Abstract
Both Calvin-Benson-Bassham (C3) and Hatch-Slack (C4) cycles are most important autotrophic CO2 fixation pathways on today’s Earth. C3 cycle is believed to be originated from cyanobacterial endosymbiosis. However, studies on evolution of different biochemical variants of C4 photosynthesis are limited to tracheophytes and origins of C4-cycle genes are not clear till now. Our comprehensive analyses on bioinformatics and phylogenetics of novel transcriptomic sequencing data of 21 rhodophytes and 19 Phaeophyceae marine species and public genomic data of more algae, tracheophytes, cyanobacteria, proteobacteria and archaea revealed the origin and evolution of C4 cycle-related genes. Almost all of C4-related genes were annotated in extensive algal lineages with proteobacterial or archaeal origins, except for phosphoenolpyruvate carboxykinase (PCK) and aspartate aminotransferase (AST) with both cyanobacterial and archaeal/proteobacterial origin. Notably, cyanobacteria may not possess complete C4 pathway because of the flawed annotation of pyruvate orthophosphate dikinase (PPDK) genes in public data. Most C4 cycle-related genes endured duplication and gave rise to functional differentiation and adaptation in different algal lineages. C4-related genes of NAD-ME (NAD-malic enzyme) and PCK subtypes exist in most algae and may be primitive ones, while NADP-ME (NADP-malic enzyme) subtype genes might evolve from NAD-ME subtype by gene duplication in chlorophytes and tracheophytes.
Introduction
The Calvin-Benson-Bassham (CBB) cycle is the most important autotrophic CO2 fixation pathway on today’s Earth, and widely distributes among all land plants, algae and cyanobacteria [1]. The characteristic enzyme involved in CBB cycle is ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO), which catalyzes the primary carboxylation of ribulose 1,5-bisphosphate (RuBP) and yields two molecules of 3-phosphoglycerate (PGA), a C3 compound [2]. Therefore, the CBB cycle is also called C3 cycle [2]. Numerous reviews have summarized the understandings of this important pathway [2], [3].
Another important CO2 fixation pathway is Hatch-Slack (H-S) cycle, found in sugarcane in 1965 [4]. It is also well known as C4 cycle because, in counterpart with the C3 cycle, the first enzyme involved in this cycle is phosphoenolpyruvate carboxylase (PEPC), which catalyzes the primary carboxylation of phosphoenolpyruvate (PEP) to form a 4-carbon acid compound oxaloacetate (OAA) as the first photosynthetic product. As compared with C3 plants, the C4 cycle develops novel and efficient CO2 concentration mechanisms, on anatomical and biochemical function, to enhance RubisCO performance even at limited ambient CO2 levels and result in significant decreases in photorespiration, improvement of photosynthetic efficiency and water use efficiency during CO2 fixation [5]. Thus, C4 plants are capable of growing in habitats that may be too harsh for C3 species, such as rock outcrops and hypersaline or arid soils of low latitude, and contribute about a quarter of the primary productivity on the planet [6].
For land plants, C4 plants can be divided into three distinct biochemical variants based on different decarboxylation modes: NADP-malic enzyme (NADP-ME) type, NAD-malic enzyme (NAD-ME) type, and phosphoenolpyruvate carboxykinase (PCK) type [7]. Each C4 subtype consists of two shared enzymes (PEPC and PPDK) and two or three other unique enzymes of their own, which are all encoded by nuclear genome and transported to different position of cell to catalyze corresponding reaction.
Through the phylogenetic studies in species of Flaveria (Asteraceae) and Brassica gravinae, C4 plants are believed to have evolved gradually from C3 plants through several intermediate stages of C3–C4 plants [8]. However, C4-cycle genes and pathway analysis are mostly limited to land plants till recently. It is widely accepted that land plants evolved from streptophyte algae (a diverse group of green, fresh water algae) [9]. Algae are the principal primary producers in oceanic and freshwater communities, and also are responsible for the net flux of about 2 gigatons of carbon per year from the atmosphere to the lithosphere [10]. Furthermore, some C4-cycle genes and intermediates were found in a few algal species [11]–[13], although the presence of the whole pathway was not confirmed in algae. Therefore the phylogenetic analysis of C4 pathway-related (C4-related) genes using recently released algal genomic and transcriptomic data has important scientific values for understanding the origin and evolution of photosynthesis.
With the development of the new generation sequencing (NGS) technology, more algal genome and transcriptome sequencing data were released (Table S1, including their references), including several species of marine phytoplankton such as diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum, green algae Ostreococcus tauri and Micromonas, brown alga Ectocarpus siliculosus, and red algae Pyropia haitanensis and Pyropia yezoensis. In addition, as a part of the recent 1000 Plant (OneKP) Project (http://www.onekp.com), we provided 19 marine phaeophytes and 21 marine rhodophytes for transcriptome sequencing. These new released algal sequencing data provide us more valuable gene information and more extensive algal lineages to screen the key genes in algae. Therefore, in this study, by analyzing these sequencing data, we confirmed the existence of C4-related genes in extensive algal lineages. We further resolved to perform a comprehensive phylogenetic analysis using a much larger dataset (especially including much more diverse algal lineages, land plants, cyanobacteria, γ-proteobacteria and archaea) to elucidate the evolution of C4-related genes and possible pathway types. Our analyses, for the first time, support a non-cyanobacterial origin for almost all of the C4-related genes, with the exception of phosphoenolpyruvate carboxykinase gene (PCK) and aspartate aminotransferase (AST), which have both cyanobacterial endosymbiont and non-cyanobacterial origin. This result is quite different from the previous study on C3 photosynthesis origin from a cyanobacterial primary endosymbiont.
Results
Sequencing yield and annotation of unigenes
The transciptomic sequencing of 21 red and 19 brown algal species yielded a total of 503,310,608 raw reads, equal to the length of 89.2 Gb with an average sequence length of 180 bases. The reads were assembled into 2,161,986 scaffolds, with an average length of 717 bp and an N50 of 1751 bp. All sequences were aligned against the local nr protein database downloaded from NCBI using the BLASTx algorithm for gene annotation. When the E-value cutoff was set at 10−5, a total of 585,247 unigenes had significant BLAST matches.
