Abstract
Blacks experience morbidity and mortality earlier in the life course compared to whites. Such premature declines in health may be indicative of an acceleration of the aging process. The current study uses data on 7,644 black and white participants, ages 30 and above, from the third National Health and Nutrition Examination Survey, to compare the biological ages of blacks and whites as indicated from a combination of ten biomarkers and to determine if such differences in biological age relative to chronological age account for racial disparities in mortality. At a specified chronological age, blacks are approximately 3 years older biologically than whites. Differences in biological age between blacks and whites appear to increase up until ages 60-65 and then decline, presumably due to mortality selection. Finally, differences in biological age were found to completely account for higher levels of all-cause, cardiovascular and cancer mortality among blacks. Overall, these results suggest that being black is associated with significantly higher biological age at a given chronological age and that this is a pathway to early death both overall and from the major age-related diseases.
Keywords: Aging, Racial Disparities, Biomarkers, Mortality Selection, Life Expectancy
Introduction
Race is linked to striking health disparities in the United States. Overall, blacks experience death and disease much earlier in the life course than do whites, which may suggest that on average blacks are aging faster (Hayward, Crimmins, Miles, et al., 2000). Because the progression of physiological deterioration that accompanies aging may be strongly related to environmental factors (Finch & Tanzi, 1997), it is conceivable that the various social, economic, mental, and physical factors encountered by many racial minorities throughout their lives may be capable of causing an acceleration of the aging process.
A number of factors have been shown to contribute to racial differences in morbidity and mortality: socioeconomic status (SES) (Hayward, Crimmins, Miles, et al., 2000; Franks, Muennig, Lubetkin, et al., 2006), neighborhood (Williams & Collins, 2001; Acevedo-Garcia, Ospuk, McArdle, et al., 2008), availability of quality healthcare (Mayberry, Mili, & Ofili, 2000; 2008 National Healthcare Disparities Report, 2009), behaviors (Jackson, Knight, & Raffery, 2010), and psychological stress (McEwen, 1998). Over time, these factors have the ability to get “under the skin” and alter physiological functioning (Taylor, Repetti, & Seeman, 1997; Kuzawa & Sweet, 2009). Blacks also experience more discrimination, have less economic security and often live in worse neighborhoods, offering fewer nutritional options, worse air quality, and less access to recreational activities (Krieger, Rowley, Herman, et al, 1993; Ellen, Mijanovich, & Dillman, 2001; Bell & Ebisu, 2012). These experiences may lead to higher levels of both physical and psychological stress with the potential to cause a myriad of biological changes with implications for aging. Finally, the higher prevalence of dangerous health behaviors, such as obesity, among blacks relative to whites (Flegal, Carroll, Kit, et al., 2012), are also believed to contribute to progressive breakdowns in biological tissues and systems, leading to widening gaps in physiological function Growing disparity in physiological functioning due to the continual exposure to adverse conditions is the premise of the “Weathering Hypothesis”, which suggests that the negative effects of exposure to hazardous physical, social, and economic environments of socially disadvantaged racial groups accumulate over the lifespan and contribute to premature health deterioration, which may be indicative of an acceleration of the biological aging process (Geronimus, Hicken, Keene, et al., 2006).
The pace of age-related deterioration, potentially resulting from the accumulation of tissue and cellular damage to molecules like DNA and proteins, may be strongly influenced by the amount of wear and tear the body undergoes over time. (Selye & Tuchweber, 1976). As a result, individuals exposed to hazardous environments may presumably age quicker, causing them to appear biologically older at a given chronological age. In fact, previous research examining race differences in cumulative biological risk have shown that on average, blacks have the same number of “high-risk” (indicated using clinically established cutoffs) physiological indicators as whites who are significantly older chronologically (Geronimus, Hicken, Keene, et al., 2006; Crimmins, et al. 2007).
