Abstract
The fruit fly, Drosophila melanogaster, innately avoids even low levels of CO2. CO2 is part of the so-called Drosophila stress odor produced by stressed flies, but also a byproduct of fermenting fruit, a main food source, making the strong avoidance behavior somewhat surprising. Therefore, we addressed whether feeding states might influence the fly’s behavior and processing of CO2. In a recent report, we showed that this innate behavior is differentially processed and modified according to the feeding state of the fly. Interestingly, we found that hungry flies require the function of the mushroom body, a higher brain center required for olfactory learning and memory, but thought to be dispensable for innate olfactory behaviors. In addition, we anatomically and functionally characterized a novel bilateral projection neuron connecting the CO2 sensory input to the mushroom body. This neuron was essential for processing of CO2 in the starved fly but not in the fed fly. In this Extra View article, we provide evidence for the potential involvement of the neuromodulator dopamine in state-dependent CO2 avoidance behavior. Taken together, our work demonstrates that CO2 avoidance behavior is mediated by alternative neural pathways in a context-dependent manner. Furthermore, it shows that the mushroom body is not only involved in processing of learned olfactory behavior, as previously suggested, but also in context-dependent innate olfaction.
Keywords: Drosophila, olfaction, antennal lobe, mushroom body, neuromodulation, dopamine
Drosophila CO2 Sensory System
Flies instinctively avoid CO2 already at very low levels. This avoidance behavior is odor-specific and easily assayed in laboratory choice assays. Therefore, CO2 avoidance represents a great model to study the neural circuits and mechanisms between sensory input and motor output. In Drosophila, CO2 is detected by a dedicated pair of chemoreceptors, Gr21a and Gr63a, present in ab1c sensory neurons of the basiconic sensilla on the antenna.1,2 These neurons project their axons to a single glomerulus known as V-glomerulus in the ventral most region of the antennal lobe (AL), the primary olfactory center. A previous study had identified a single projection neuron (PN) that conveys CO2 information exclusively from the V-glomerulus in the AL to a higher brain center.3 This higher brain center, called the lateral horn (LH) has been known to process innate behaviors. Based on the stereotyped innate behavior and the presence of a dedicated neural pathway, the CO2 sensory system of the fly was considered hard-wired or “a labeled line” for information processing that is similar to the processing of sex pheromones or the more recently described microbial volatile odorant geosmin.4-6
Innate reactions to sensory stimuli can be modulated by context, such as internal state or the environment.7 Thus, context-dependent change of choice behavior is important for many animals. Behavioral modification according to the external stimuli and internal state enables the animals to respond appropriately and enhances their chances of survival in their environment. However, the neural basis of this state-dependent behavioral modification is poorly understood. Again, CO2 choice behavior represents a great model to identify the neural basis of context-dependent choice behavior as outlined below.
CO2 as a ubiquitous gaseous molecule, released in various concentrations from different short- or long-range sources, is an important sensory cue in the life of many insects.8 As mentioned above, when fruit flies encounter CO2 above background level, they show an innate stereotyped avoidance response.9 This reaction could be explained by the fact that CO2 is one of the main components of the Drosophila stress odor.9 Interestingly, CO2 is also produced in varying amounts during fruit ripening and by yeast that infests rotting fruit. Therefore, an interesting question arises: how do Drosophila melanogaster, which feed and oviposit mainly on rotting fruit, evaluate the presence of rising amounts of CO2 produced during fermentation? A recent investigation on this question shed some light by showing that some of the volatiles released by fruit, at sufficient concentration, can inhibit CO2 detection directly at the sensory neuron level.10 However, how internal state-dependent needs, such as hunger, may modify CO2 avoidance behavior remained elusive.
Parallel Pathways Involved in CO2 Avoidance Behavior Modification
In a recent report we used CO2 choice behavior to unravel the impact of feeding state on olfactory choice behavior and the underpinning neural circuit mechanisms11. First, we hypothesized that the feeding-state of the fly may modulate the representation of CO2 in the brain, in particular in the context of feeding on ripening or rotting fruits.