KEGG pathway analysis of CO2 fixation genes in diverse algal lineages
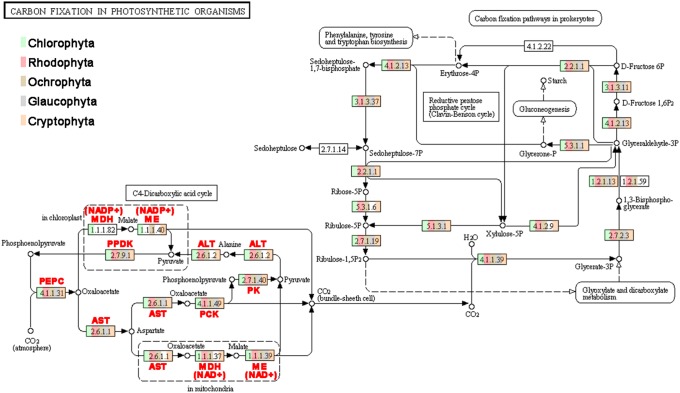
These transcriptomic data were further performed on KEGG pathway analysis. We also included genomic data from 19 sequenced algae (Table S1) for a thorough identification of genes encoding enzymes related to the carbon fixation pathways in detail and all enzymes in C3 and C4 pathways among diverse algal groups (Chlorophyta, Rhodophyta, Ochrophyta, Glaucophyta and Cryptophyta) (Fig. 1). The numbers of gene in C3 and C4 pathways in algal species and some representative tracheophytes are listed in Table 1. Our results provide an unequivocal molecular evidence that most of the C3-pathway and C4-pathway genes are actively transcribed in these algal groups and demonstrate the possibility of the extensive existence of different photosynthetic pathways in algae.
Figure 1. Genes identified for coding enzymes in CO2 fixation pathways in algal species generated by KEGG.
Different colors in the small boxes represent the genes identified from Rhodophyta, Ochrophyta, Chlorophyta, Glaucophyta and Cryptophta, respectively. The numbers within the small boxes are the enzyme commission (EC) number. PEPC: phosphoenolpyruvate carboxylase; PPDK: pyruvate, orthophosphate dikinase; ALT: alanine transaminase; MDH: malate dehydrogenase; ME: malic enzyme; AST: aspartate aminotransferase; PK: pyruvate kinase; PCK: phosphoenolpyruvate carboxykinase.
Table 1. The numbers of gene in C3 and C4 pathways in algae and tracheophytes species.
| Taxonomy | Organism | ALT | AST | MDH | ME | PCK | PEPC | PK | PPDK |
| Chlorophyta | Bathycoccus prasinos | 1 | |||||||
| Chlorophyta | Chlamydomonas reinhardtii | 2 | 5 | 4 | 2 | 1 | 2 | 2 | 2 |
| Chlorophyta | Chlorella variabilis | 2 | 2 | 2 | |||||
| Chlorophyta | Coccomyxa subellipsoidea | 2 | |||||||
| Chlorophyta | Micromonas pusilla | 1 | |||||||
| Chlorophyta | Micromonas sp. | 1 | |||||||
| Chlorophyta | Ostreococcus lucimarinus | 1 | 2 | 2 | 3 | 1 | |||
| Chlorophyta | Ostreococcus tauri | 2 | 2 | 1 | 1 | 1 | |||
| Chlorophyta | Volvox carteri f nagariensis | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 |
| Cryptophyta | Guillardia theta | 2 | 7 | 3 | 6 | 1 | 2 | 2 | 1 |
| Glaucophyta | Cyanophora paradoxa | 4 | 4 | 1 | 1 | 1 | |||
| Ochrophyta | Aureococcus anophagefferens | 1 | 2 | ||||||
| Ochrophyta | Nannochloropsis gaditana | 5 | 1 | 2 | 2 | 1 | 5 | ||
| Ochrophyta | Nannochloropsis oceanica | ||||||||
| Ochrophyta | Colpomenia sinuosa | 1 | 2 | 2 | 1 | 1 | 1 | 3 | |
| Ochrophyta | Desmarestia viridis | 1 | 3 | 1 | 2 | 1 | 3 | 1 | |
| Ochrophyta | Dictyopteris undulata | 1 | 2 | 2 | 2 | 3 | 1 | ||
| Ochrophyta | Ectocarpus siliculosus | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 1 |
| Ochrophyta | Ishige okamurae | 1 | 1 | 1 | 3 | 1 | |||
| Ochrophyta | Petalonia fascia | 1 | 3 | 1 | 2 | 1 | 2 | ||
| Ochrophyta | Phaeodactylum tricornutum | 1 | 4 | 1 | 2 | 1 | 2 | 2 | |
| Ochrophyta | Punctaria latifolia | 1 | 3 | 2 | 3 | 1 | 3 | ||
| Ochrophyta | Saccharina japonica | 1 | 2 | 2 | 1 | 1 | 2 | ||
| Ochrophyta | Saccharina sculpera | 1 | 3 | 2 | 2 | 1 | |||
| Ochrophyta | Sargassum fusiforme | 1 | 2 | 1 | 1 | ||||
| Ochrophyta | Sargassum hemiphyllum var. chinense | 1 | 3 | 2 | 1 | 3 | 1 | ||
| Ochrophyta | Sargassum henslowianum | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Ochrophyta | Sargassum horneri | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 |
| Ochrophyta | Sargassum integerrimum | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 1 |
| Ochrophyta | Sargassum muticum | 1 | 2 | 2 | 2 | 1 | |||
| Ochrophyta | Sargassum thunbergii | 1 | 4 | 2 | 1 | 1 | 1 | 2 | 1 |
| Ochrophyta | Sargassum vachellianum | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 1 |
| Ochrophyta | Scytosiphon dotyi | 1 | 2 | 2 | 2 | 1 | 3 | ||
| Ochrophyta | Scytosiphon lomentaria | 1 | 3 | 2 | 1 | 1 | 1 | 3 | 1 |
| Ochrophyta | Thalassiosira oceanica | 3 | 1 | 1 | 1 | ||||
| Ochrophyta | Thalassiosira pseudonana | 3 | 2 | 1 | 1 | 1 | 1 | ||
| Ochrophyta | Undaria pinnatifida | 1 | 4 | 2 | 1 | 1 | 2 | ||
