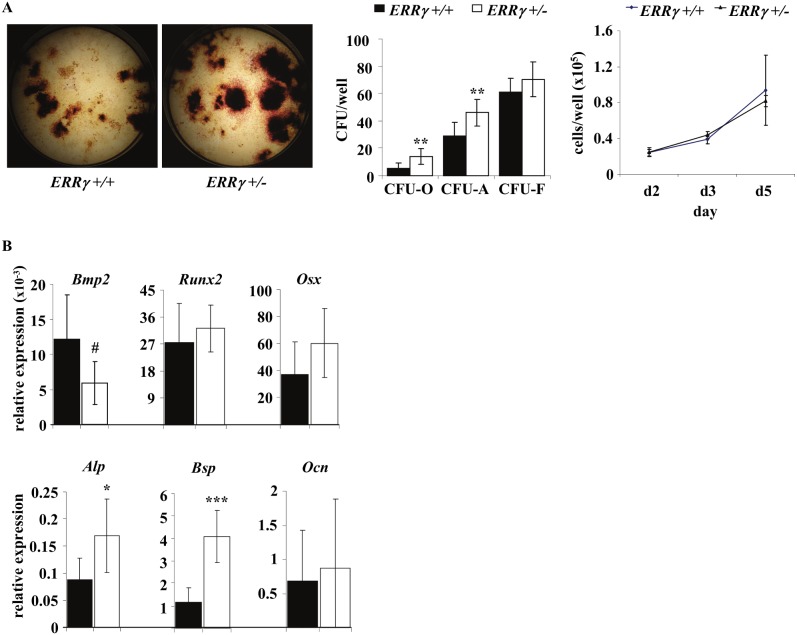
The data thus far suggest that ERRγ negatively regulates bone formation through regulation of osteoblast number. We therefore next assessed osteoblast differentiation in primary cultures of bone marrow stromal cells from ERRγ +/− and ERRγ +/+ mouse hind limbs. Colony forming unit-osteoblast (CFU-O) and CFU-alkaline phosphatase (CFU-ALP) numbers were increased in ERRγ +/− versus ERRγ +/+ stromal cell cultures, without an increase in CFU-fibroblast (CFU-F) number (Figure 5A). Consistent with the in vivo analyses (Figure 3B), no difference was detectable in proliferation of stromal cells between genotypes (Figure 5A).
Figure 5. Osteoblast differentiation is increased in ERRγ +/− stromal cell cultures.
(A) Bone marrow stromal cells cultured from ERRγ +/− mice had significant increases in CFU-O and CFU-ALP, with no change in CFU-F, compared to cells from ERRγ +/+ mice. Cell counts performed during the proliferation phase revealed no differences between genotypes (N = 8 ERRγ +/+; N = 8 ERRγ +/−). (B) Gene expression analysis of ERRγ +/− versus ERRγ +/+ cultured stromal cells revealed a trend to decreased expression of Bmp, and a significant increase in Alp and Bsp (N = 6 ERRγ +/+; N = 6 ERRγ +/−). All values are expressed as mean ± SD. # = p = 0.055; * = p<0.05; ** = p<0.01; ***p<0.001.
Because ERRγ has previously been implicated as a negative regulator of BMP-stimulated osteogenesis in vitro [15], we next assessed expression of Bmp and its downstream targets in vivo and in vitro. Runx2 expression was unchanged in stromal cell cultures (Figure 5B), consistent with the results of Jeong et al. [15], or in trabecular bone (Figure 4B) of 14-week old ERRγ +/− versus ERRγ +/+ male mice. Bmp2 expression was also either unchanged (Figure 4B) or exhibited a downward trend (p = 0.06) (Figure 5B) in ERRγ +/− versus ERRγ +/+ trabecular bone and differentiated stromal cells respectively. Expression of Alp and Bsp but not Osx or Ocn was increased in both trabecular bone (Figure 4B) and stromal cells (Figure 5B) of ERRγ +/− versus ERRγ +/+ mice. There were no significant differences in expression of osteocyte markers (Dmp1 and Sost) or in ERα, ERβ, ERRα or ERRβ in either stromal cell cultures or in trabecular bone samples (data not shown). Taken together, the data suggest that ERRγ negatively regulates osteoblast differentiation and matrix mineralization at a stage after osteoblast commitment (i.e., after upregulation of Runx2 and Osx expression) but before osteoblast maturation or terminal differentiation to osteocytes (i.e., prior to acquisition of Ocn expression).
