Abstract
Episodic memory loss is one of the hallmarks of age-related cognitive decline and a major symptom of Alzheimer's disease. The persistence and strength of memories is determined by modulatory factors such as emotional arousal. Whether emotional memories are preserved with age or if these memories are just as susceptible to loss and forgetting is not well understood. We have recently shown that emotion alters how similar memories are stored using non-overlapping representations (i.e. pattern separation), in an emotional mnemonic discrimination task. Here, we extend this work to testing young and older adults at two time-points (immediately after encoding and 24 hours later). Overall, older adults performed worse than young adults, a memory deficit that was not secondary to perceptual or attentional deficits. When tested immediately, older adults were impaired on neutral target recognition but intact on emotional target recognition. We also found that a pattern we previously reported in young adults (reduced emotional compared to neutral discrimination of similar items) was reversed in older adults. When tested after 24-hours, young adults exhibited less forgetting of emotional targets compared to neutral, while older adults exhibited more forgetting of emotional targets. Finally, discrimination of highly similar positive items was preserved in older adults. These results suggest that emotional modulation of memory interacts with age in a complex manner such that the emotion-induced memory trade-off reported in young adults is reversed in older adults. These findings shed light on how emotion and memory interact in the aging brain.
Keywords: emotion, memory, interference, hippocampus, age, pattern separation
Introduction
Changes in episodic memory performance are a hallmark feature of aging and a major symptom of mild cognitive impairment (MCI) and Alzheimer's disease (AD). Episodic memory, or memory for personal events, is frequently reported to decline in the elderly (Craik & Simon, 1980; Glisky, 2007). The medial temporal lobes (MTL), which include the hippocampus, amygdala, and surrounding cortices, are crucial for the formation of episodic memories (Milner, Squire, & Kandel, 1998)and is one of the first sites of degeneration in aging and AD (Gómez-Isla et al., 1996). This network is implicated in age-related deficits in neurocognitive functioning. While general impairment of episodic memory has been well established, it is not clear whether there are existing mechanisms that may allow older adults to compensate for this memory loss by altering modulatory influences. For example, altering the focus of attention, increasing repetition of events, or increasing the significance of events may alter the degree of memory impairment in older adults.
While episodic memory deficits are a hallmark characteristic of aging, emotion's modulatory influence on memory remains less well characterized in aging. Decades of research in rodents have shown that emotional experiences are better remembered than non-emotional experiences (McGaugh, 2004; LeDoux, 2007). However, animal and human studies are inconsistent with respect to age-related changes in emotional modulation of memories. Several stimulus-specific factors may contribute to these discrepancies, such as valence, capacity to increase arousal, and degree of detail (Foster, DeFazio, & Bizon, 2012; McGaugh, 2006). Moreover, when deficits in the emotional modulation of memory manifest in aging, it is not clear whether these deficits are due to age-related hippocampal alterations, changes in the brain regions thought to play a modulatory role, such as the amygdala, or both.
Several human studies have shown that memory for emotional experiences is preserved with age (Denburg, Buchanan, Tranel, & Adolphs, 2003; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002). However, not all aspects of an emotional event are remembered equally. Emotional arousal can enhance memory for the general theme or ‘gist-based’ information but impair memory for detailed information (Adolphs, Denburg, & Tranel, 2001; Kensinger, 2009). This trade-off likely plays a critical role in the storage of long-lasting memories and may distinguish the aspects of those memories that are remembered with better accuracy. In aging, some studies have suggested that there is a “positivity effect,” where older adults may be more likely to attend to positive information in the environment (Dolcos et al., 2012; Mather & Carstensen, 2005). In contrast, some have suggested that memory for detailed information in older adults reveals no such positivity effect and may actually be biased towards remembering negative details (Kensinger, Garoff-Eaton, & Schacter, 2007). This suggests that the relationship between aging and emotional modulation of memory is complex and requires a more thorough investigation.
The hippocampus, which plays a key role in episodic memory (Milner, Squire, & Kandel, 1998), is thought to be involved in pattern separation – the process of reducing interference among similar inputs by using non-overlapping representations (Marr, 1971; McClelland, McNaughton, & O'Reilly, 1995; O'Reilly & Norman, 2002; Treves & Rolls, 1994; Yassa & Stark, 2011). Older individuals show deficits in paradigms that tax the ability to discriminate highly similar information (i.e. a putative behavioral measure of hippocampal pattern separation) (Toner, Pirogovsky, Kirwan, & Gilbert, 2009; Yassa et al., 2010). We have also recently reported a specific perturbation in hippocampal activity thought to be reflective of impaired pattern separation in older adults, which is associated with poor discrimination performance (Yassa, Mattfeld, Stark, & Stark, 2011; Yassa et al., 2010). This may be closely linked to degraded input from the perforant path (Kalus et al., 2006; Rogalski et al., 2009; Yassa, Muftuler, & Stark, 2010).
The impact of emotional arousal on mnemonic computations, such as pattern separation, has not been examined in detail in older humans and has only been investigated recently in young adults (Leal et al., in press). While pattern separation allows for the orthogonalization of information to distinguish highly similar events and experiences in our lives, the persistence and strength of memories is determined by modulatory factors such as emotional arousal. In the current study, we utilized an emotional mnemonic discrimination task similar to tasks used in the past (see Segal et al., 2012; Stark et al., 2013; Yassa et al., 2011) to examine the influences of emotional arousal on discrimination of emotional information. Our goal was to understand the emotional modulation of episodic memory in aging by first examining this putative modulation behaviorally in young and older adults. Furthermore, emotional arousal is thought to influence the consolidation of information over time, thus, we tested participants either immediately after encoding or 24 hours later.