| Rhodophyta | Ahnfeltiopsis flabelliformis | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Betaphycus philippinensis | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Ceramium kondoi | 1 | 2 | 1 | 2 | ||||
| Rhodophyta | Chondrus crispus | 2 | 1 | 2 | 1 | 2 | |||
| Rhodophyta | Cyanidioschyzon merolae | 1 | 2 | 2 | 1 | 1 | 3 | ||
| Rhodophyta | Dumontia simplex | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Eucheuma denticulatum | 1 | 2 | 1 | 2 | 1 | 2 | 1 | |
| Rhodophyta | Galdieria sulphuraria | 1 | 3 | 3 | 1 | 2 | 1 | 4 | |
| Rhodophyta | Gloiopeltis furcata | 1 | 1 | 2 | 2 | ||||
| Rhodophyta | Gracilaria blodgettii | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Gracilaria chouae | 1 | 1 | 1 | 1 | 2 | 1 | ||
| Rhodophyta | Gracilaria vermiculophylla | 1 | 2 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Gracilariopsis lemaneiformis | 1 | 1 | 2 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Grateloupia catenata | 1 | 1 | 1 | 1 | 1 | 2 | 1 | |
| Rhodophyta | Grateloupia chiangii | 1 | 1 | 1 | 1 | 2 | 1 | ||
| Rhodophyta | Grateloupia filicina | 1 | 1 | ||||||
| Rhodophyta | Grateloupia livida | 1 | 1 | 1 | 1 | 1 | |||
| Rhodophyta | Grateloupia turuturu | 1 | 2 | 1 | 1 | 2 | 1 | ||
| Rhodophyta | Heterosiphonia pulchra | 1 | 1 | 1 | 1 | 1 | |||
| Rhodophyta | Kappaphycus alvarezii | 1 | 1 | 1 | |||||
| Rhodophyta | Mazzaella japonica | 1 | 3 | 2 | 1 | 2 | |||
| Rhodophyta | Neosiphonia japonica | 1 | 1 | 1 | 2 | 1 | 1 | 1 | |
| Rhodophyta | Pyropia yezoensis | 3 | 1 | 1 | 1 | 2 | |||
| Rhodophyta | Symphyocladia latiuscula | 1 | 1 | 2 | 1 | 1 | 2 | 1 | |
| Tracheophytes | Arabidopsis thaliana | 2 | 6 | 11 | 6 | 4 | 4 | 13 | 1 |
| Tracheophytes | Oryza sativa | 2 | 6 | 10 | 7 | 4 | 6 | 10 | 4 |
| Tracheophytes | Sorghum bicolor | 1 | 3 | 7 | 11 | 3 | 4 | 11 | 3 |
| Tracheophytes | Zea mays | 9 | 5 | 2 | 1 | 1 | 3 | 11 | 1 |
| Bryophytes | Physcomitrella patens | 8 | 3 | 11 | 4 | 4 | 7 | 15 | 5 |
Phylogenetic analysis of C4-related genes
In addition of diverse algal species and tracheophytes, wealth of candidate C4 gene sequences (Table S2) were also detected among archaea, proteobacteria and cyanobacteria. Therefore, we built phylogenetic trees that display relationships of full amino acid sequences of C4 related genes from archaea, proteobacteria, cyanobacteria, tracheophytes, and algae based on Bayesian method (only representative candidates are included to save space). The results show that the eukaryotic C4 enzymes have an archaeal/proteobacterial core (Fig. 2, 3, and 4). The phylogenetic trees of PEPC (phosphoenolpyruvate carboxylase), PPDK (pyruvate, orthophosphate dikinase), ALT (alanine transaminase), MDH (malate dehydrogenase), ME (malic enzyme), and PK (pyruvate kinase) (see Figs. 2, 3, and 4) support their non-cyanobacterial origin in primary endosymbiotic algae. However, almost all eukaryotic PCKs have a cyanobacterial origin through endosymbiosis gene transfer (EGT). Some genes of ochrophytes and cryptophytes potentially have a red algal origin (e.g., ME) as expected under the secondary endosymbiosis hypothesis, and others have archaeal/proteobacterial origins inherited from their endosymbiosis host genomes or acquired from non-cyanobacterial archaea or proteobacteria via horizontal gene transfer (HGT) (e.g., PEPC).
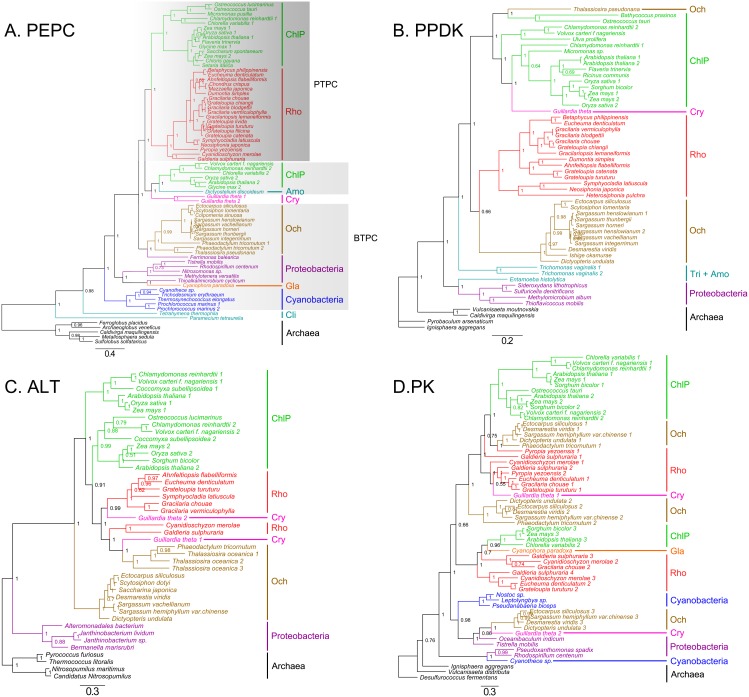
Figure 2. Bayesian phylogenetic trees of PEPC (A) PPDK (B), ALT (C) and PK (D) enzymes with bootstrap values (when >50%) indicated at the nodes.
The PEPCs are classified as PTPCs (in dark gray boxes) and BTPCs (in light gray boxes). ChlP, chlorophytes and plants. Gla, glaucophytes. Rho, rhodophytes. Och, ochrophytes. Cry, cryptophytes. Tri, trichomonad. Amo, amoeba. Cil, ciliates.
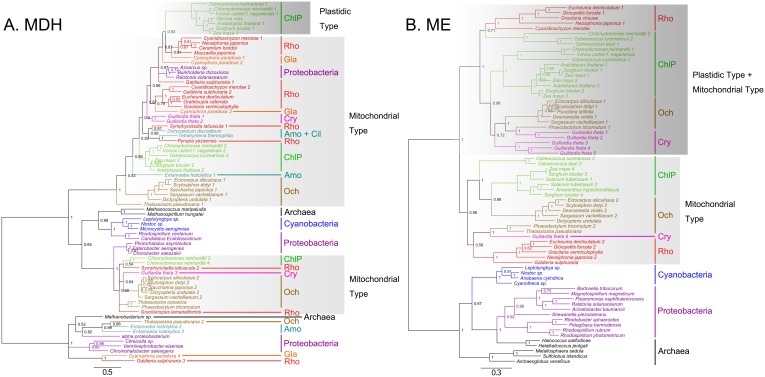
Figure 3. Bayesian phylogenetic trees of MDH (A) and ME (B) enzymes with bootstrap values (when >50%) indicated at the nodes.
The plastic type MDHs are in dark gray boxes, and mitochondrial types in light gray boxes. Eukaryotic MEs cluster in two clades, one clade in dark gray boxes consists of both plastic and mitochondrial type, the other one only contains mitochondrial type. ChlP, chlorophytes and plants. Gla, glaucophytes. Rho, rhodophytes. Och, ochrophytes. Cry, cryptophytes. Tri, trichomonad. Amo, amoeba. Cil, ciliates.