Based on a previous finding that feeding state can be integrated at the level of the mushroom body (MB) during appetitive learning and memory,12 in our work we explored the role of the MB in feeding state-dependent CO2 avoidance behavior 11. The MB has been studied to a great extent for its essential role in various behaviors. Best understood to date is its function as a brain center for olfactory learning and memory. While other behaviors have been linked to this structure as well, for example, sleep and courtship conditioning, the MB was thought to be dispensable for innate olfactory behavior.13 Given that the MB receives input from a variety of neurons such as neuromodulatory neurons, it is in an ideal position to integrate context also during innate olfaction.14 With this in mind, we analyzed CO2 avoidance behavior in fed and starved flies with and without functional MB output. To this aim, we expressed the temperature-sensitive version of dynamin (shibirets1) in MB intrinsic cells, the Kenyon cells (KC) to block its synaptic output. Shibirets1 allowed blocking neurons transiently by shifting flies to 32 °C (restrictive) while not causing any effect at 25 °C (permissive). In fed flies, we found that blocking output of all classes of KCs had no effect on CO2 avoidance behavior. Blocking the same neurons in starved flies, however, abolished CO2 avoidance completely. The MB contains several populations of KCs, α/β, α`/β`, and γ, that are distinguished by their anatomy and also in part by their differential requirement during learning and memory.14-16 Dissecting the population of KCs further, using the same method combined with more specific expression drivers for the subpopulations, revealed that α`/β` KC cells are more important than the other populations for processing CO2 avoidance behavior, since blocking other KCs had no effect on CO2 avoidance behavior in starved or fed flies. Similarly to the internal state of hunger, the presence of vinegar as an external context rendered the behavior MB-dependent. When hungry or fed flies were given the choice between air and a mix of CO2 and the food odor vinegar, they required the MB to process the CO2 component of the odor mix. Blocking the MB resulted in an attraction of fed flies to the mixture, similarly to what was observed in the context of starvation. Taken together these behavioral experiments demonstrated that the context of either starvation or the presence of a food odor changed neural processing of CO2 from MB-independent to -dependent. In support of these behavioral experiments, using in vivo calcium imaging experiments we showed that CO2 stimulation elicits a calcium response in KCs in a concentration-dependent manner. Thus, the MB is required for processing innate olfactory behavior exclusively in the context of internal state or additional external stimuli11.
By using GFP-photoconversion experiments, and an extensive search of a GAL4-database, we identified a bilateral ventral projection neuron (biVPN) that connects the V-glomerulus to the higher brain centers. Interestingly, the biVPN appeared to be the only neuron connecting the V-glomerulus to the MB, while 2 other neurons that we identified only projected to the lateral horn (LH). Clonal analysis and 3D reconstructions showed that the cell body of this neuron was located directly lateral to the subesophageal ganglion (SOG), a brain area innervated by axons of gustatory sensory neurons. Its dendrites innervate the V-glomerulus and the axon projected both to the LH and to the MB calyx, the primary MB region for incoming olfactory information. In the MB calyx, the axon of the biVPN appeared to form synapses with all types of KCs including the α`/β` subtype. Using in vivo calcium imaging, we demonstrated that CO2 led to a calcium rise in the biVPN cell body and in the V-glomerulus. Finally, behavioral experiments showed that the biVPN was essential upon starvation for 24 or 42 h, but dispensable for CO2 avoidance under fed conditions. These findings clearly demonstrated that this atypical PN is critical for context-dependent CO2 avoidance by connecting CO2 sensory input to the MB.
A recent report by Lin et al., which appeared at the same time as our original publication, independently identified several CO2-responsive PNs, including the biVPN (referred to as PNv-1).17 Different from our report, Lin et al. implicated this neuron in processing of concentration-dependent CO2 avoidance behavior. Their results suggested that the biVPN was required only at lower CO2 concentrations (0.5%), but not for avoidance of higher concentrations (2%). For avoidance of 2% CO2 another VPN (PNv-2), which connects the V-glomerulus to the bilateral superior dorsofrontal protocerebrum (SDFP), appeared to be essential. However, the study did not address the role of internal state or context. In light of these 2 reports, it is clearly evident that CO2 information is conveyed to higher brain centers via multiple PNs with specific functional and anatomical identities. These findings changed the previous notion that the V-glomerulus was connected to the LH only and that the MB was not required during CO2 avoidance.3,9 How exactly these 2 different roles for the same neuron can be explained and connected to each other will be addressed in future experiments.