Materials and Methods
Participants
Participants were recruited from Johns Hopkins University as well as the local Baltimore community via local campus announcements, flyers, and ads in local newspapers. Participants were between the ages of 18-35 for the young adult groups and 60-85 for the older adult groups. Participants received either course credit or monetary remuneration for their participation. The young adult group tested immediately consisted of 24 participants (mean age 21 SD 3, 16 female). The young adult group tested 24 hours later consisted of 14 participants (mean age 21 SD 1, 6 female). The data from young participants in this paper have already been reported in Leal et al. (in press). The older adult group tested immediately consisted of 22 participants (mean age 67 SD 4, 14 female). The older adult group tested 24 hours later consisted of 16 participants (mean age 71 SD 8, 12 female). Informed consent was obtained from all participants, with all procedures approved by the Johns Hopkins University Institutional Review Board.
Inclusion/exclusion criteria
All participants were screened against major medical or psychiatric morbidities as well as substance abuse history. Older participants received a neuropsychological evaluation during their visit. The battery was designed to examine memory function, as well as other aspects of general cognition. The assessment included the following: (1) Mini-Mental State Exam (MMSE) to assess global cognitive status, (2) Rey Auditory Verbal Learning Test (RAVLT) to assess verbal learning, immediate and delayed recall, and recognition, (3) Digit Span backwards and forwards to assess working memory, (4) Trail Making Tests A and B to assess attention, visual search, and mental processing speed, (5) Beck Depression Inventory-II (BDI-II) to assess depressive symptoms, and (6) Geriatric Depression Scale (GDS) to assess depressive symptoms more specific to older adults (see Table 1). There were no significant differences between the older adult groups in age, education, and all other neuropsychological measures. All participants had normal or corrected to normal vision.
Table 1. Older Participant Demographics and Neuropsychological Test Rexittts.
| Groups | Older immediate | Older 24-hr delay | ||
|---|---|---|---|---|
|
| ||||
| Sample Size | 22 | 16 | ||
| M : F | 8 : 4 | 4 : 12 | ||
| variables | Mean | SEM | Mean | SEM |
| Age | 66.7 | 0.9 | 70.6 | 1.9 |
| Education | 16.0 | 0.5 | 15-4 | 0.7 |
| Beck depression invientory-II | -i.l | 0.5 | 5.1 | 1.I |
| Geriatric depression wale | 1.3 | 0.2 | 1,1 | 0,4 |
| RAVLT* ast immediale recall | 10.3 | 0.5 | 9.7 | 0.8 |
| RAVL1 delayed recall | 10,0 | 0.6 | 9A | 0.8 |
| Digit span forward | 12.5 | 0.5 | 11.0 | 0.6 |
| Digit span backward | 6.3 | 0.5 | 7 1 | 0.5 |
| Mini mental state exam | 2S.6 | 0.4 | 27.8 | 0.3 |
| Trail making test A | 28.6 | 2 1 | 34.7 | 3.7 |
| Trail making test B | 69.8 | 2.3 | 89.1 | 10.4 |
= RAVLT: rey auditory verbal learning test.
Emotional mnemonic discrimination task
The stimulus set was comprised of novel scenes freely available online, sized to a width of 600 pixels. Images were categorized a priori for emotional valence (negative, neutral, positive), arousal (very calming to very exciting), and similarity (median split of similarity ratings into “high” and “low”) (Leal et al., in press). The experimental paradigm consisted of 149 images during the encoding phase and 291 images during the retrieval phase. Targets, lures (similar but not identical to images shown during encoding), and foils were roughly evenly distributed across emotional valence and similarity level (high similarity (HS) and low similarity (LS) lures). An Apple iMac equipped with MATLAB (Version R2010a, Natick, MA) software and PsychToolbox version 3.0 was used to present the stimuli and record keyboard responses. Each trial consisted of 2 displays: an image display (images were presented on the center of the screen with a black background for 2500 ms) and a fixation display (a white fixation cross on the center of the screen with a black background for 500 ms). Timing was identical for encoding and retrieval phases.
Each participant was given oral and written instructions. Participants underwent an incidental encoding phase where they were shown emotional and non-emotional images, presented in randomized order, and were asked to rate the images for emotional valence. Young adults rated images on a 1-9 point scale (1 being most negative, 9 being most positive, and 5 being neutral) and older adults used a similar scale, but without the large range of responses (limited to 3 button responses of 1 for negative, 5 for neutral, and 9 for positive). In pilot studies, we observed that allowing older adults the full 9-point scale to respond led to frustration and increased latency beyond the response window. Thus, we chose to limit the response options in order to make the decision process easier and remove this potential difficulty. Participants were given a subsequent surprise memory test either immediately after encoding or after a 24-hour delay, in which participants saw another series of stimuli, some of which were seen once before in the incidental task (targets), some were similar to ones seen in the incidental task but not identical (lures), and some were new (foils). Participants were asked to indicate whether items were “old” or “new” by button responses on the keyboard. Participants were explicitly told that in order for an image to be called “old,” it had to be the exact same image they saw before (Fig. 1). Participants who returned 24 hours later were told that they would be coming in for another hour of testing and were not explicitly told what tasks they would be performing.