The earlier onset of aging-related deteriorations in physiological functioning is believed to also give rise to premature incidence of mortality. It has been reported that life expectancy for blacks is about 5 years less than whites. (Arias 2007) and contributes to approximately 100,000 excess deaths per year (Levine, Foster, Fullilove, et al., 2001). Additionally, dramatic racial disadvantages have been found across multiple domains of health. Even in middle-age, blacks have been shown to have significantly higher prevalence of both fatal and non-fatal chronic conditions than whites (Hayward, Crimmins, Miles, et al., 2000) and being black is often associated with earlier onset of many age-related chronic diseases (Bibbins-Domingo, Pletcher, Lin, et al., 2009). This shift in the age curve—in which the onset of death and disease occurs earlier in life—is thought to explain why racial disparities in mortality risks cross-over in late life, resulting from mortality selection at earlier ages (Johnson, 2000; Manton, Poss, & Wing, 1979). Taken together, this may suggest that a majority of those in the black population may be aging faster than the white population in the U.S.
Biological age measures were developed to quantify multi-system age-related changes on a physiological level and may be useful as proxies for the pace or extent of aging of an individual. While the concept of combining multiple measures into a single variable to model the rate of aging was proposed over fifty years ago, recent techniques have been found to be promising predictors of aging-related health outcomes (Cho, Park, & Lim, 2010; Levine, 2013). These measures utilize information from multiple biomarkers to determine where an individual lies on an aging trajectory. Typically, the trajectory is determined using a data driven approach that calculates age-associated differences in the various markers within a large representative sample (Bae, Kang, Kim, et al., 2008; Krøll & Saxtrup, 2000; Nakamura & Miyao, 2007). As a result, biological age reflects the chronological age which on average is characterized by the specified biological profile. For example, someone with a biological age of 50 has the physiological functioning of the average 50 year old within the population. An individual's chronological age can be subtracted from biological age to determine whether the pace of aging for an individual or group is accelerated (i.e. they are older biologically than they are chronologically). For this reason, although it is not an actual marker of mortality risk, the concept of biological age may be useful for examining health disparities, as it allows us to directly estimate the degree of aging, or the difference between biological and chronological age, of disadvantaged groups, as well as compare biological age for race groups at varying chronological ages.
Using data from the National Health and Nutrition Examination Survey (NHANES III), this study examines 1) the racial difference in the pace of aging across ages and by ten-year age groups, to determine if blacks are aging biologically faster than whites and whether disparities in the pace of aging decline or cross-over in later life; and 2) whether these differences in the pace of aging, account for racial disparities in age-specific risks of all-cause mortality, cardiovascular disease (CVD) mortality, and cancer mortality. Overall, we hypothesize that blacks will have higher levels of accelerated aging compared to whites. However, these differences should decrease with age since the most disadvantaged are selected out of the population earlier. Finally, we hypothesize that racial differences in pace of aging will account for the higher mortality risk among blacks.
Materials and Methods
Study Population
We use data from NHANES III, a nationally representative, cross-sectional study conducted by the National Center for Health Statistics (NCHS) between 1988 and 1994. Data were collected from at-home interviews and examinations taking place at a Mobile Examination Center (MEC). Further details of recruitment, procedures, population characteristics and study design are available through the Centers for Disease Control and Prevention (U.S. Department of Health and Human Services, 2001). Our analytic sample (N=7,587) was restricted to black and white subjects ages 30-89. Hispanics were excluded because, although they have slightly higher life expectancy than whites, nativity is believed to be may be a major factor in this observation, and there is evidence to believed that these differences may be explained by the “salmon hypothesis” which suggests that many Hispanics may return to their country of origin once they become ill and thus their mortality is not observed (Crimmins et al.,). Those over age 89 were excluded given that NHANES III top-codes age at 90. Complete biomarker data was available for approximately 70% of the age-eligible sample. However, excluded subjects were more likely to be black, have lower education, were older, and were more likely to die between baseline and follow-up.
Biological Age Measure
Our estimation was calculated using information for ten biomarkers—C-Reactive Protein (CRP), Serum Creatinine, Glycosylated Hemoglobin (HbAlc), Systolic Blood Pressure, Serum Albumin, Total Cholesterol, Cytomegalovirus Optical Density (CMV), Serum Alkaline Phosphatase, Forced Expiratory Volume at 1 second (FEV1), and Serum Urea Nitrogen. These markers were selected because they had been suggested as potential biomarkers of aging, used in prior estimations of Biological Age using the NHANES III sample (Levine, 2013), or had been found to significantly correlate with chronological age at r> 0.10. Together, these biomarkers provide an indication of metabolic, cardiovascular, inflammatory, kidney, liver, and lung functioning.