ERRγ interacts with RUNX2, and knockdown of RUNX2 in vitro leads to at least partial rescue of the ERRγ +/− osteogenic phenotype
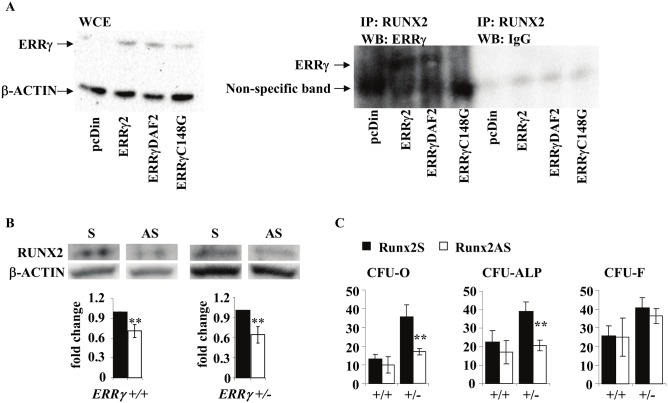
ERRγ has been reported to outcompete P300 as a co-factor for RUNX2, resulting in repression of RUNX2 transactivity [15]. However, it has not been reported which region(s) of ERRγ is/are required for this interaction, but both the DNA binding domain (DBD) and ligand binding domain (LBD) contain dimerization interfaces that modulate the regulation of target genes [26]. We therefore next explored the potential interaction between ERRγ and RUNX2 proteins by doing co-immunoprecipitation (Co-IP) of ERRγ and RUNX2 in MC3T3-E1 preosteoblast cell lines, transfected with either a full length ERRγ (ERRγ2), a LBD mutant (ERRγΔAF2), or a DBD mutant (ERRγC148G). All three ERRγ proteins were expressed, but only ERRγ2 and ERRγΔAF2 and not ERRγC148G co-immunoprecipitated with RUNX2 (Figure 6A), confirming the physical interaction of ERRγ and RUNX2 and revealing that the DBD is required for this interaction.
Figure 6. ERRγ interacts with RUNX2 through the DBD and modulates osteoblast differentiation.
(A) The MC3T3-E1 preosteoblastic cell line was stably transfected with the indicated plasmids. Whole cell extracts (WCE) from these cell lines were probed with anti-ERRγ, confirming protein expression in the stable constructs. A co-immunoprecipitation assay revealed interaction between RUNX2 and full length ERRγ (ERRγ2), as well as with the LBD mutant form of ERRγ (ERRγΔAF2), but not the ERRγ DBD mutant (ERRγC148G). (B) Decreased protein levels of RUNX2 in Runx2 antisense (Runx2AS), compared to Runx2 sense (Runx2S) samples. (C) CFU-O and CFU-ALP in ERRγ +/− bone marrow stromal cells treated with Runx2 antisense oligonucleotides (Runx2AS) were significantly reduced while no changes in either CFU-O or CFU-ALP were observed in ERRγ +/+ cultures. CFU-F numbers were unchanged regardless of treatment and genotype. All values are expressed as mean ± SD. Graphs shown in (C) are of one representative sample, done in triplicate. In all cases, statistical analyses were performed on a minimum of 3 biological samples. ** = p<0.01.
To determine whether RUNX2 is required for the ERRγ +/− osteoblast phenotype, we treated bone marrow stromal cells with antisense Runx2 oligonucleotides post-confluence throughout the differentiation/mineralization period. Runx2 AS but not S oligonucleotides significantly decreased RUNX2 expression (Figure 6B). Consistent with the observation that abrogation of Runx2 expression has no overt effect on cells in cells at certain differentiation stages, i.e., in cells already committed to the osteoblast lineage [27], we observed no decrease in CFU-O or CFU-ALP in Runx2 antisense-treated ERRγ +/+ cultures treated in the time window tested (Figure 6C). In contrast, CFU-O and CFU-ALP were significantly decreased in ERRγ +/− cultures (Figure 6C). Taken together, the data suggest that the enhanced osteoblast differentiation seen in ERRγ +/− mice was both RUNX2-dependent and differentiation stage-dependent.
Discussion
We report that whereas ERRγ-deficient mice manifested no detectable defects in embryonic or early postnatal development, ERRγ-deficient male mice exhibited increased trabecular, but not cortical bone volume in 14-week animals due to RUNX2-dependent negative regulation of the progression of osteoblast differentiation. Female mice were unaffected by ERRγ deficiency.