In summary, our work showed that the MB is functionally involved in modification of CO2 avoidance behavior in a context-dependent manner. Together with the discovery that distinct types of VPNs process CO2 sensory information, this lead to the conclusion that the CO2 circuitry in the fly brain is complex and adaptable to internal stage and external stimuli. One of the factors that determine which one of these parallel pathways is utilized during CO2 processing is hunger.
Dopamine Release is Involved in Context-Dependent CO2 Behavior
An outstanding question arising from our work is which signals determine the choice of neural pathway. We used behavioral analysis and functional imaging to address this question.
A prolonged lack of nutrition changes the behavior of both the adult as well as larval Drosophila melanogaster drastically. Hunger increases activity as well as food searching behavior in the fly.18,19 Starvation can also induce the release of different neuromodulators in the nervous system. By acting on the peripheral and central nervous systems, neuromodulators can bring about synaptic changes that facilitate neuromodulation in animals. There are several neurochemicals that can act as neuromodulators in the insect nervous system. Notably among them are different neuropeptides and biogenic amines such as dopamine.20,21 Previous studies have shown that neuropeptides such as tachykinin and sNPF modulate olfactory information processing at the level of sensory neurons and the AL to increase the sensitivity toward food odors.19,22 However, these peptide systems not only influence olfaction at the early stages of processing but also in higher brain centers. Hunger also plays an important role in olfactory learning and memory.12 The authors showed that neuropeptide dNPF gates hunger-dependent appetitive memory by acting on dopaminergic neurons. These neurons, in turn, release their inhibition on KCs so that memory processing can go forth. Thus, these dopaminergic neurons represent a form of motivational gate: only a starved fly is sufficiently interested in the expression of food related memories. Hunger gating may hence be necessary, because certain odors may only be associated with food, if the animal has encountered them during actual feeding, which in turn requires the motivation to feed. An equivalent situation occurs during CO2 related behavior, if CO2 is presented in the context of a food odor 11. In fed conditions, the food-related vinegar odor does not present an incentive to overcome the aversive CO2 cue. Once the fly is starved, however, it is motivated by its alternate internal state to process food-related cues differentially, and now prioritizes appetitive fragrances over a potential source of danger. As a result, more flies overcome their aversion to CO2 and approach the potential food source.
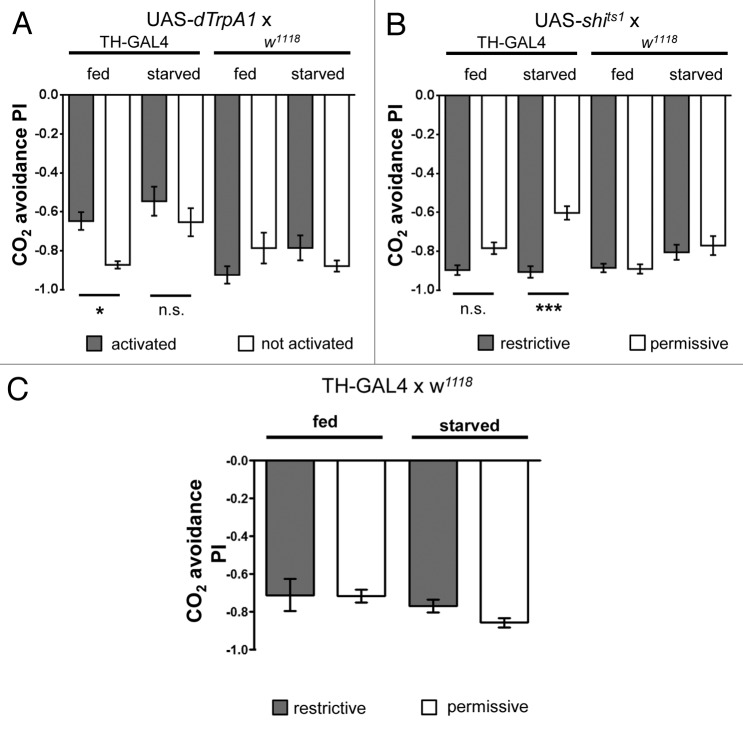