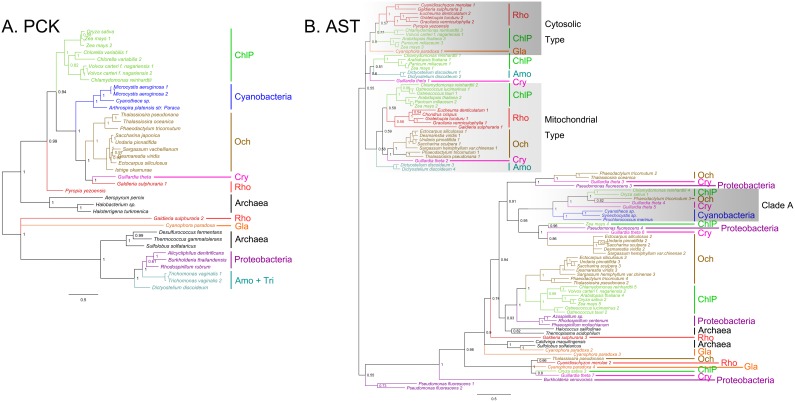
Figure 4. Bayesian phylogenetic trees of PCK (A) and AST (B) enzymes with bootstrap values (when >50%) indicated at the nodes.
The cytosolic types of AST are in dark gray boxes and mitochondrial types are in light gray boxes. Besides this two clades, some chlorophyte, plant and cryptophyte ASTs are grouped with cyanobacteria (Clade A), which are also in light gray boxes. ChlP, chlorophytes and plants. Gla, glaucophytes. Rho, rhodophytes. Och, ochrophytes. Cry, cryptophytes. Tri, trichomonad. Amo, amoeba. Cil, ciliates.
PEPC originated from archaea/proteobacteria and evolved into diverse types in different eukaryotic organisms
PEPC (EC 4.1.1.31) is an important enzyme for CO2 fixation in the C4-cycle and shared by three subtypes of C4-pathway metabolisms. The enzyme is present in almost all plants, green algae, cyanobacteria, most archaea, and non-photosynthetic bacteria, but is absent from animals and fungi [14]. In this study, we, for the first time, find its homologous sequences widely exist in various algae species, including chlorophytes, ochrophytes, rhodophytes, glaucophytes and cryptophytes (Fig. 1).
There are two homologous of PEPC genes in chlorophytes and tracheophytes, termed as bacterial-type (BTPC) and plant-type (PTPC) according to their amino acid sequences and structures [15]. Especially, the deduced PEPC polypeptides are readily classified as a BTPC or PTPC based on their C-terminal tetrapeptide, which is either (R/K) NTG for BTPCs or QNTG for PTPCs [16]. We compared the full deduced sequence of PEPCs among all above five algal groups (chlorophytes, ochrophytes, rhodophytes, glaucophytes and cryptophytes), cyanobacteria, proteobacteria and archaea. The results show that rhodophytes only possess PTPCs, whereas ochrophytes, glaucophytes, proteobacteria, and cyanobacteria only contain BTPCs. PEPCs of archaea, cryptophytes and ciliates do not have the typical C-terminal tetrapeptide and can not be classified into any known types.
Phylogenic analysis suggests that PEPC broadly distributes among algae and plants and is likely to have a non-cyanobacteria origin. Within the strict consensus tree, archaeal PEPCs is the first group to diverge at the base of the tree, followed by ciliates clade, BTPCs clusters, cryptophytes clade, and a large group including PTPCs of chlorophytes and rhodophytes which form a single well-resolved clade in two clusters with strong support (Bayesian posterior probability, PP = 0.98) (Fig. 2A). This topology suggests that BTPCs maybe more primitive than PTPCs and the latter one presumably have arisen from the BTPC homologs. Furthermore, PTPC can be classified into C3-type, C3-like-type, C3–C4 intermediate type, and C4-type according to the sequence context (the amino acid residue at 774 or those around it at the C-end of PEPCs) [17]. All C4-type PTPCs investigated to date harbor a serine (S) residue at the corresponding position but this very site is replaced by an alanine (A) residue in all non-photosynthetic PEPCs. Site-specific mutagenesis studies demonstrated that this amino acid residue plays a key role in enzyme kinetics [18]. In addition, we found that the differentiation between non-C4-type and C4-type is not limited in PTPCs but also happens in BTPCs (Fig. 5). According to the criteria mentioned above, BTPCs of chlorophytes and tracheophytes are identified as non-C4-type, whereas in ochrophytes, diatoms possess non-C4-type site and Phaeophyceae algae prefer to C4-type. Interestingly, fractions of cyanobacteria and archaea also have the C4-type site (Fig. 5).
Figure 5. Alignments of partial amino acid sequences of PEPC isoenzymes between species of Rhodophyta, Ochrophyta, chlorophytes, Cryptophta, bacteria and archaea.

The deduced PEPC sequences were aligned using ClustalX 1.83 software. Numbering indicates the position of the first and last residue in each aligned sequence. Semi-colons and asterisks indicate identical and conserved amino acids respectively. The C-terminal tetrapeptide (R/K)NTG for BTPCs is highlighted in blue color, whereas QNTG for PTPCs is highlighted in orange. The amino acid residue of No. 774 or around it is highlighted in green (non-photosynthetic PEPCs) or red (C4-type PEPCs).
PPDK, ALT, and PK have a non-cyanobacteria archaea/proteobacteria origin
The enzyme PPDK (EC 2.7.9.1), another shared enzyme in the C4 cycle (Fig. 1), catalyzes the reversible phosphorylation of pyruvate and inorganic phosphate yielding P-enolpyruvate and inorganic pyrophosphate at the expense of a single molecule of ATP [19]. Using present gene data in NCBI, PPDK homologs have not been detected in the available cyanobacterial genomes. Actually we find that all published cyanobacterial PPDK are not real PPDK, for they lack the PPDK N-terminal nucleotide-binding domain. Instead, they should be classified as other PEP-utilizing genes, such as phosphoenolpyruvate synthetase (PEPS; pyruvate, water dikinase) (Fig. 6).
Figure 6. Partial sequence alignment of the N-terminal domains of PEPS and PPDK enzymes.
Numbering indicates the position of the first and last residue in each aligned sequence. Proteins with biochemically confirmed enzyme activity are indicated by gray shading. Grey shading indicate the identified sequence signatures specific for PEPS and PPDK respectively.
In the consensus tree of PPDK (Fig. 2B), archaeal sequences also cluster at the base like the PEPC tree, neighbored by clades of proteobacteria and protists, indicating its origin from archaea/proteobacteria. A separate clade is formed by rhodophytes with moderate bootstrap support, while Phaeophyceae algae (Ochrophyta) cluster independently and appear as the sister taxon of rhodophytes. Tracheophytes, chlorophytes, diatoms, and cryptophytes form another large clade. Therefore, primary endosymbiotic algal PPDK gene seems originated from archaea or proteobacteria, while Phaeophyceae algae possibly inherit the gene from red algal endosymbionts.