Fig. 1. Emotional mnemonic discrimination task.
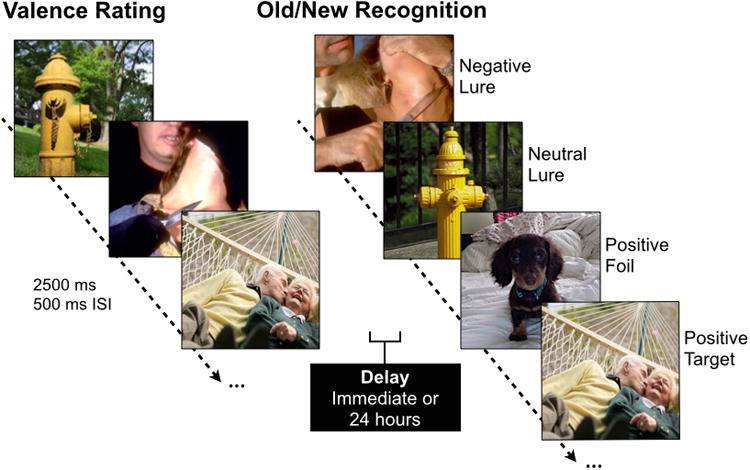
During encoding, participants rated images according to their emotional valence. Each image was presented for 2500 ms with a 500 ms inter-stimulus-interval (ISI). Immediately after study or 24 hours later, participants underwent a surprise recognition test where they viewed negative, neutral, and positive targets, foils, and lures varying in similarity and were asked to indicate whether items were “old” or “new”.
Our two key outcome measures of interest were target recognition (D′) and lure discrimination index (LDI). Target recognition was measured by a discriminability index (D′), which was calculated as z(Hits) – z(False Alarms), which is thought to assess gist knowledge or general familiarity (Norman, 2010; Yonelinas, Aly, Wang, & Koen, 2010). This was calculated for each emotion (negative, neutral, and positive). From a computational perspective, this process requires pattern completion but not pattern separation (Kim & Yassa, 2013; Yassa & Stark, 2011). In order to measure how well participants discriminated similar items (lures), we examined performance using a response bias-corrected LDI operationalized as p(‘New’|Lure) – p(‘New’|Target). One potential problem with using correct rejections (i.e. ‘New’|Lure) as a behavioral correlate for pattern separation is that it is likely contaminated by rejections that are the result of insufficient or inattentive encoding (i.e. misses, ‘New’|Target). In order to account for this possibility, we subtract the probability of rejecting an old item (which quantifies such misses) from the probability of rejecting a lure to generate the LDI metric we use in the task. This corrected for the general tendency to reject (i.e. call an item ‘New’) and is similar to other metrics we used in prior work (Leal et al., 2014; Yassa & Stark, 2011; Yassa et al., 2010a; Yassa et al., 2010b). This was also calculated for each emotion (negative, neutral, and positive). From a computational perspective, this process requires both pattern completion as well as pattern separation (Kim & Yassa, 2013; Yassa & Stark, 2011). Raw response proportions and reaction time (RT) data for all experimental groups are in Supplementary Information (Table S1 and Fig. S1).
Match-to-sample task
It is possible that behavioral performance on the discrimination task is affected by perceptual/attentional deficits that manifest with aging. To control for these potential confounds, we employ a match-to-sample task using the same stimuli, except yoking similar image pairs together (separated by a static noise mask to eliminate sensory memory). Two separate samples of young and older adults were tested on a match-to-sample task using the same exposure time as in the primary experiment (2500 ms) (Young: N = 18, 22 ± 3, 12 female, Old: N = 13, 65 ± 6, 7 female). The young adult group is from Leal et al. (in press). The match-to-sample task consisted of trials that were composed of 4 sequential displays: first image (presented for 2500 ms), followed by a pixelated noise mask display for 1000 ms, a second image for 2500 ms, and finally an inter-trial fixation display (500 ms). Images were identical to those used in the discrimination experiments. Participants were told to determine whether the two images were exactly the same or different via button press on the keyboard. Subjects were told to respond while the second image was still on the screen. Image pairs were either identical (repetitions) or similar (lures). We have used a similar design in our past work to control for perceptual or attentional influences, which can vary with age (Yassa et al., 2010; Ly, Murray, & Yassa,2013).
Statistical analysis
All statistical analyses were conducted in SPSS v. 20.0 (IBM Corp., released 2011, Armonk, NY). For immediate testing, 2-way repeated-measures ANOVAs (age and emotion) were performed for target recognition and lure discrimination index (high and low similarity ANOVAs conducted separately). For 24-hour delay testing, 3-way repeated-measures ANOVAs (age, emotion, time of testing) were performed for target recognition and lure discrimination index (again, high and low similarity ANOVAs conducted separately). Forgetting rates were calculated post hoc for visualization purposes only (i.e. no statistics were run on the forgetting rates). This was a between-subjects design, thus, forgetting rates were calculated on the group means (e.g. mean D′ (immediate) – mean D′ (delay) and not on individual subject scores). Repeated measures tests were corrected for error non-sphericity using Greenhouse-Geisser correction where appropriate. Post hoc statistical tests were corrected for multiple comparisons using Scheffé's correction, with critical F values indicated in the text corresponding to the degrees of freedom (df) of the F-test (mentioned only once for each pair of df's). Statistical values were considered significant at a final corrected alpha level of .05, which appropriately controlled for Type I error.