Biological age was calculated in accordance with the method proposed by Klemera and Doubal (2006). This method has been shown to predict death more accurately than other well-known Biological Age algorithms, such as Multiple Linear Regression and Principle Component Analysis, and was found to be a better indicator of mortality risk than chronological age (Levine, 2013). The estimated Biological Age calculation combines information from m=10 regression lines of the m=10 biomarker indicators regressed on chronological age (Eq. 1).
| (1) |
In Equation 1, kj and qj are the slope and intercept, respectively, for each biomarker regressed on chronological age, Xj is the participant's measured value for a given biomarker, sj is the root mean squared error of a biomarker regressed on chronological age, and CA represents chronological age. Additionally, is the variance of the random variable, RBA, which represents the difference between participants' biological and chronological age.
Sociodemographic Characteristics
Chronological age, race, sex, education, and smoking were based on self-reports. Subjects were categorized into two race groups—Non-Hispanic White and Non-Hispanic Black. Education was used as an indicator of SES. Reported school years completed were used to create four education groups: <12 years (less than a high school education), 12 years (high school degree), 13-15 years (some college), and 16+ years (college degree). Next, three smoking groups were created based on subjects' answers to two questions: non-smokers (reported not having smoked at least 100 cigarettes during their life time), former-smokers (not currently smoking but reported having smoked at least 100 cigarettes during their life time), and current smokers. Finally, BMI was calculated as measured weight (in kg) divided by measured height (in meters) squared, and used to classify participants as underweight (BMI<18.5), normal weight (BMI 18.5-24.9), overweight (BMI 25-29.9), and obese (BMI 30+).
Mortality
Mortality follow-up and person-months of follow-up were available for all participants from linked records from the National Death Index through 2006 (U.S. Department of Health and Human Services, 2001). Information was provided for 113 potential underlying causes of death (UCOD-113), and used to code for all-cause mortality, cardiovascular disease (CVD) mortality, and cancer mortality. For all mortality analyses, violent, accidental or HIV related deaths were censored given that our study is concerned with age-related mortality that can be linked to chronic diseases.
Statistical Analysis
Using OLS regression, we compared the biological ages of blacks and whites adjusting for chronological age and sex, and then again after adjusting for chronological age, sex, and additional covariates such as education, BMI and smoking. Results from these models were then used to estimate adjusted mean biological age for the two groups. Biological ages of blacks and whites were then compared in ten-year age groups to determine whether differences converged later in life. Finally we examined whether Biological Age could explain racial disparities in mortality using Cox Proportional Hazard Models. First, models were run only controlling for chronological age and sex to determine the association between race and mortality (baseline models). Next we reran models, adjusting for biological age to determine whether it mediated the association between race and mortality and then reran these models with the inclusion of covariates such as education, BMI and smoking. We also examined whether covariates such as alcohol consumption, physical activity, and prevalence of CVD, cancer, COPD, and diabetes influenced our results. Overall, we found that they had no significant influence on our results (not shown), and as a result we did not include them in our final models given that they contributed to more participants being excluded due to missingness. Cause-specific mortality was examined using a competing-risks framework. All analyses were run in STATA and used sample weights and appropriate survey procedures for dealing for complex sampling design.
Results
Sample Description
As shown in Table 1, both chronological and biological age had means of 50.2 years; however, as expected the standard deviation was slightly larger for biological age (15.5) than chronological age (14.9). The sample is mostly made up of whites, with only about 11% blacks. Approximately 10% of the sample never attended high school, one-quarter attended but did not graduate from high school, one-third completed high school, one-fifth had some college education, and one-quarter completed at least four years of college. Just over half of the subjects are female (53%). Overall, the majority of subjects had a normal BMI (38.6%), while 35.1% were overweight, 24.5% were obese, and approximately 2% were underweight. Over half the sample has a history of smoking, with 31% reporting they were former smokers, and 28% reporting that they were current smokers. Finally, over the 18-year follow-up, 20% of subjects died overall, 9% died from CVD, and 5.5% died from cancer. The analysis is based on a total of 97,557 person-years of exposure for 7,587 subjects.