Similar to PPDK, neither dose ALT have any cyanobacterial homolog, indicating the eukaryotic ALT is also non-cyanobacterium-derived gene (Fig. 2C). Cryptophytes and diatoms formed a well supported clade with rhodophytes, which indicates their revolution relationship with red algae endosymbionts.
The analysis of PK genes is much more complicated due to its enormous duplications in eukaryotes. Our phylogenetic analysis also excluded the cyanobacterial origin of PK in algae and tracheophytes (Fig. 2D). Rhodophytes, glaucophytes, chlorophytes, and tracheophytes obtained this gene from archaea/proteobacteria, and gene duplication occurred extensively at least twice in these species. Cryptophytes and Phaeophyceae algae have two types of PK, one is inherited from red algae through EGT (eg. Guillardia theta 1, EKX52520.1), another is transferred from some proteobacteria or archaea (eg. Guillardia theta 2, EKX43540.1). The latter type is not found in diatoms, and this absence suggests different evolutionary pattern, such as gene lost, between Phaeophyceae algae and diatoms.
Note that Sogin and Zillig et al. theorized that the eukaryotic nucleus arose from the cellular fusion between both a bacterium and an archaeon [20]. Lang et al. indicated several hundred eukaryotes genes are acquired from the mitochondrial ancestor derived from an endosymbiotic alpha-proteobacterium that was engulfed by a eukaryotic- or archaebacteria-like cell more than one billion years ago [21]. Therefore, we suggest that the archaea/proteobacteria-derived C4-related genes in eukaryotic organisms may inherit from the endosymbiotic host genome, which had acquired numerous genes from bacterium and archaeon or transferred from archaea/proteobacteria through HGT.
MDH and ME originated from archaea/proteobacteria and evolved into different homologs after duplication
The phylogenic analysis of MDH and ME has encountered difficulties from sequence similarity between NADP- and NAD-dependent homologs. Therefore, we built phylogenies separately for MDH and ME, albeit they probably shared a common ancestry (Fig. 3). According to the phylogenetic tree, it is clear that eukaryotic MDHs are considered essentially archaeal/proteobacterial in provenance (Fig. 3A). Targeting signal predictions for plastid and mitochondrion genes in algae and plants suggest that the gene encoding plastid-targeted MDH in tracheophytes and chlorophytes likely evolved from eukaryotic algal mitochondrial homologs via gene duplication and acquisition of a plastid-import signal. Ochrophyte MDH has two separate origins: one from red algae endosymbionts (eg. Saccharina japonica 2, KM113483), the other from secondary endosymbiotic hosts or archaea/proteobacteria through HGT (eg. Saccharina japonica 1, KM113482). Cryptophytes only acquired MDH from red algal endosymbionts (Fig. 3A).
The strict ME consensus tree displays a relatively simple topology compared to the MDH tree. It shows that the ME genes are also obtained very early in algal evolution but start to duplicate before the emergence of primary endosymbiotic algae, might be in its last eukaryotic common ancestor. After duplication, some Archaeplastida (including red algae, green algae and plants) obtained two homologs of ME from archaea/proteobacteria, and these genes are likely to be transmitted into ochrophytes and cryptophytes by EGT from their red algal endosymbionts (Fig. 3B). Targeting signal predictions suggest two clades with different organellar locations. One clade is composed of plastid target genes and the other consists of both plastid and mitochondrion target genes.
PCK and AST originated from both cyanobacteria and archaea/proteobacteria
The remaining C4 genes, PCK and AST, have a complex evolutionary history. Our phylogenetic analysis shows they may have arisen from multiple origins (Fig. 4A). For instance, two copies of PCKs from Galdieria sulphuraria cluster into separate clades: one is related to archaea and proteobacteria (eg. Galdieria sulphuraria 2, EME28834.1), the other groups with cyanobacteria (eg. Galdieria sulphuraria 1, EME27660.1). Therefore, rhodophytes may acquire PCK from two different ancestors. In contrast, glaucophytes possess PCKs of archaea/proteobacteria origin. Ochrophytes and cryptophytes may obtain PCKs from red algal endosymbionts.
The evolution of eukaryotic AST genes appears much more complicated. In our phylogenetic tree, the enzymes clustered into two separate clades. One clade consists of cytosolic and mitochondrial ASTs. In the constricted tree (Fig. 4B), mitochondrial AST is present in tracheophytes, chlorophyte, rhodophyte, ochrophyte, and cryptophyte algae, and the cytoplasmic AST is present in archaeplastida (Rhodophyta, Virideplantae and Glaucophyta) and forms a monophyletic group without any secondary endosymbiotic algae. These species all group with homologs of proteobacteria, suggesting their proteobacteria origin. However interestingly, in our phylogenetic tree, in addition to the mitochondrial and cytoplasmic types, ASTs of some chlorophyte and cryptophyte algae are grouped into another clade (clade A) with cyanobacteria, indicating their cyanobacteria endosymbiont origin.
Discussion
C4 genes distributed among diverse algae mostly with archaeal/proteobacterial origins
Our new data allow the identification of almost all the enzymes necessary for C4 photosynthesis widely distributing among different eukaryotic algal lineages (Fig. 1). Furthermore, we find that most of eukaryotic C4 genes have an archaeal/proteobacterial core but algal PCKs and ASTs originated from both cyanobacteria and archaea/proteobacteria, based on our phylogenetic analyses by using diverse genes from archaea, proteobacteria, cyanobacteria, tracheophytes and various algae groups (Fig. 2, 3, 4).
Notably, we found that cyanobacteria do not possess complete C4 pathway. Though PEPCs are proved existed in cyanobacteria (Fig. 2A), the cyanobacterial PPDK sequences published in GenBank are excluded from our analysis (Fig. 2B), because we for the first time found that the published cyanobacterial PPDK sequences should actually be annotated as PEPS or other PEP-utilizing genes because of the lacking of the PPDK N-terminal nucleotide-binding domain [22]. Neither are ALT and PK found their homologs in cyanobacteria (Fig. 2C, D), suggesting that the C4 cycle dose not arisen from a cyanobacterial endosymbiosis. Our further phylogenic analysis strongly supports this hypothesis that almost all of the C4-related genes have a non-cyanobacteria origin (Fig. 2B).
We also searched the cyanobacterium-like plastid (also termed as cyanelle) genome of Glaucophyta Cyanophora paradoxa, which is considered as a “living fossil” and a paradigm for the invasion of a eukaryotic cell by a cyanobacterium [23], but did not find any complete C4 pathway, except for partial sequences of PPDk and ALT homologs (data not shown). As nucleomorphs are considered as the enslaved red or green algal nucleus residuals, we further searched the nucleomorph genomes of Cryptophyta Guillardia theta (AF165818.4, AJ010592.2, AF083031.2), Hemiselmis andersenii (CP000881.1, CP000882.1, CP000883.1), Cryptomonas paramecium (CP002172.1, CP002173.1, CP002174.1) and Cercozoa Bigelowiella natans (DQ158856.1, DQ158857.1, DQ158858.1), and did not find any C4 genes, neither. The results are consistent with our hypothesis that the C4 cycle is not evolved from cyanobacterial-endosymbiotic event.