Results
Impaired neutral but preserved emotional target recognition in older adults
We investigated the effect of emotion (negative, neutral, and positive) on target recognition when tested immediately across age groups (young and old) using a two-way ANOVA, which revealed a significant effect of emotion [F(2,88) = 5.3, P = .01], where negative targets were better remembered compared to neutral and positive targets [F(1,88) = 9.7, critical Scheffé = 6.2]. There was a significant effect of age where young adults performed better than older adults [F(1,44) = 7.7, P = .01]. In addition, there was a significant interaction between emotion and age [F(2,88) = 3.7, P = .04]. We parsed this interaction using a post-hoc contrast examining the effect of emotional valence (positive and negative vs. neutral) across groups (young vs. old). We found that older adults were impaired relative to young adults on neutral target recognition but were preserved on emotional (positive and negative) target recognition, on par with young adults [F(1,88) = 6.7, critical Scheffé = 6.2; Fig. 2A). We will refer to “emotional target recognition or lure discrimination” as comprised of both negative and positive stimuli unless otherwise noted.
Fig. 2. Performance in young and older adults.
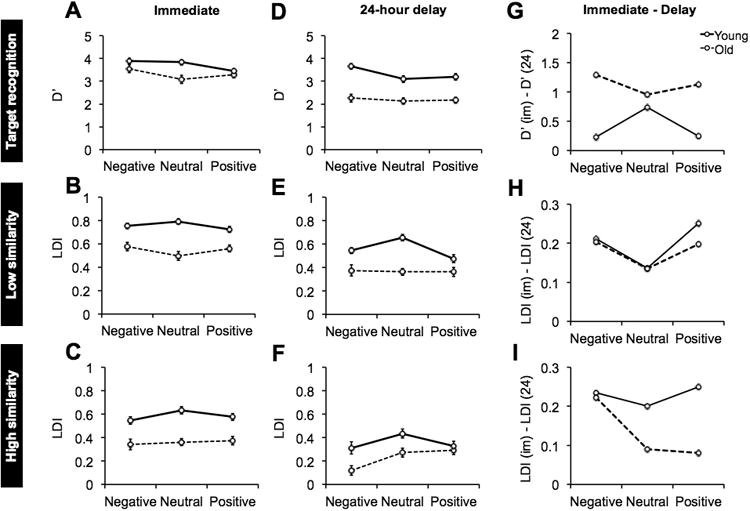
(A) Target recognition (D′) in the immediate condition; (B) Low interference Lure Discrimination Index (LDI) in the immediate testing condition; (C) High interference LDI in the immediate testing condition; (D) Target recognition (D′) in the 24-hour delay condition; (E) Low interference LDI in the 24-hour delay condition; (F) High interference LDI in the 24-hour delay condition; (G) Target recognition forgetting rate plotted as the difference between immediate target recognition [(D′ (im)] and 24-hour delay target recognition [D′ (24)]; (H) Low interference lure discrimination forgetting rate plotted as the difference between immediate lure discrimination [(LDI(im)] and 24-hour delay lure discrimination [LDI(24)]; (I) High interference lure discrimination forgetting rate plotted as the difference between LDI(im) and LDI(24). See main text for data interpretation and statistical comparisons.
Reversed emotional modulation of lure discrimination in older adults
Next, we assessed the effect of emotion on discrimination of low similarity lures (LS-LDI) across age groups using a two-way ANOVA, which revealed a significant effect of age, in which young adults performed better than old adults [F(1,44) = 47.6, P < .001]. We also found a significant interaction between emotion and age [F(2,88) = 4.5, P = .02]. We parsed this interaction using a post-hoc contrast examining the effect of emotional valence (positive and negative vs. neutral) across groups (young vs. old). We found that older adults show increased emotional versus neutral lure discrimination, while young adults show reduced emotional versus neutral lure discrimination [F(1,88) = 8.2, critical Scheffé = 6.2; Fig. 2B). While discrimination of emotional stimuli was better compared to neutral stimuli in older adults, it is important to note that even with a boost in memory performance for emotional items, older adults do not overcome the overall memory deficit.
We evaluated the effect of emotion on high similarity LDI (HS-LDI) across age groups using a two-way ANOVA, which revealed a significant effect of age [F(1,44) = 47.6, P < .001], where young adults performed better than older adults. There were no significant differences between age groups for emotion or an interaction between age and emotion. While young adults show a similar pattern of reduced emotional versus neutral lure discrimination, older adults show no differences in discrimination across highly similar emotional and non-emotional items (Fig. 2C).
Emotional modulation not secondary to attention or perceptual effects
A potential interpretation of the emotional modulation effects we report is that age-related changes in attentional focus or working memory capacity could influence behavioral performance. For example, participants may not have perceptually encoded all of the details of the emotional images during the encoding phase and this lack of attention to detail may have affected subsequent memory performance. Consistent with this idea, Mather and Sutherland (2011) proposed that arousal during an event can either enhance or impair memory for events, depending on attentional factors that bias competition in favor of high priority stimuli (Mather & Sutherland, 2011).