The increase in trabecular bone volume seen in ERRγ +/− male mice was due to increased trabecular number and thickness, as a consequence of an increased number of osteoblasts on the bone surface, increased BFR and increased MS/BS ratio, indicating an overall increase in active osteoblasts occupying the bone surface. We found no significant change in osteoclast number or surface, suggesting that ERRγ regulates bone formation and osteoblast differentiation, but not bone resorption. This was further supported by the observation of increased expression of certain osteoblast markers in the trabecular bone, with no change in markers of osteoclast formation or activity. Our findings contrast with ERα deficiency in male mice, which also leads to increased trabecular bone volume, but due to decreased bone turnover, with decreases in both bone formation and resorption [25]. On the other hand, ERRγ deficiency also results in increased trabecular bone volume, due to increased bone formation, resulting from increased osteoblast differentiation; this was variously reported to be accompanied by no differences in CFU number or proliferation in female mice only [9] or with an increase in osteoblast proliferation, with no increase in CFU number in both male and female mice; these discrepancies may reflect differences in mouse strains and/or the targeting strategies used [10]. In any case, we detected no significant changes by µCT analysis in ERRγ +/− female mice at the same ages at which differences were seen in ERRγ +/− versus ERRγ +/+ male mice.
The fact that a bone consequence of ERRγ haploinsufficiency is seen only in adult male mice, and not in developing or neonatal mice or in female mice at any age tested is of interest. This is likely not due to age-related changes in expression levels of ERRγ, since we have found ERRγ to be expressed at relatively low levels in male and female bone (similar to ERα levels) at all ages tested, and expression is flat/constitutively low in cultures undergoing osteoblast differentiation (data not shown). It is possible that ERRγ has a redundant role in endochondral bone or growth plate development and bone growth, but we also observed no change in BV/TV in newborn mice, which suggests that ERRγ is not necessary for osteoblast differentiation or precursor fate at developmental or neonatal stages, but rather plays a regulatory role in mice only at sexual maturity. This suggests that negative regulation of osteoblast differentiation by ERRγ is sex hormone-dependent. Regulation of sex hormone receptor-dependent bone phenotypic outcomes is complex and multifaceted, but it is notable that sex-dependent adult bone phenotypes have also been seen in ER knockout mice. For example, ERα ablation decreased bone turnover and increased trabecular bone mass in both male and female mice, whereas ERβ ablation decreased bone resorption and increased trabecular bone mass only in female mice [25]. The elevated BV/TV observed in the male gonad-intact ER knockouts was found to be due to the high serum testosterone levels and androgen receptor (AR) function [25]. We therefore tested but found no difference in serum testosterone levels between ERRγ +/− and ERRγ +/+ mice. While complete elucidation of the underlying direct and indirect mechanisms for sex-dependent effects on bone of ERs or AR in a background of altered ERRγ expression is ongoing, interactions between ER and ERR signaling pathways and transcriptional activity would not be unexpected to play roles in mice with altered expression of ERRs. Specifically with respect to ERRγ, estrogen via ERα has been shown to regulate ERRγ expression in breast cancer cells [28]. Transcriptional coactivators such as peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1 α (PGC-1α) and PGC-1β amongst others, play important roles in both ER- and ERR-mediated transcription [29]–[32], suggesting that transcriptional cofactors are partially shared between ERs and ERRs, such that altered levels of ERRγ may alter cofactor stoichiometry for ERs and other ERRs. ERRs can bind to both estrogen response elements (EREs) and ERR response elements (ERREs) [2], [33]–[35], suggesting that ERRs can also affect ER-mediated signaling. Consistent with this, ERRγ has been shown to modulate ERα responsiveness in prostate, breast, and uterine endometrial cancers [28], [36], [37]. Thus, we postulate that ERRγ haploinsufficiency may alter the actions of ERα in male mouse bone, an effect potentially abrogated by ERβ activities and estrogen signaling in females. Of course, since ERRs can form not only homo- but heterodimers with each other and ERs [2], [34], [38]–[42], we cannot rule out ER-independent effects of ERRγ on bone target genes and bone or a role for ERRα or potentially ERRβ in the bone phenotype seen.