C4-related genes of diverse subtypes in different algal lineages
In land plants, C4 photosynthesis are typically divided into three subtypes-NADP-ME, NAD-ME, and PCK-based on different decarboxylation models. Different C4 species often use one of the three subtypes nearly exclusively [24]. Some experimental results suggest that the PCK subtype is maximal in biomass production and CO2 fixation [25], [26], and others show that the NAD-ME and PCK subtypes are more adaptive than the NADP-ME subtype for species in arid environments [24].
By surveying a selection of cyanobacterial and algal species to determine the gene composition (Table S3), we found all chlorophytes own all three varieties of C4-related genes, just like their derived lineage, tracheophytes. However, virtually all species of rhodophytes and ochrophytes, including diatoms which have been proved to be able to incorporate CO2 into the C-4 carboxyl of C4 acids [11], own NAD-ME and PCK subtypes genes, but lack the MDH (NADP) of NADP-ME subtype. This result suggests that diatoms, red algae and brown algae quite possibly have similar C4-related genes, and NADP-ME type C4 genes may emerge in chlorophytes after they separated from other algal lineages.
In tracheophytes, the cytosolic and mitochondrial AST activities are connected by C4 photosynthesis and they both participate in the NAD-ME subtype of C4 pathway, while cytosolic AST itself can function as the PCK type [27]. In our analysis, Archaeplastida all evolved to have both cytosolic AST and mitochondrial AST except that glaucophytes only have cytosolic AST (Fig. 4B). In addition, no known C4 cytosolic AST homolog has been annotated in ochrophytes and cryptophytes, but ASTs of these secondary endosymbiotic algae are grouped into another clade with proteobacteria/archaea (Fig. 4B), indicating different evolution of C4-related genes between primary and secondary endosymbiotic algae.
Gene duplication of C4-related enzymes
Our phylogenic analyses also suggest that most C4-related genes undergo gene duplications subsequently at different evolutionary time scale (Fig. 2, 3, 4). Furthermore, there are diverse gene duplications among different eukaryotic lineages. For instance, there are two types of PEPC termed as PTPC and BTPC in chlorophytes and tracheophytes, which demonstrate that the PEPC gene duplication had already occurred in the ancestral chlorophytes, with BTPCs being the ancestral type.
Our study showed that the primary endosymbiotic algae evolved to have both cytosolic AST and mitochondrial AST except glaucophytes may only have cytosolic AST (Fig. 4B), suggesting AST duplicated and functional differentiated in a common algal ancestor before the primary endosymbiosis, and the absence of mitochondrial AST in glaucophytes may be due to gene lost. Another group of AST found in the secondary endosymbiotic algae, including diatoms, Phaeophyceae and cryptophytes (Fig. 4B), may also be the results of gene lost and functional differentiation after gene duplication which happened in their common ancestor before the secondary endosymbiosis.
Gene duplication and evolution in MDH and ME are even more fascinating. NADP-dependent and NAD-dependent MDHs of the two separate C4 subtypes seem to share the same ancestor, and duplication took place in the last common ancestor of chlorophytes and tracheophytes (Fig. 3A). The same event happened during evolution of ME, yet duplication maybe occurred earlier in the last eukaryotic common ancestor (Fig. 3B). The ancestral mitochondrial enzymes endured duplication and gave rise to homologs which evolved plastid target signal peptide. These plastid MDH and ME genes finally took part in the construction of NADP-ME type C4 pathway in tracheophytes.
Since gene duplication is a necessary contribution to genetic novelty and adaptation and requirement [28], the multiple copies of C4-related genes should have arisen accompanying the emergence of different C4 cycle which made the regulation more delicate during the evolution of C4 photosynthesis.
Origin and evolution of C4 pathway
Our analysis suggests that the C4-related genes (except for PCK and AST) in eukaryotic organisms originated from archaea/proteobacteria. It is widely accepted that the eukaryotic nucleus may have arisen from the cellular fusion between either a bacterium or “protoeukaryote” and an archaeon [20]. Therefore, a substantial number of archaea/proteobacteria-derived genes are retained in the eukaryotic nuclear genome. Another mechanism for algae and plants acquiring archaea/proteobacteria genes is horizontal gene transfer (HGT) from archaea or proteobacteria that occurred throughout the history of eukaryotes [29]. Thus, the archaea/proteobacteria-derived C4-related genes may inherit from the endosymbiotic host genome or transferred from archaea/proteobacteria through HGT.
Previous study showed that photosynthetic eukaryotes (i.e., algae and plants) gained C3 photosynthesis from a cyanobacterial primary endosymbiont [30]. The eukaryotic C3 pathway consists of eleven enzymes, and molecular phylogenetic analyses show that recruitment of single enzymes from different pathways could be the driving force for C3 pathway evolution in chlorophytes, tracheophytes and rhodophytes [31], [32]. Therefore, we would like to propose a hypothesis on C4 cycle formation.
First, when a cyanobacterium was engulfed and retained by a heterotrophic eukaryote, its PCK and AST genes transferred into the host nucleus and were inherited by the primary endosymbiotic algae to lay out the main framework of PCK subtype of C4 pathway with some archaea/proteobacteria-derived C4 genes founding in plants.
In addition to the PCK subtype, NAD-ME subtype was also constructed as the result of a patchwork assembly in tracheophytes, but it is difficult to predict which one is more primitive. However, the present work proved that NADP-ME subtype should be a derivant of NAD-ME subtype according to our phylogenic analysis of MDH and ME genes. The plastid MDHs in chlorophytes and tracheophytes are originated from its mitochondrial homologs via duplication (Fig. 3A), and a clade contains both mitochondrial and plastid ME homologs are originated from mitochondrial ones (Fig. 3B).
The changing CO2 concentration may also be a major environmental driving force for eukaryotes to develop C4 metabolism, to suppress photorespiration. Throughout Earth’s geological history, eukaryotes had been exposed to much higher CO2 at the beginning of evolutional history but then became starved by a steep decrease of CO2 and increase of O2 as the outcome of appearance of C3 photosynthesis. Some plants can dramatically change the photosynthetic and anatomical traits to meet different environment pressure. For example, Eleocharis vivipara, an amphibious sedge, changes its photosynthetic pathway from C3 to C4 under conditions of CO2 deficiency [33]. So did in aquatic environments, in order to reduce the impact of increasing oxygen pressures, aquatic photosynthetic organisms evolved special mechanisms to efficiently maintain carbon fixation. It has been demonstrated that many aquatic photosynthetic organisms can take up both CO2 and HCO3− from the surrounding media and this capacity is greatly strengthened under CO2-limiting conditions, which is generally known as the inorganic carbon-concentrating mechanism (CCM) [34].