To examine the possibility of extra-mnemonic influences on task performance, we tested 31 new participants (13 older adults) on a match-to-sample (MTS) task using the same stimuli that were used in the emotional mnemonic discrimination task. Participants were shown yoked pairs of similar or repeated images with a static noise mask in between and asked to determine if the images were the same or different. We measured target hit rate and lure rejection rate and found a main effect of age for target hit rate [F(1,29) = 6.88, P = .014; Fig.S2a], where older adults performed slightly better than young adults. There were no significant differences across age groups for lure rejection rate (all P's > .05; Fig. S2b). This suggests that while attention may play a role in emotional processing, it did not significantly contribute to the effects observed here. A related possibility is that encoding and consolidation mechanisms interact so that emotionally arousing items are differentially processed during encoding, in such a manner that their long-term consolidation is also altered (Hamann, 2001).
Increased forgetting of emotional compared to neutral targets after 24 hours in older adults
Next, we had separate groups of young and older adults perform the same task, but perform the surprise memory test 24 hours later. We investigated the effect of emotion, age, and time of testing (immediate vs. 24-hour delay) on target recognition using a three-way ANOVA, which revealed a significant effect of emotion [F(2,144) = 8.8, P <.001], where negative targets were better remembered compared to neutral and positive targets [F(1,144) = 14.3, critical Scheffé = 6.12]. There was a significant effect of age where young adults performed better than older adults [F(1,72) = 49.8, P < .001]. There was a significant effect of time of testing where immediate performance was better than after 24 hours [F(1,72) = 49.1, P < .001]. In addition, there was a significant interaction between age and time of testing [F(1,72) = 10.8, P = .002]. We parsed this interaction using a post-hoc contrast examining the effect of time of testing (immediate vs. delay) across groups (young vs. old). We found that old and young adults performed similarly on target recognition when tested immediately, while older adults had worse target recognition (i.e. more forgetting) after a 24-hour delay. Interestingly, we found a three-way interaction between time of testing, emotion, and group [F(2,144) = 3.5, P = .037]. We performed a post-hoc contrast examining the effect of time of testing (immediate vs. delay) and emotional valence (negative and positive vs. neutral) across groups (young vs. old). We found that young adults exhibited less forgetting of emotional targets compared to neutral, while older adults exhibited more forgetting of emotional targets compared to neutral [F(1,144) = 6.8, P = .011; Fig. 2D,G].
Increased false recognition of emotional compared to neutral lures after 24 hours across both young and older adults
We also assessed the effect of emotion, age, and time of testing for low similarity lure discrimination index using a three-way ANOVA, which revealed a significant effect of emotion [F(2,144) = 3.2, P = .048], where positive and negative lures were more poorly discriminated compared to neutral lures [F(1,144) = 3.5, P < .05]. There was a significant effect of age, where young adults performed better than older adults [F(1,72) = 69.9, P < .001]. There was a significant effect of time of testing, where performance was better when tested immediately versus at a 24 hour delay [F(1,72) = 61.1, P < .001]. We also found a significant interaction between emotion and time of testing. We performed a post-hoc contrast examining the interaction between emotional valence (negative and positive vs. neutral) and time of testing (immediate vs. delay), where emotional lures were more falsely recognized over time compared to neutral lures across age groups [F(1,144) = 6.0, P = .017; Fig. 2E,H]. We also found a significant interaction between emotion and age [F(2,144) = 9.3, P < .001], where a post-hoc contrast of emotional valence across age groups showed that older adults have increased emotional versus neutral lure discrimination, while young adults have decreased emotional versus neutral lure discrimination [F(1,144) = 18.0, P < .001], suggesting that the aforementioned reversal of the emotional modulation of lure discrimination in older adults continues at a delay of 24 hours.
Preserved discrimination of highly similar positive lures in older adults
We assessed the effect of emotion, age, and time of testing on high similarity LDI using a three-way ANOVA, which revealed a significant effect of emotion [F(2,144) = 10.5, P <.001], where discrimination of highly similar emotional lures was worse than neutral lure discrimination [F(1,144) = 12.2, P =.001]. There was a significant effect of age [F(1,72) = 34.6, P < .001], where young adults performed better than older adults. There was a significant effect of time of testing [F(1,72) = 35.5, P < .001], where performance was better when tested immediately versus after a 24 hour delay. Additionally, we found an emotion by age interaction [F(2,144) = 3.1, P =.048]. A post-hoc contrast of emotional valence across age groups revealed that young adults had worse discrimination on both positive and negative items relative to neutral. Older adults, on the other hand had worse discrimination only on negative items relative to neutral, but were preserved on positive items [F(1,144) = 3.5, P < .05; Fig F,I].
In order to determine if older adults show greater impairment when discriminating high similarity lures compared to low similarity lures, we conducted an additional ANOVA in older adults with emotion and similarity as within-subject factors and time as a between-subject factor and found a significant effect of similarity, where older adults were more impaired when discriminating high similarity lures compared to low similarity lures [F(1,36) = 109.11, P < .001].