Our observations on bone marrow stromal cells in vitro indicate that ERRγ deficiency leads to an increase in osteoblast differentiation (increase in CFU-ALP and CFU-O), without a change in the number of mesenchymal precursors (CFU-F) or proliferation. Further, we observed an increase in Alp and Bsp, markers of osteoblast differentiation and activity. Although we cannot rule out the possibility that ERRγ directly regulates these genes, previously, Jeong et al. showed that ERRγ repressed the transcriptional activity of RUNX2 on Bsp and Ocn [15]; they also showed that ERRγ physically interacted with RUNX2, an interaction that repressed RUNX2 transactivity on a 6x OSE-luc promoter construct [15]. We now show that ERRγ interacts with RUNX2 in vitro, and that the DBD is necessary for this protein interaction, indicating that the DBD is responsible not only for binding to response elements, but also for proper protein-protein interaction. It has been reported previously that both the LBD and the DBD contain regions for dimerization [42]. Interestingly, it has been shown that ERα interacts with RUNX2 in MCF7 breast cancer cell line, and the MC3T3-E1 pre-osteoblast cell line, and that this interaction was estrogen dependant, and resulted in the inhibition of RUNX2 activity [43]. Further, these authors reported that the DBD harboured the interaction motif that directly binds RUNX2. Thus, one can hypothesize that a larger transcription complex, including ERRγ and ERα may form to modulate RUNX2 activity, and regulate osteoblast differentiation/mineralization. Our data suggest that ERRγ may bind to DNA while interacting with RUNX2. There is no reported putative ERRE within the 6x OSE response element, and it has not yet been shown that ERRγ can bind this region directly.
Alp and Bsp, but not Runx2, Osx or Ocn are upregulated in ERRγ +/− compared to ERRγ +/+ trabecular bone samples and in differentiating stromal cell cultures, suggesting that ERRγ negatively regulates osteoblast differentiation and matrix mineralization at a stage after osteoblast commitment (i.e., after upregulation of Runx2 and Osx expression) but before osteoblast maturation or terminal differentiation to osteocytes (i.e., prior to acquisition of Ocn expression, and expression of osteocyte markers Dmp1 and Sost [data not shown]). This, together with the observation that Runx2 antisense treatment of post-confluent differentiating stromal cell cultures significantly reduced CFU-O and CFU-ALP in ERRγ +/− cultures compared to sense controls, but not in ERRγ +/+ cultures, supports the conclusion not only that RUNX2 is required for the ERRγ +/− osteoblast phenotype but that ERRγ's role is differentiation stage-dependent. This is interesting in view of recent data that Runx2 also has differentiation stage-dependent effects, with no effect of knockdown or knockout at certain developmental times. Thus, although RUNX2 was originally thought to be necessary at all stages of osteoblast differentiation, it has now been shown via analysis of the α1(I)-collagen-Cre;Runx2flox/flox (2.3 kb α1(I)-collagen promoter) conditional knockout mouse that Runx2 does not have an overt effect in cells already committed to the osteoblast lineage [27]. On the other hand, several kinds of data, including results from the global Runx2 knockout and an OG2-ΔCbfa1 transgenic mouse indicate that RUNX2 is required in uncommitted osteoprogenitors [44] and in mature OCN-expressing osteoblasts [45]. The lack of effect of Runx2 antisense knockdown in ERRγ +/+ cells is consistent with the fact that the majority of osteoblast precursors in the mouse cultures under the experimental conditions used are already committed and differentiating at the time of antisense treatment [46]. Further analysis of additional genetically-modified mouse models will be useful for extending the evidence that ERRγ is a differentiation stage-dependent negative regulator of osteoblast differentiation.
In summary, this is the first report of the consequences of ERRγ ablation and haploinsufficiency on bone metabolism in vivo and extends previous reports of ERRγ function in osteoblasts to the whole animal level. Our data indicate that ERRγ is a sex-dependent and RUNX2-dependent negative regulator of osteoblast differentiation, and that its regulatory activity may also be differentiation stage-dependent.