Table 1. Sample Characteristics for the Full Sample and by Race.
| Characteristic | Full Sample (N=7,587) | Whites (N=4,851) | Blacks (N=2736) |
|---|---|---|---|
| Biological Age, μ (s.d.) | 50.2 (15.5) | 50.2 (15.5) | 50.0 (15.0) |
| Chronological Age, μ (s.d.) | 50.2 (14.9) | 50.6 (15.0) | 47.0 (13.6) |
| Female (%) | 52.9 | 52.5 | 56.3 |
| 0-8 years schooling (%) | 9.5 | 8.8 | 15.0 |
| Some High School (%) | 12.5 | 11.7 | 19.2 |
| High School Degree (%) | 34.8 | 34.7 | 35.6 |
| Some College (%) | 20.0 | 20.1 | 18.6 |
| College Degree (%) | 23.2 | 24.6 | 11.6 |
| Underweight (%) | 1.8 | 1.74 | 1.8 |
| Normal BMI (%) | 38.6 | 39.5 | 31.4 |
| Overweight (%) | 35.1 | 35.2 | 34.5 |
| Obese (%) | 24.5 | 23.6 | 32.3 |
| Former Smoker (%) | 30.8 | 32.2 | 19.8 |
| Current Smoker (%) | 27.6 | 26.6 | 36.1 |
| All-Cause Mortality (%) | 19.8 | 19.9 | 19.5 |
| CVD Mortality | 8.7 | 8.8 | 7.9 |
| Cancer Mortality | 5.5 | 5.4 | 5.9 |
| Person-Years (Total) | 104,641 | 93,495 | 11,146 |
Biological Age by Race
Adjusted means for Biological Age by race are shown in Table 2. When controlling for chronological age and sex, blacks were found to have biological ages that were significantly higher than whites (P<.001). On average, the biological age for blacks was 53.16 years, which was more than a 3 year increase over whites, who had an average biological age of 49.84 years. Next, in addition to controlling for chronological age and sex, means were also adjusted for SES (as measured by years of education), BMI, and smoking, to determine if these accounted for the difference in biological age between blacks and whites. Results showed that even when controlling for SES and health behaviors, blacks were still found to have higher biological ages than whites (P<.001). Nevertheless, the difference was slightly reduced (2.83 years), with blacks having a mean of 52.72 years compared to 49.89 years for whites. Overall, this suggests that on average blacks have the physiological functioning of whites who are more than three years older chronologically. Furthermore, even when accounting for the effects of SES, obesity, and smoking, some of the differences were attenuated; however, with chronological age, SES and health behaviors controlled blacks are still biologically older than whites.
Table 2. Mean Biological Age by Race (N=7,587).
| Mean Biological Age (S.E.) | ||
|---|---|---|
|
| ||
| Adjusted for Chronological Age and Sex | Adjusted for Chronological Age, Sex, SES, BMI, and Smoking | |
|
| ||
| Non-Hispanic White | 49.84 | 49.89 |
| Non-Hispanic Black | 53.16 | 52.72 |
|
| ||
| Difference | 3.32 (P<.001) | 2.83 (P<.001) |
To test whether differences in biological age by race varied across the age range, we compared adjusted means for Biological Age by race within ten year age groups (controlling for chronological age and sex). As shown in Figure 1, differences in biological age between blacks and whites increased up until ages 60-69—when blacks were found to be 4.82 years older biologically than whites (P<.001). However, after ages 60-69 the difference in biological age between black and whites steadily declined until there was only a 1.17 year difference (P=.054) for those 80-89 years old.
Figure 1. Racial Differences in Adjusted Mean Biological Age by 10-year Chronological Age Groups.
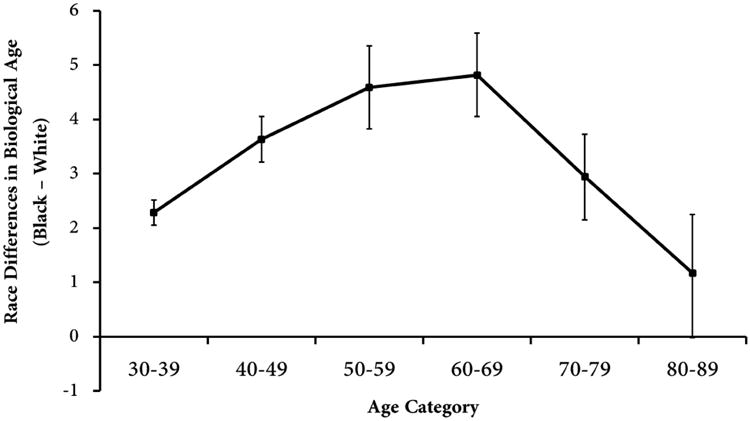
The difference in biological age between blacks and whites increased with chronological age prior to the age of 70. Blacks in their thirties, forties, fifties, and sixties had biological ages that were 2.28, 3.63, 4.59, and 4.82 years, respectively, higher than whites. However, for those in their seventies and eighties racial differences in biological age decreased to 2.94 and 1.17, respectively, and were no longer significant for persons ages 80-89. Models were adjusted for age, sex, education, BMI, and smoking. Bars represent stand errors of adjusted means.