Discussion
While general impairment of episodic memory in age-related cognitive decline has been well established, it is not clear whether there are existing mechanisms that may allow older adults to compensate for this memory loss by altering modulatory systems in the brain. The current study examined whether there were age-related changes in the emotional modulation of memory, specifically in the context of interference and pattern separation changes associated with aging. Since emotion's influence on memory can occur during encoding and furthermore during consolidation, we tested separate groups of participants immediately after encoding and 24 hours later.
Emotional modulation of memory encoding in aging
When testing participants immediately after encoding, we found an overall enhancement in young adult performance compared to older adults in general recognition and discrimination. Older adults showed a preservation of emotional target recognition compared to young adults, but showed an impairment of neutral target recognition compared to young adults. This finding is consistent with previous findings that emotional memory may be preserved across the lifespan (Denburg et al., 2003; Kensinger, Krendl, & Corkin, 2006; Waring & Kensinger, 2009). While older adults show a general decline in episodic memory, memories tied to an emotional context are remembered with more fidelity. Although forgetting increases with age, these results and others suggest that there is a selective remembering of emotional experiences, serving to create lasting memories of our more important experiences.
Whereas older adults were impaired in discriminating similar items relative to young adults overall, the effect of emotion was reversed with age. Young adults were more likely to falsely recognize similar emotional lures than older adults. This effect was evident at least for the low similarity lure items. The shift in emotional modulation could be due to at least two possible explanations: 1) a compensatory effect such that emotional arousal can boost discrimination performance on similar items and help increase memory for more important emotional events or 2) an aberration of emotional-mnemonic processing in older adults such that the boost in emotional discrimination is actually maladaptive, since it would be better to remember the gist for emotional events rather than the details. Young adults' discrimination (ability to suppress false recognition) was enhanced on neutral items compared to emotional items presumably due to a trade-off between gist and detail. Thus, it may be more adaptive to forget minute details of emotional experiences in favor of retaining the bigger picture (Adolphs et al., 2001; Kensinger, 2009; Loftus, Loftus, & Messo, 1987). Older adults, on the other hand, appear to suppress false alarms better for emotional items, suggesting that they may be engaging in a more costly mnemonic operation without clear adaptive value.
Emotional modulation of memory consolidation in aging
We then conducted the study with a 24-hour delay between the study and test phase and compared immediate and delayed performance across groups. Young adults exhibited less forgetting of emotional targets compared to neutral after 24 hours, while older adults exhibited more forgetting of emotional targets after 24 hours. Thus, with the passage of time, emotional gist memory seems to be preserved in young adults and reduced in older adults. Initially, memories tied to an emotional context seem to be better remembered in older adults, but these memories may not undergo consolidation to the same extent as in young adults. Over time, emotional arousal does not seem to provide an additional boost for remembering the same image seen before.
For stimuli that were somewhat similar but not identical to previously viewed stimuli (i.e. low similarity lures), false recognition was higher for emotional compared to neutral items across both age groups. This suggests that both young and older adults discriminate emotional and neutral information similarly after consolidation has occurred (i.e. both age groups falsely recognize more emotional versus neutral information over time). For stimuli that were very similar but not identical to previously viewed stimuli (i.e. high similarity lures), false recognition of negative items (but not neutral or positive items) was higher in older adults. The ability of older adults to recall the details of positive stimuli to correctly discriminate them from highly similar lures seems to be unaffected by age. This is consistent with the positivity bias reported in past literature (Mather & Carstensen, 2005). In addition, the effects we find on memory consolidation may be associated with changes in the sleep-wake cycle, where typical findings have shown that older adults have irregular sleep patterns (Buckley & Schatzberg, 2005), which may affect their ability to consolidate emotional memories (Payne & Kensinger, 2010).
Potential mechanisms for shifts in emotional modulation of memory with age
Paradigms that vary mnemonic interference offer a robust empirical framework by which hippocampal function can be assessed (Hunsaker & Kesner, 2013). Indeed, much work has already been done using this framework including the assessment of changes in neurocognitive aging (Stark, Yassa, & Stark, 2010; Toner et al., 2009; Yassa & Stark, 2011; Yassa et al., 2010), mild cognitive impairment (Yassa et al., 2010c), perforant path degradation (Yassa et al., 2011; Yassa, Muftuler, & Stark, 2010), and neurogenesis loss of function (Clelland et al., 2009) as well as gain of function (Sahay et al., 2011). In human high-resolution BOLD fMRI studies, behavior on discrimination tasks has been specifically associated with pattern separation signals in the hippocampal DG and CA3 (Yassa et al., 2011) as well as the integrity of the perforant path input to the hippocampus from the entorhinal cortex (Yassa et al., 2010). Here, we extended the pattern separation framework to investigate the impact of emotional modulation on hippocampal memory in aging. Although pattern separation was not directly assessed here, our manipulation of the similarity of lure stimuli allows us to examine a potential behavioral correlate of hippocampal pattern separation (Yassa & Stark, 2011).