Acknowledgments
We are grateful to Ruolin Guo for the µCT data collection, Ralph Zirngibl for the ERRγ2FL, ERRγΔAF2, ERRγC148G stable MC3T3-E1 cell lines, and Renée Bernatchez in the Centre for Bone and Periodontal Research for performing methylmethacrylate embedding and histological staining. We also thank other members of the Aubin lab for helpful discussions.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The work was supported by the following: Canadian Institutes of Health Research operating grant (FRN 88104) JEA; and Canadian Institutes of Health Research Doctoral Research Award MC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Giguere V (2002) To ERR in the estrogen pathway. Trends Endocrinol Metab 13: 220–225. [DOI] [PubMed] [Google Scholar]
- 2. Yang N, Shigeta H, Shi H, Teng CT (1996) Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem 271: 5795–5804. [DOI] [PubMed] [Google Scholar]
- 3. Giguere V, Yang N, Segui P, Evans RM (1988) Identification of a new class of steroid hormone receptors. Nature 331: 91–94. [DOI] [PubMed] [Google Scholar]
- 4. Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, et al. (2007) The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6: 25–37. [DOI] [PubMed] [Google Scholar]
- 5. Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, et al. (2007) Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab 5: 345–356. [DOI] [PubMed] [Google Scholar]
- 6. Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, et al. (2007) ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6: 13–24. [DOI] [PubMed] [Google Scholar]
- 7. Bonnelye E, Aubin JE (2013) An energetic orphan in an endocrine tissue: a revised perspective of the function of estrogen receptor-related receptor alpha in bone and cartilage. J Bone Miner Res 28: 225–233. [DOI] [PubMed] [Google Scholar]
- 8. Bonnelye E, Merdad L, Kung V, Aubin JE (2001) The orphan nuclear estrogen receptor-related receptor alpha (ERRalpha) is expressed throughout osteoblast differentiation and regulates bone formation in vitro. J Cell Biol 153: 971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delhon I, Gutzwiller S, Morvan F, Rangwala S, Wyder L, et al. (2009) Absence of estrogen receptor-related-alpha increases osteoblastic differentiation and cancellous bone mineral density. Endocrinology 150: 4463–4472. [DOI] [PubMed] [Google Scholar]
- 10. Teyssier C, Gallet M, Rabier B, Monfoulet L, Dine J, et al. (2009) Absence of ERRalpha in female mice confers resistance to bone loss induced by age or estrogen-deficiency. PLoS One 4: e7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajalin AM, Pollock H, Aarnisalo P (2010) ERRalpha regulates osteoblastic and adipogenic differentiation of mouse bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 396: 477–482. [DOI] [PubMed] [Google Scholar]
- 12. Elfassihi L, Giroux S, Bureau A, Laflamme N, Cole DE, et al. (2010) Association with replication between estrogen-related receptor gamma (ESRRgamma) polymorphisms and bone phenotypes in women of European ancestry. J Bone Miner Res 25: 901–911. [DOI] [PubMed] [Google Scholar]
- 13. Pirih FQ, Abayahoudian R, Elashoff D, Parhami F, Nervina JM, et al. (2008) Nuclear receptor profile in calvarial bone cells undergoing osteogenic versus adipogenic differentiation. J Cell Biochem 105: 1316–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roforth MM, Liu G, Khosla S, Monroe DG (2012) Examination of nuclear receptor expression in osteoblasts reveals Rorbeta as an important regulator of osteogenesis. J Bone Miner Res 27: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong BC, Lee YS, Park YY, Bae IH, Kim DK, et al. (2009) The orphan nuclear receptor estrogen receptor-related receptor gamma negatively regulates BMP2-induced osteoblast differentiation and bone formation. J Biol Chem 284: 14211–14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim EJ, Kang IH, Lee JW, Jang WG, Koh JT (2013) MiR-433 mediates ERRgamma-suppressed osteoblast differentiation via direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci 92: 562–568. [DOI] [PubMed] [Google Scholar]
- 17. Parr BA, McMahon AP (1995) Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature 374: 350–353. [DOI] [PubMed] [Google Scholar]
- 18. Rossert J, Eberspaecher H, de Crombrugghe B (1995) Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol 129: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scutt A, Beier N, Fittschen C (2004) EMD273316 & EMD95833, type 4 phosphodiesterase inhibitors, stimulate fibroblastic-colony formation by bone marrow cells via direct inhibition of PDE4 and the induction of endogenous prostaglandin synthesis. BMC Pharmacol 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wai PY, Mi Z, Gao C, Guo H, Marroquin C, et al. (2006) Ets-1 and runx2 regulate transcription of a metastatic gene, osteopontin, in murine colorectal cancer cells. J Biol Chem 281: 18973–18982. [DOI] [PubMed] [Google Scholar]