The Mortality Associated with Accelerated or Decelerated Biological Aging
Cox proportional hazard models, controlling for chronological age and sex, were run to determine the overall mortality and disease-specific mortality risks associated with being black. As shown in Table 3, subjects who are black were 46% more likely to die overall (HR: 1.46, 95%CI: 1.30-1.65), 40% more likely to die from CVD (HR: 1.40, 95%CI: 1.18-1.67), and 51% more likely to die from cancer (HR: 1.51, 95%CI: 1.24-1.85) when compared to subjects who are white. Additionally, a one year increase in chronological age was associated with an 11% increase in all-cause mortality (HR: 1.11, 95%CI: 1.10-1.11), a 12% increase in CVD mortality (HR: 1.12, 95%CI: 1.11-1.13), and an 8% increase in cancer mortality (HR: 1.08, 95%CI: 1.07-1.09). This suggests that the increased risk of mortality associated with being black is equivalent to the risks associated with a 3-6 year increase in chronological age.
Table 3. Biological Age Mediates Racial Disparities in All-Cause, CVD and Cancer Mortality (N=7,587).
| Hazard Ratio (95% Confidence Interval) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| All-Cause Mortality | CVD Mortality | Cancer Mortality | |||||||
|
| |||||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Black | 1.46 (1.30-1.65) | 0.97 (0.85-1.11) | 0.97 (0.85-1.11) | 1.40 (1.18-1.67) | 0.91 (0.75-1.11) | 0.90 (0.74-1.11) | 1.51 (1.24-1.85) | 1.20 (0.97-1.50) | 1.24 (0.99-1.55) |
| Chronological Age (years) | 1.11 (1.10-1.11) | 1.00 (0.99-1.01) | 1.02 (1.01-1.03) | 1.12 (1.11-1.13) | 1.01 (0.99-1.03) | 1.02 (1.01-1.04) | 1.08 (1.07-1.09) | 1.02 (0.99-1.04) | 1.04 (1.02-1.07) |
| Sex (Female=1) | 0.67 (0.60-0.75) | 0.54 (0.48-0.60) | 0.58 (0.51-0.66) | 0.61 (0.52-0.72) | 0.49 (0.41-0.57) | 0.49 (0.41-0/59) | 0.65 (0.52-0.80) | 0.57 (0.46-0.72) | 0.69 (0.54-0.88) |
| Biological Age (years) | 1.11 (1.09-1.12) | 1.10 (1.09-1.11) | 1.11 (1.10-1.13) | 1.11 (1.09-1.12) | 1.06 (1.04-1.08) | 1.05 (1.02-1.07) | |||
| Education (Reference=College) | |||||||||
| 0-8 Years Education | (1.11-1.66) | (0.96-1.81) | (0.79-1.78) | ||||||
| Some High School | 1.48 (1.21-1.82) | 1.46 (1.05-2.01) | 1.30 (0.87-1.94) | ||||||
| Completed High School | 1.42 (1.17-1.72) | 1.42 (1.06-1.92) | 1.39 (0.96-2.02) | ||||||
| Some College | 1.29 (1.04-1.60) | 1.38 (0.99-1.93) | 1.20 (0.80-1.80) | ||||||
| BMI (Reference=Normal) | |||||||||
| Underweight | 1.89 (1.36-2.60) | 1.30 (0.80-2.09) | 1.21 (0.49-2.97) | ||||||
| Overweight | 0.85 (0.75-0.97) | 0.87 (0.71-1.06) | 0.92 (0.71-1.18) | ||||||
| Obese | 0.93 (0.79-1.08) | 1.01 (0.81-1.27) | 1.06 (0.79-1.42) | ||||||
| Smoking (Reference=Never Smoker) | |||||||||
| Former Smoker | 1.28 (1.13-1.47) | 1.05 (0.86-1.27) | 1.99 (1.51-2.63) | ||||||
| Current Smoker | 2.04 (1.73-2.40) | 1.59 (1.26-2.02) | 3.41 (2.51-4.63) | ||||||
When models were run controlling for biological age, it completely accounted for racial disparities in all-cause, CVD, and cancer mortality. While a one year increase in biological age was associated with having an 11% greater risk for all-cause mortality (HR: 1.11, 95%CI: 1.09-1.12), being black no longer significantly increased the risk of all-cause mortality (HR: 0.97, 95%CI: 0.85-1.11). Similarly, being one year older biologically was associated with an 11% increase in the risk of CVD mortality (HR: 1.11, 95%CI: 1.10-1.13) and a 6% increase in the risk of cancer mortality (HR: 1.06, 95%CI: 1.04-1.08); however, there were no longer significant differences in the risk of either CVD or cancer mortality risks for blacks relative to whites (HRCVD: 0.91, 95%CI: 0.75-1.1; HRCancer: 1.20, 95%CI: 0.97-1.50). Furthermore, chronological age was no longer significantly associated with all-cause, CVD or cancer mortality. Even when SES, BMI