We observed a shift in the balance of processing similar emotional information with age, which may be due to a shift in amygdala-hippocampal interactions. St. Jacques and colleagues (2009) suggested that an age-related reduction in the contribution of amygdala-hippocampal mechanisms may be compensated by enhanced contribution of amygdala-prefrontal mechanisms to the formation of emotional memories (Murty et al., 2009; St Jacques, Dolcos, & Cabeza, 2009). In normal aging, the DG/CA3 regions seem to be affected such that there is hyperactivity in the CA3 region (likely driven by the region's excitatory recurrent collaterals that are disinhibited with age) concurrent with a decrease in input from the entorhinal cortex (Gallagher & Koh, 2011; Jagust, 2013; Yassa et al., 2010; Yassa, Muftuler, & Stark, 2010b). The amygdala is relatively well preserved, but still shows age-related change, especially in the basolateral amygdala (BLA) (Dere, Pause, & Pietrowsky, 2010; Herzog & Kemper, 1980; Vereecken, Vogels, & Nieuwenhuys, 1994).
It is hypothesized that emotional arousal, via norepinephrine (NE) release in the BLA, strengthens hippocampal memory representations (Gallagher, Kapp, Musty, & Driscoll, 1977; McGaugh, 2004). Decreases in peripheral epinephrine levels (Sternberg, Martinez, Gold, & McGaugh, 1985) and noradrenergic involvement in age-related memory dysfunction (Kubanis & Zornetzer, 1981) have been reported in animal models of aging. Alterations in synaptic plasticity in the amygdala-hippocampal network with age have also been reported. Young rodents display early-long term potentiation (LTP) when stimulating the perforant path, which is prolonged into late-LTP when the BLA is stimulated 15 minutes later. However, aged rodents show no enhancement of perforant path – dentate gyrus LTP after BLA stimulation (Almaguer, Estupiñán, Uwe Frey, & Bergado, 2002). A combination of deficient synaptic plasticity and alterations in the noradrenergic system may therefore impair amygdala-hippocampal interactions with aging. Additionally, studies have shown that there is no upregulation of phosphorylated CREB in the BLA and hippocampus in low and moderate intensity shock, although aged rodents do show behavioral enhancements in the moderate shock condition (Morris & Gold, 2012). Findings such as this suggest that increasing the level of arousal may allow for compensation in aged rodents. It is not clear whether these deficits are due to hippocampal alterations or an age-related decrease in activity in modulatory regions such as the amygdala. Future studies utilizing high-resolution neuroimaging methods in combination with the current task may allow us to gain insight into subtle behavioral and neurobiological changes occurring in the medial temporal lobe in aging.
There are some limitations of the current study. It is difficult to rule out the possibility that our older adult group includes individuals with preclinical Alzheimer's disease (AD), which may present with different behavioral effects on emotional memory. While we attempted to minimize this possibility by excluding any individuals who presented with deficits in neuropsychological test performance, we cannot be certain that some of the behavioral effects observed are not driven at least in part by incipient AD pathology.
Furthermore, while we screened all of our young adults against major cognitive disorders, we did not perform detailed neuropsychological testing, thus investigating correlations between task performance and neuropsychological test performance was not feasible. Additionally, sample sizes were too small to thoroughly investigate gender differences, which have been demonstrated in some prior studies of emotional memory (Cahill, 2006). Finally, we used naturalistic stimuli and not computer-generated, controlled morphs, thus specific features (e.g. orientation, color, etc.) were quite variable. It is possible that future studies with more controlled stimuli can be used to examine mnemonic asymmetry for emotional items in more detail by directly manipulating individual aspects of the images. In addition, as mentioned in the Methods section, young and older adults used different scales for rating the valence of the images seen during the study phase. Young adults rated images on a 1-9 point scale while older adults used a similar scale, but without the large range of responses (limited to 3 button responses versus 9). We chose to limit the response options for older adults to make the decision process easier and remove this difficulty, however, this could potentially underlie differences seen in task performance between young and older adults. This possibility can be examined in future studies by changing the young adults' rating scale to the same 3 button response as older adults to match across groups.
In conclusion, our data suggest that there may be age-related changes to how emotional memories are processed, such that emotional details of the experience may be remembered with higher fidelity in older adults. These results highlight an interesting behavioral phenomenon with age and a novel neuropsychological paradigm that can be used in conjunction with high-resolution neuroimaging to test the neural mechanisms of pattern separation of emotional information and how they change with age and disease.
Supplementary Material
Acknowledgments
We thank Marilyn Albert for comments on earlier drafts of this manuscript. We also thank Liz Murray and Maria Ly for help with participant recruitment and testing. This research is supported by NIA grants P50 AG05146 and R01 AG034613 as well as a NIA T32 training grant to S.L.L. (AG027668: PI M. Albert).