For algae, it is believed that the C3 cycle is predominant in the CO2 fixation pathway [35], as a result of the endosymbiotic acquisition of a cyanobacterium that evolved into the chloroplasts [31]. However, the existence of a C4 photosynthetic pathway in algae or marine phytoplankton is a long-standing debate [36]. Recent papers have reported the evidence for the operation of C4 photosynthesis as an alternative CCM in the marine diatom Thalassiosira weissflogii, using 14C-labeling experiments [11], [12]. In addition, some intermediate products of the C4 pathway had been detected in brown alga Ascophyllum nodosum, euglenoid Euglena gracilis, and dinoflagellates [13], and C4-like photosynthetic characteristics had also been detected in green alga Udotea flabellum [37]. Until recently, few studies are focused on C4-cycle genes in limited algae species, and imperfect detection method in algae may bring about ambiguous results. Our current work provides molecular evidences for further biochemical and physiological experiment validation on more algal species to prove the existence of C4-related genes, or even C4 cycle in some algal species or extensive algal lineages.
Materials and Methods
Ethics Statement
Marine brown and red algal samples were collected along the coast of China during October, 2010 to March, 2012, and were provided by Culture Collection of Seaweed at the Ocean University of China. The location is neither privately owned nor protected places. No specific permissions were required for these locations, and the study did not involve any endangered or protected species. The species information and the GPS coordinates of their specific locations are provided in Table S4.
RNA extraction
Total RNA was extracted from algal tissues using an improved CTAB method for brown algal samples and an improved Trizol method for red algal samples, and RNA quantity and quality was examined as previous reports [38], [39].
Transcriptome sequencing and de novo assembly
cDNA library construction and sequencing were performed by the BGI (Shenzhen, China) on Illumina (San Diego, USA) HiSeq instruments in accordance with the manufacturer’s instructions. Strict reads filtering was performed before de novo assembly using SOAP denovo-Trans (http://soap.genomics.org.cn/SOAPdenovo-Trans.html). Pair-end reads with primer or adaptor sequences were removed. Reads with more than 10% of the bases below Q20 quality or more than 5% of unknown nucleotides (Ns) were filtered from total reads. Gapcloser was further used for gap filling of the scaffolds.
C4-cycle gene identification and KEGG pathway analysis
The assembled sequences were BLASTx against the nr protein database downloaded from NCBI with E-value<10−5. Deduced algal C4 protein coding sequences were further examined for their homology by using BLAST X and BLAST P available at NCBI. These sequences were uploaded to GenBank database, and their accession numbers were listed in Table S2. All other publicly available C4 enzymes sequences were obtained from GenBank databases (Table S2). The accession numbers of gene in C3 and C4 pathways in tracheophytes species were listed in Table S5. To reconstruct the metabolic pathways, high-quality assembled algal sequences and 19 sequenced algal genome sequences downloaded from GenBank database (Table S1) were assigned to KEGG Automatic Annotation Server (www.genome.jp/tools/kaas/) to provide functional annotation of genes.
Sequence alignments and phylogenetic analyses
Sequences were aligned with ClustalX 1.83 software before Bayesian analysis using MrBayes 3.1.2 software. Analyses were performed as two independent runs, each with four incrementally heated Metropolis-coupled Monte-Carlo Markov Chains running for 5,000,000 generations. Trees were sampled every 100 generations. A total of 50,001 trees in one files were read and the first 25% of trees were discarded as the burn-in. The average standard deviation of split frequencies at the end of the run was below 0.01, indicating stationary conditions.
Cellular target prediction
The probability of plastid or mitochondrion targeting was assessed according to the on-line prediction service at CBS (http://www.cbs.dtu.dk/services/) with the ChloroP V1.1 and TargetP V1.1 servers using the default values.
Supporting Information
Update information of publicly available algal genome sequences.
(XLSX)
Taxonomical list of taxa included in this study. The sequences used to reconstruct phylogenetic trees are written in bold letters, the other Rhodophyta and Ochrophyta full-length sequnces in regular letters are also obtained in present study. Sequence data of Cyanophora paradoxa are acquired from Cyanophora Genome Database.
(XLSX)
Genes identified for coding enzymes of each biochemical variant of C4 photosynthesis in cyanobacteria and algae species.
(XLSX)
Species information of 18 brown algae and 21 red algae for transcriptome sequencing.
(XLSX)
The accession numbers of gene in C3 and C4 pathways in tracheophytes species.
(XLSX)
Acknowledgments
The authors thank Cui Liu, Yalan Zhang and Guoliang Wang for valuable technical advices.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are available from the GenBank database (accession numbers are listed in Supporting Information files).
Funding Statement
This work was supported by the National Natural Science Foundation of China (NSFC No. 41206116 and No. 31271397, China Postdoctoral Science Foundation (No. 2011M501167), National Public Benefit Research Foundation of China (No. 201105021). The algal transcriptome sequencing was supported by 1KP Project (www.onekp.com). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Raven JA (2009) Conributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat Microb Ecol 56: 177–192. [Google Scholar]
- 2. Tabita FR (2004) Research on carbon dioxide fixation in photosynthetic microorganisms (1971–present). Photosynth Res 80: 315–332. [DOI] [PubMed] [Google Scholar]
- 3. Falkowski PG, Fenchel T, DeLong EF (2008) Themicrobial engines that drive Earth’s biogeochemical cycles. Science 320: 1034–1039. [DOI] [PubMed] [Google Scholar]
- 4. Hatch MD, Slack CR (1966) Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J 101(1): 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuoka M, Furbank RT, Fukayama H, Miyao M (2001) Molecular engineering of C4 photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 52: 297–314. [DOI] [PubMed] [Google Scholar]
- 6.Brown RH (1999) Agronomic implications of C4 photosynthesis. In: SageRF, MonsonRK, eds. C4 Plant biology. USA: Academic Press 473–507.