In light of the aforementioned previous work on olfactory learning and memory, we asked whether manipulation of the dopaminergic system during innate choice behavior resulted in a change of strength of CO2 avoidance. To this aim we employed the TH-GAL4 line to manipulate a large portion of the dopaminergic system in the fly. This GAL4 is expressed under the control of an enhancer element stemming from the endogenous tyrosine hydroxylase (TH) locus, a gene necessary for dopamine biosynthesis. The resulting TH-GAL4 line has been reported to express in around 70% of the total dopaminergic neuron population within the fly brain.23 To trigger neuronal activity of these cells in behaving animals, we utilized dTrpA1, a cation channel which promotes neuronal firing at 32 °C, but has no effect at 25 °C.24 These properties allowed us to design an experiment, in which we compared flies of the same genotype with or without artificially activated TH-positive neurons in a T-maze assay. Activating dopaminergic neurons via TH-GAL4 driven expression of UAS-dTrpA1 resulted in decreased CO2 avoidance in fed flies (Fig. 1A). This decrease led to a similar PI as observed in starved control flies 11. Activating these neurons in starved flies, however, did not result in further decrease in avoidance. To corroborate this data, we also blocked dopaminergic neurons in both starved and fed flies via expression of temperature sensitive shibirets1 as described above.25 Blocking neurons in fed flies had no effect on CO2 avoidance when comparing the experimental and respective control groups (Fig. 1B). In starved flies, however, we observed an increase in CO2 avoidance to a level that resembled the PI observed in fed control flies 11. TH-GAL4 flies without UAS-shits1 or UAS-dTrpA1 in contrast did not show any significant differences in CO2 avoidance between fed and starved flies (Fig. 1C).
Figure 1. (A) CO2 avoidance of flies after activating TH-GAL4 neurons via dTRPA1 (B) or after blocking dopaminergic output of TH-GAL4 neurons via shits1. (C) CO2 avoidance of TH-GAL4/w1118 control flies. Flies were either starved 42 h (starved) or kept on food (fed). Error bars indicate s.e.m. (n = 9 in A, B, and C). One asterisk, P < 0.05, 3 asterisks, P < 0.001 (analysis of variance, Bonferroni's multiple comparison test).
Thus, both results are complimentary to each other. They suggest that dopamine release modulates the behavior of fed flies to resemble the one of starved flies, while blocking dopaminergic signaling returns CO2 avoidance of starved flies back to the level of fed flies. It is, however, important to note that we only observed differences in behavior of starved and fed control flies in some genetic backgrounds, such as the combination of TH-GAL4 and the UAS-effector. TH-GAL4 only or UAS-effector only control flies showed no significant reduction in CO2 avoidance behavior in starved compared with fed flies (Fig. 1A–C). Nevertheless, permissive temperature control flies of the same genotype carrying both TH-GAL4 and the UAS-effector represent in our opinion the best controls, because these flies have undergone the exact same treatments and originate from the same parent cross. Starvation sensitivity, for instance, as measured by the rate of fly death strongly depends on prior treatment and genetic background 11. Analysis of more specific lines that contain only subsets of dopaminergic neurons will reveal which role dopamine plays in context-dependent CO2 avoidance.