References
- Adolphs R, Denburg NL, Tranel D. The amygdala's role in long-term declarative memory for gist and detail. Behavioral Neuroscience. 2001;115:983–992. doi: 10.1037//0735-7044.115.5.983. [DOI] [PubMed] [Google Scholar]
- Almaguer W, Estupiñán B, Uwe Frey J, Bergado JA. Aging impairs amygdala-hippocampus interactions involved in hippocampal LTP. Neurobiology of aging. 2002;23(2):319–324. doi: 10.1016/s0197-4580(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory consolidation. Am J Geriatr Psychiatry. 2005;13:344–352. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, et al. Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Simon E. Age differences in memory: The roles of attention and depth of processing. New directions in memory and aging. 1980:95–112. [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion Washington Dc. 2003;3(3):239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dere E, Pause BM, Pietrowsky R. Emotion and episodic memory in neuropsychiatric disorders. Behavioural brain research. 2010;215(2):162–71. doi: 10.1016/j.bbr.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Dolcos S, Denkova E, Morey R, Wang L, McCarthy G, Dolcos F. Brain imaging investigation of the impairing effect of emotion on cognition. J Vis Exp. 2012:pii–2434. doi: 10.3791/2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, DeFazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Frontiers in Aging Neuroscience. 2012 doi: 10.3389/fnagi.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Current opinion in neurobiology. 2011;21(6):929–34. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky E. Changes in Cognitive Function in Human Aging. In: Riddle D, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton: CRC Press; 2007. pp. 1–10. [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT, M DW., Jr Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer's Disease Teresa Go. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Arch Neurol. 1980;37:625–629. doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and biobehavioral reviews. 2013;37(1):36–58. doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Jagust W. Review Vulnerable Neural Systems and the Borderland of Brain Aging and Neurodegeneration. Neuron. 2013;77(2):219–234. doi: 10.1016/j.neuron.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, et al. Kiefer C. Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia. NeuroImage. 2006;30(3):713–20. doi: 10.1016/j.neuroimage.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Krendl AC, Corkin S. Memories of an emotional and a nonemotional event: effects of aging and delay interval. Experimental aging research. 2006;32(1):23–45. doi: 10.1080/01902140500325031. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Remembering the Details : Effects of Emotion. Emotion Reviews. 2009;1(2):99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Emotion. Vol. 2. Washington Dc: 2002. Effects of normal aging and Alzheimer's disease on emotional memory; pp. 118–134. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. The journals of gerontology Series B Psychological sciences and social sciences. 2007;62:208–15. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kim J, Yassa MA. Assessing recollection and familiarity of similar lures in a behavioral pattern separation task. Hippocampus. 2013;23(4):287–94. doi: 10.1002/hipo.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubanis P, Zornetzer SF. Age-related Behavioral and Neurobiological Changes: A Review with an Emphasis on Memory. 1981 doi: 10.1016/s0163-1047(81)91195-x. [DOI] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Yassa MA. Assymetric effect of emotion on mnemonic interference. Neurobiology of Learning and Memory. doi: 10.1016/j.nlm.2014.02.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux J. The amygdala. Current Biology. 2007;17(20):868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Loftus EF, Loftus GR, Messo J. Some facts about “weapon focus”? Law and Human Behavior. 1987;11:55–62. [Google Scholar]
- Ly M, Murray E, Yassa MA. Perceptual Versus Conceptual Interference and Pattern Separation of Verbal Stimuli in Young and Older Adults. Hippocampus. 2013;23:425–430. doi: 10.1002/hipo.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on psychological science a journal of the Association for Psychological Science. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological review. 1995;102(3):419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual review of neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. TICS. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive Neuuroscience and the Study of Memory. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Morris KA, Gold PE. Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training. Mechanisms of ageing and development. 2012;133(5):291–9. doi: 10.1016/j.mad.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, et al. Mattay VS. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of cognitive neuroscience. 2009;21(10):1920–33. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20:1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends in Cognitive Sciences. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Payne JD, Kensiger EA. Sleep's role in the consolidation of emotional episodic memories. Curr Dir in Psych Sci. 2010;19:290–295. [Google Scholar]
- Rogalski EJ, Murphy CM, deToledo-Morrell L, Shah RC, Moseley ME, Bammer R, Stebbins GT. Changes in parahippocampal white matter integrity in amnestic mild cognitive impairment: a diffusion tensor imaging study. Behavioural neurology. 2009;21(1):51–61. doi: 10.3233/BEN-2009-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek Ma, Burghardt NS, et al. Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA. Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiology of Learning and Memory. 2012;97:465–469. doi: 10.1016/j.nlm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: a network analysis of functional magnetic resonance imaging data. Psychological Science. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia. 2013:1–8. doi: 10.1016/j.neuropsychologia.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CEL. Learning & memory. 6. Vol. 17. Cold Spring Harbor; N.Y.: 2010. Individual differences in spatial pattern separation performance associated with healthy aging in humans; pp. 284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg DB, Martinez JL, Gold PE, McGaugh JL. Age-related memory deficits in rats and mice: enhancement with peripheral injections of epinephrine. Behavioral and neural biology. 1985;44(2):213–20. doi: 10.1016/s0163-1047(85)90212-2. [DOI] [PubMed] [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Learning & memory. 5. Vol. 16. Cold Spring Harbor; N.Y.: 2009. Visual object pattern separation deficits in nondemented older adults; pp. 338–42. [DOI] [PubMed] [Google Scholar]
- Treves a, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–91. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Vereecken THLG, Vogels OJM, Nieuwenhuys R. Neuron loss and shrinkage in the amygdala in Alzheimer's disease. Neurobiology of Aging. 1994;15:45–54. doi: 10.1016/0197-4580(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Waring JD, Kensinger EA. Effects of emotional valence and arousal upon memory trade-offs with aging. Psychology and aging. 2009;24(2):412–22. doi: 10.1037/a0015526. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010;000:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34(10):515–25. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MichaelA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8873–8. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MichaelA, Muftuler LT, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MichaelA, Muftuler LT, Stark CEL. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107(28):12687–91. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MichaelA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. NeuroImage. 2010c;51(3):1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20(11):1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