and smoking were included in the hazard models, biological age was found to be a strong predictor of all-cause, CVD and cancer mortality, while race remained non-significant. Finally, we examined the interaction between race and biological age and also ran race-stratified mortality models to determine whether the association between biological age and mortality differed for blacks and whites. Interactions between race and biological age were not statistically significant for any of the mortality outcomes. The stratified models also showed very little race differences across the three mortality outcomes. For instance, a one-year increase in biological age was associated with a 10% increase in all-cause mortality for whites, and an 8% increase in all-cause mortality for blacks. For CVD, and cancer mortality, the risks for whites increased by 11% and 5%, respectively, with every one year increase in biological age, while the risks for blacks increased by 9% and 3%, respectively, with every one year increase in biological age.
Discussion
Our results suggest that being black is associated with significantly higher biological age and that this is a pathway to early death overall, and from CVD or cancer. We have long known that race is linked to earlier mortality and morbidity. Life expectancy at age 25 is about 5-6 years lower for U.S. blacks than it is for whites (Arias, 2007). Furthermore, disease incidence has also been found to occur significantly earlier for blacks (Bibbins-Domingo, Pletcher, et al., 2009). However, this study is novel in offering evidence that racial differences in the pace of aging—as signified by biological age— may be a central mechanism for the earlier overall and disease-specific mortality of black individuals.
Given that the physiological changes associated with the aging process lead to an increase in susceptibility to disease onset and death (Yin & Chen, 2005), mortality and morbidity is likely to occur significantly earlier for individuals who are aging faster. Our results showed that on average blacks tend to be more than 3 years older biologically than whites. This is consistent with findings from previous studies reporting that blacks tend to have levels of biological risk factors that are indicative of someone significantly older chronologically (Geronimus, Hicken, Keene, et al., 2006)—providing further evidence that the pace of aging may be accelerated.
Everyday stressors associated with being black may negatively impact physiological functioning and under chronic exposure, accumulate over the lifespan and contribute to growing disparities in biological risk. Furthermore, if such environmental, behavioral and mental factors contribute to an acceleration of the aging process, we would expect that persons who are aging the fastest should have the highest risk of mortality and thus be selected out of the population at younger ages due to their lower life expectancy. The presence of mortality selection should also lead to a convergence in biological risk between advantaged and disadvantaged populations (Vaupel & Yashin, 1985). This is consistent with the current study, which showed that racial disparities in biological age systematically varied across the age range. We found that with increasing age, the gap in biological age between blacks and whites widened up until participants were nearing old age, after which point it began to converge. This suggests that the most disadvantaged blacks may be accumulating poorer and poorer health as they age; however, as those who are worst off are selected out of the population by mortality, disparities between blacks and whites steadily decrease. Similar findings have been shown when examining differences in indexes of biological risk by SES (Crimmins, Kim, & Seeman, 2009).