- 7. Hatch MD, Kagawa T, Craig S (1975) Subdivision of C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2(2): 111–128. [Google Scholar]
- 8. Ueno O (2011) Structural and biochemical characterization of the C3–C4 intermediate Brassica gravinae and relatives, with particular reference to cellular distribution of Rubisco. J Exp Bot 62(15): 5347–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiu YL, Palmer JD (1999) Phylogeny of early land plants: insights from genes and genomes. Trends Plant Sci 4: 26–30. [DOI] [PubMed] [Google Scholar]
- 10. Dorrell RG, Smith AG (2011) Do red and green make brown?: perspectives on plastid acquisitions within chromalveolates. Eukaryot Cell 10(7): 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reinfelder JR, Kraepiel AML, Morel FMM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407: 996–999. [DOI] [PubMed] [Google Scholar]
- 12. Reinfelder JR, Milligan AJ, Morel FMM (2004) The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol 135: 2106–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan XL, Fang YJ, Hu SN, Wang GC (2007) Generation and analysis of 5318 expressed sequence tags from the filamentous sporophyte of Porphyra haitanensis (Rhodophyta). J Phycol 43: 1287–1294. [Google Scholar]
- 14. Patel HM, Kraszewski JL, Mukhopadhyay B (2004) The phosphoenolpyruvate carboxylase from Methanothermobacter thermautotrophicus has a novel structure. J Bacteriol 186: 5129–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Leary B, Park J, Plaxton WC (2011) The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem J 436(1): 15–34. [DOI] [PubMed] [Google Scholar]
- 16. Gennidakis S, Rao SK, Greenham K, Uhrig RG, O’Leary B, et al. (2007) Bacterial- and plant-type phosphoenolpyruvate carboxylase polypeptides interact in the hetero-oligomeric class-2 PEPC complex of developing castor oil seeds. Plant J 52: 839–849. [DOI] [PubMed] [Google Scholar]
- 17. Westhoff P, Gowik U (2004) Evolution of C4 phosphoenolpyruvate carboxylase. Genes and proteins: a case study with the genus Flaveria . Ann Bot 93(1): 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engelmann S, Bläsing OE, Westhoff P, Svensson P (2002) Serine 774 and amino acids 296 to 437 comprise the major C4 determinants of the C4 phosphoenolpyruvate carboxylase of Flaveria trinervia . FEBS Lett 524(1–3): 11–14. [DOI] [PubMed] [Google Scholar]
- 19. Reeves RE, Menzies RA, Hsu DS (1968) The pyruvate-phosphate dikinase reaction. The fate of phosphate and the equilibrium. J Biol Chem 243(20): 5486–5491. [PubMed] [Google Scholar]
- 20. Brown JR, Doolittle WF (1997) Archaea and the prokaryote-to-eukaryote transition. Microbiol Mol Biol Rev 61(4): 456–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang BF, Gray MW, Burger G (1999) Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet 33(1999): 351–397. [DOI] [PubMed] [Google Scholar]
- 22. Tjaden B, Plagens A, Dörr C, Siebers B, Hensel R (2006) Phosphoenolpyruvate synthetase and pyruvate, phosphate dikinase of Thermoproteus tenax: key pieces in the puzzle of archaeal carbohydrate metabolism. Mol Microbiol 60(2): 287–298. [DOI] [PubMed] [Google Scholar]
- 23. Stirewalt VL, Michalowski CB, Löffelhardt W, Bohnert HJ, Bryant DA (1995) Nucleotide sequence of the cyanelle genome from Cyanophora paradoxa . Plant Molecular Biology Reporter 13: 327–332. [Google Scholar]
- 24. Taub DR (2000) Climate and the U.S. distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. Am J Bot 87(8): 1211–1215. [PubMed] [Google Scholar]
- 25. Fravolini A, Williams DG, Thompson TL (2002) Carbon isotope discrimination and bundle sheath leakiness in three C(4) subtypes grown under variable nitrogen, water and atmospheric CO(2) supply. J Exp Bot 53(378): 2261–2269. [DOI] [PubMed] [Google Scholar]
- 26. Wang C, Guo L, Li Y, Wang Z (2012) Systematic comparison of c3 and c4 plants based on metabolic network analysis. BMC Syst Biol 2: S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taniguchi M, Kobe A, Kato M, Sugiyama T (1995) Aspartate aminotransferase isozymes in Panicum miliaceum L., an NAD-malic enzyme-type C4 plant: comparison of enzymatic properties primary structures, and expression patterns. Arch Biochem Biophys 318(2): 295–306. [DOI] [PubMed] [Google Scholar]
- 28. Hittinger CT, Carroll SB (2007) Gene duplication and the adaptive evolution of a classic genetic switch. Nature 449(7163): 677–681. [DOI] [PubMed] [Google Scholar]
- 29.Qiu H, Yoon HS, Bhattacharya D (2013) Algal endosymbionts as vectors of horizontal gene transfer in photosynthetic eukaryotes. Front Plant Sci 19(4): 366. eCollection 2013. [DOI] [PMC free article] [PubMed]
- 30. Bhattacharya D, Yoon HS, Hackett JD (2004) Photosynthetic eukaryotes unite: endosymbiosis connects the dots. BioEssays 26: 50–60. [DOI] [PubMed] [Google Scholar]
- 31. Martin W, Schnarrenberger C (1997) The evolution of the Calvin cycle from prokaryotic to eukaryotic chromosomes: a case study of functional redundancy in ancient pathways through endosymbiosis. Curr Genet 32(1): 1–18. [DOI] [PubMed] [Google Scholar]
- 32. Reyes-Prieto A, Bhattacharya D (2007) Phylogeny of Calvin cycle enzymes supports Plantae monophyly. Mol Phylogenet Evol 45(1): 384–391. [DOI] [PubMed] [Google Scholar]
- 33. Ueno O (1996) Structural characterization of photosynthetic cells in an amphibious sedge, Eleocharis vivipara, in relation to C3 and C4 metabolism. Planta 199: 382–393. [Google Scholar]
- 34. Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C (2011) Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana . Photosynth Res 109: 205–221. [DOI] [PubMed] [Google Scholar]
- 35. Tsuji Y, Suzuki I, Shiraiwa Y (2009) Photosynthetic carbon assimilation in the coccolithophorid Emiliania huxleyi (Haptophyta): evidence for the predominant operation of the C3 cycle and the contribution of b-carboxylases to the active anaplerotic reaction. Plant Cell Physiol 50: 318–329. [DOI] [PubMed] [Google Scholar]
- 36.Falkowski PG, Raven JA (1997) Aquatic Photosynthesis. Blackwell Science, Malden, MA.
- 37. Reiskind JB, Bowes G (1991) The role of phosphoenolpyruvate carboxykinase in a marine macroalga with C4-like photosynthetic characteristics. Proc Natl Acad Sci USA 88: 2883–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li TY, Ren L, Zhou G, Liu C, Chi S, et al. (2012) A suitable method for extracting total RNA from red algae (in Chinese). Transactions of Oceanology and Limnology 4: 64–71. [Google Scholar]
- 39. Johnson MT, Carpenter EJ, Tian Z, Bruskiewich R, Burris JN, et al. (2012) Evaluating methods for isolating total RNA and predicting the success of sequencing phylogenetically diverse plant transcriptomes. PLoS One 7: e50226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Update information of publicly available algal genome sequences.
(XLSX)
Taxonomical list of taxa included in this study. The sequences used to reconstruct phylogenetic trees are written in bold letters, the other Rhodophyta and Ochrophyta full-length sequnces in regular letters are also obtained in present study. Sequence data of Cyanophora paradoxa are acquired from Cyanophora Genome Database.
(XLSX)
Genes identified for coding enzymes of each biochemical variant of C4 photosynthesis in cyanobacteria and algae species.
(XLSX)
Species information of 18 brown algae and 21 red algae for transcriptome sequencing.
(XLSX)
The accession numbers of gene in C3 and C4 pathways in tracheophytes species.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are available from the GenBank database (accession numbers are listed in Supporting Information files).