- 21.Zirngibl RA, Chan JS, Aubin JE (2013) Divergent regulation of the Osteopontin promoter by the estrogen receptor-related receptors is isoform- and cell context-dependent. J Cell Biochem. [DOI] [PubMed]
- 22. Luo J, Sladek R, Carrier J, Bader JA, Richard D, et al. (2003) Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol 23: 7947–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, et al. (2000) Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci U S A 97: 5474–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sims NA, Clement-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, et al. (2003) A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest 111: 1319–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, et al. (2002) Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30: 18–25. [DOI] [PubMed] [Google Scholar]
- 26. Perlmann T, Umesono K, Rangarajan PN, Forman BM, Evans RM (1996) Two distinct dimerization interfaces differentially modulate target gene specificity of nuclear hormone receptors. Mol Endocrinol 10: 958–966. [DOI] [PubMed] [Google Scholar]
- 27. Takarada T, Hinoi E, Nakazato R, Ochi H, Xu C, et al. (2013) An analysis of skeletal development in osteoblast-specific and chondrocyte-specific runt-related transcription factor-2 (Runx2) knockout mice. J Bone Miner Res 28: 2064–2069. [DOI] [PubMed] [Google Scholar]
- 28. Ijichi N, Shigekawa T, Ikeda K, Horie-Inoue K, Fujimura T, et al. (2011) Estrogen-related receptor gamma modulates cell proliferation and estrogen signaling in breast cancer. J Steroid Biochem Mol Biol 123: 1–7. [DOI] [PubMed] [Google Scholar]
- 29. Hentschke M, Susens U, Borgmeyer U (2002) PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun 299: 872–879. [DOI] [PubMed] [Google Scholar]
- 30. Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, et al. (2003) PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A 100: 12378–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J Biol Chem 278: 9013–9018. [DOI] [PubMed] [Google Scholar]
- 32. Tcherepanova I, Puigserver P, Norris JD, Spiegelman BM, McDonnell DP (2000) Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem 275: 16302–16308. [DOI] [PubMed] [Google Scholar]
- 33. Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, et al. (1997) Estrogen-related receptor alpha 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol 11: 342–352. [DOI] [PubMed] [Google Scholar]
- 34. Vanacker JM, Bonnelye E, Chopin-Delannoy S, Delmarre C, Cavailles V, et al. (1999) Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-alpha). Mol Endocrinol 13: 764–773. [DOI] [PubMed] [Google Scholar]
- 35. Vanacker JM, Pettersson K, Gustafsson JA, Laudet V (1999) Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J 18: 4270–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu S, Wang X, Ng CF, Chen S, Chan FL (2007) ERRgamma suppresses cell proliferation and tumor growth of androgen-sensitive and androgen-insensitive prostate cancer cells and its implication as a therapeutic target for prostate cancer. Cancer Res 67: 4904–4914. [DOI] [PubMed] [Google Scholar]
- 37. Yamamoto T, Mori T, Sawada M, Kuroboshi H, Tatsumi H, et al. (2012) Estrogen-related receptor-gamma regulates estrogen receptor-alpha responsiveness in uterine endometrial cancer. Int J Gynecol Cancer 22: 1509–1516. [DOI] [PubMed] [Google Scholar]
- 38. Bonnelye E, Vanacker JM, Dittmar T, Begue A, Desbiens X, et al. (1997) The ERR-1 orphan receptor is a transcriptional activator expressed during bone development. Mol Endocrinol 11: 905–916. [DOI] [PubMed] [Google Scholar]
- 39. Xie W, Hong H, Yang NN, Lin RJ, Simon CM, et al. (1999) Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol 13: 2151–2162. [DOI] [PubMed] [Google Scholar]
- 40. Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, et al. (2002) Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell 9: 303–313. [DOI] [PubMed] [Google Scholar]
- 41. Hentschke M, Susens U, Borgmeyer U (2002) Domains of ERRgamma that mediate homodimerization and interaction with factors stimulating DNA binding. Eur J Biochem 269: 4086–4097. [DOI] [PubMed] [Google Scholar]
- 42. Huppunen J, Aarnisalo P (2004) Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun 314: 964–970. [DOI] [PubMed] [Google Scholar]
- 43. Khalid O, Baniwal SK, Purcell DJ, Leclerc N, Gabet Y, et al. (2008) Modulation of Runx2 activity by estrogen receptor-alpha: implications for osteoporosis and breast cancer. Endocrinology 149: 5984–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, et al. (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89: 755–764. [DOI] [PubMed] [Google Scholar]
- 45. Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, et al. (1999) A Cbfa1-dependent genetic pathway controls bone formation beyond embryonic development. Genes Dev 13: 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malaval L, Modrowski D, Gupta AK, Aubin JE (1994) Cellular expression of bone-related proteins during in vitro osteogenesis in rat bone marrow stromal cell cultures. J Cell Physiol 158: 555–572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.