We also showed that differences in biological age completely accounted for the increased risk of all-cause, CVD, and cancer mortality experienced by blacks, thus implying that the black participants with the highest biological ages may also the one's contributing to the increased mortality risk among the black population. This is supported by our result showing no interaction between race and biological age. Higher biological age was associated with higher mortality risks among blacks and whites, suggesting that biological age operates similarly for both races. Finally, when comparing the mortality between blacks and whites who have equivalent biological ages, no significant differences are present.
While not a direct marker of mortality risk, the concept of biological age may do a good job estimating an individual's degree of physiological decline and dysregulation. Conventionally, cumulative risk scores or allostatic load have been used to examine disparities in physiological function (Geronimus, Hicken, Keene, et al., 2006, Karlamangla, Merkin, Crimmins, et al., 2010; Merkin, Basurto-Dávila, Karlamangla, et al, 2009). However, racial differences in such measures have only been able to account for part of the association between race disparities in mortality (Duru, Harawa, Kermah, et al., 2012). Given that cumulative risk or allostatic load measures rely on counts of biomarkers for which a subject falls into a “high risk” category (Seeman, McEwen, Rowe, et al., 2001) they may lose some of the information that could be gained from continuous measures. For instance, aging-related breakdowns within various physiological systems tend to increase progressively over the lifespan, and as a result, race differences in the timing and age patterns of disease and mortality may be better explained by continuous measures that are more highly associated with the pace of biological aging.
Given that physiological declines associated with social inequalities are believed to accumulate and build over the lifecourse, biological aging may be a useful measure for studying health disparities, particularly from a cumulative disadvantage framework. The theory of cumulative disadvantage describes how disadvantage, beginning in early life, or in prior generations, may intensify over time, leading to a divergence in health among various social groups (Merton, 1968). While measures of allostatic load or cumulative risk based on clinical cut-points capture dysregulation after it has reached a critical point, they ignore the process of decline leading up to it, as well as the continuous progression of decline thereafter. From a life course perspective, measures of biological age may allow us to examine the shape of aging trajectories at different stages in life, to determine the role of early life or prenatal conditions, as well as the accumulation of disadvantage over time.
Additionally, differences in biological age could also reflect genetic differences between groups, or gene by environment interactions. For instance, populations with different ancestries may possess different frequencies of protective or risk alleles. As a result, biological age measures may serve as a useful phenotype for examining genetics of human aging. Additionally, it may also allow us to identify resilient persons—those who appear biologically younger than expected. Ultimately, this could facilitate our ability to study how genetic or environmental factors enable some individuals to cope with disadvantage.
There are limitations in the present study that should be acknowledged. First, biomarker data for NHANES respondents were only available for a single time point, preventing us from looking at trajectories of biological age. Next, due to missing biomarker data, our analytic sample only included approximately 70% of NHANES participants ages 30-89 who were Non-Hispanic black or Non-Hispanic white. Overall those excluded from our analysis were 54% more likely to be black, 7 years older, had one less year of schooling and were 2.5 times as likely to die. As a result, our estimates of race differences are likely to be somewhat conservative.
The findings presented here provide evidence that blacks may be aging at a faster pace than whites and that biological aging is an important factor in explaining racial disparities in overall, CVD, and cancer mortality. In moving forward, the use of biological age may allow us to examine how social, behavioral, environmental, economic, and political factors contribute to health disparities, and how these disparities are affected by the accumulation of disadvantage over the life course.
Research Highlights.
Blacks appear to be biologically older than whites of the same chronological ages.
Biological age differences by race increase up until ages 60-69, and then decline.
Race differences in biological age show evidence for mortality selection.
Higher biological ages among blacks fully account for their higher mortality risk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo-Garcia D, Ospuk T, McArdle N, Williams DR. Toward a policy relevant analysis of geographic and racial/ethnic health disparities. Health Affairs. 2008;27:321–333. doi: 10.1377/hlthaff.27.2.321. [DOI] [PubMed] [Google Scholar]
- Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(1):108–45. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 2008 National Healthcare Disparities Report. Agency for Healthcare Research and Quality. Rockville, MD: 2009. [Google Scholar]
- Arias E. United States life tables. Natl Vital Stat Rep 2004. 2007;56:1–39. [PubMed] [Google Scholar]
- Bae CY, Kang YG, Kim S, et al. Development of models for predicting biological age (BA) with physical, biochemical, and hormonal parameters. Arch Gerontol Geriatr. 2008;47:253–265. doi: 10.1016/j.archger.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699–704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IH, Park KS, Lim CJ. An empirical comparative study on biological age estimation algorithm with an application of Work Ability Index (WAI) Mech Ageing Dev. 2010;131:69–78. doi: 10.1016/j.mad.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Alley D, et al. Hispanic Paradox in Biological Risk Profiles. American Journal of Public Health. 2007;97(7):1305–10. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Seeman TE. Poverty and Biological Risk: The Earlier “Aging of the Poor. J Gerontol A Biol Med Sci. 2009;64A(92):286–292. doi: 10.1093/gerona/gln010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104(1-2):89–95. doi: 10.1016/s0027-9684(15)30120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen I, Mijanovich GT, Dillman K. Neighborhood effects on health: exploring the links and assessing the evidence. J Urban Aff. 2001;23:391–408. 11. [Google Scholar]
- Finch CE, Tanzi RE. Genetics of Aging. Science. 1997;278(5337):407–11. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Franks P, Muennig P, Lubetkin E, Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Soc Sci Med. 2006;62:2469–2478. doi: 10.1016/j.socscimed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. ‘Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Crimmins EM, Miles TP, Yu Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review. 2000;65:910–930. [Google Scholar]
- Jackson JS, Knight KM, Raffery JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–9. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA. The racial crossover in comorbidity, disability, and mortality. 2000;37(3):267–283. [PubMed] [Google Scholar]
- Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and ethnic disparities in cardiovascular risk in the United States, 2001-2006. Annals of Epidemiology. 2010;20:617–628. doi: 10.1016/j.annepidem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127:240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Krieger N, Rowley DL, Herman A, et al. Racism, sexism, and social class: Implications for studies of health, disease, and well-being. American Journal of Preventive Medicine. 1993;9:82–122. [PubMed] [Google Scholar]
- Krøll J, Saxtrup O. On the use of regression analysis for the estimation of human biological age. Biogerontology. 2000;1:363–368. doi: 10.1023/a:1026594602252. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Sweet E. Epigenetics and the embodiment of race: developmental origins of US racial disparities in cardiovascular health. Am J Hum Biol. 2009;21:2–15. doi: 10.1002/ajhb.20822. [DOI] [PubMed] [Google Scholar]
- Levine ME. Modeling the Rate of Senescence: Can Estimated Biological Age Predict Mortality More Accurately Than Chronological Age? J Gerontol A Biol Sci Med Sci. 2013;86(6):667–74. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RS, Foster JE, Fullilove RE, et al. Black-White Inequalities in Mortality and Life Expectancy, 1933-1999: Implications for Healthy People 2010. Public Health Reports. 2001;116(5):474–483. doi: 10.1093/phr/116.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton KG, Poss SS, Wing S. The Black/White mortality crossover: investigation from the perspective of the components of aging. Gerontologist. 1979;19(3):291–300. doi: 10.1093/geront/19.3.291. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and Damaging Effects of Stress Mediators. New England Journal of Medicine. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Basurto-Dávila R, Karlamangla AS, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults. NHANES III Ann Epidemiol. 2009;19(3):194–201. doi: 10.1016/j.annepidem.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton RK. The Matthew effect in science: The reward and communication system of science. Science. 1968;199:55–63. [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol A Biol Sci Med Sci. 2007;62:1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, et al. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci. 2001;98(8):4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H, Tuchweber B. In: Hypothalamus, Pituitary and Aging. Everitt A, Burgess JThomas, editors. Springfield, IL: 1976. [Google Scholar]
- Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–47. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (DHHS) National Center for Health Statistics Third National Health and Nutrition Examination Survey, 1988-1994, NHANES III. Hyattsville, MD: Centers for Disease Control and Prevention; 2001. [Google Scholar]
- Vaupel JW, Yashin A. Heterogeneity's Ruses: Some Surprising Effects of Selection on Population Dynamics. The American Statistician. 1985;39(3):176–185. [PubMed] [Google Scholar]
- Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Chen K. The essential mechanisms of aging: Irreparable damage accumulation of biochemical side-reactions. Exp Gerontol. 2005;40:455–465. doi: 10.1016/j.exger.2005.03.012. [DOI] [PubMed] [Google Scholar]