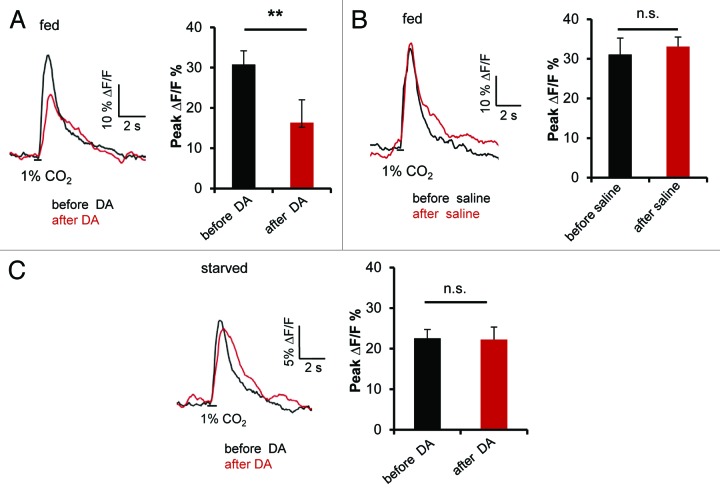
Still, these behavioral results encouraged us to further analyze the potential role of dopamine in starvation-dependent CO2 avoidance. Since we found that blocking MB output of, in particular, the α`/β` lobes, lead to a reduction of CO2 avoidance in starved flies, we next asked whether dopamine modulates the response of this MB subpopulation to the CO2 stimulus. To address this question, we measured CO2 stimulated Ca2+ signals from the MB lobes before and after dopamine application in in vivo calcium imaging experiments. Fed flies expressing GCaMP5.0 in α`/β` KCs (using Split-GAL4 line MB 186B) were stimulated with 1% CO2 for 500 ms before and after a 5 min incubation with 10 mM dopamine added via bath application to the brain within the head capsule of a living fly. We observed a significant decrease in Ca2+ signal upon CO2 stimulation after application of dopamine but not after application of saline (Fig. 2A and B). Interestingly, when we repeated the same experiment with flies that were starved for 42 h, no change in the Ca2+ signal before and after application of dopamine was observed (Fig. 2C). The selective decrease in CO2 induced Ca2+ signal in α`/β` lobes of fed flies upon application of dopamine is consistent with the hypothesis that dopamine reduces the level of responsiveness of KCs to CO2 in a metabolic state-dependent manner. However, additional ongoing experiments are required to understand the relationship of dopamine signaling and context-dependent CO2 avoidance.
Figure 2. (A) Averaged time course and peak fluorescent intensity change in α`/β` lobe of MB186B:GCaMP5.0 flies (fed) to 1% CO2 stimulation before and after treatment with dopamine (DA).. Error bars indicate s.e.m. (n = 9). Two asterisks, P < 0.005 (Mann–Whitney test). (B) Averaged time course and peak fluorescent intensity change in α`/β`of MB186B:GCaMP5.0 flies (fed) to 1% CO2 stimulation before and after treatment with imaging saline. Error bars indicates s.e.m. (n = 6). n. s., not significant (Paired t test). (C) Averaged time course and peak fluorescent intensity change in α`/β` of MB186B:GCaMP5.0 flies (42 h starved) to 1% CO2 stimulation before and after treatment with dopamine. Error bars indicate s.e.m. (n = 9). n. s., not significant (Paired t test).
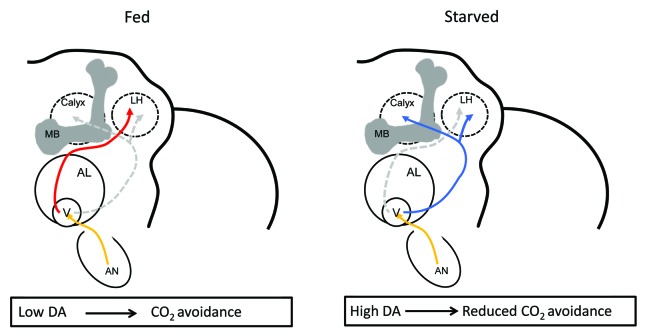
Our results thus far suggest that the neuromodulator dopamine could act as a molecular signal for metabolic state-dependent CO2 avoidance behavior (Fig. 3). Dopamine is widely known for its roles in motivation, reinforcement, and associative memory formation in mammals. For instance, it regulates feeding behavior in mice.26,27 But the number and types of dopaminergic neurons in the mammalian brain make the understanding of the underlying neural circuits difficult. Complementing the genetic and behavioral studies in human and mouse, research in the fly showed that this neurotransmitter can act on many different levels of neural processing of sensory information and internal stage or external context.28-31 For example, dopamine strongly impacts feeding behavior in the fly at different levels. In a starved fly, a single dopaminergic neuron in the SOG modulates proboscis extension behavior.32 Temperature-dependent activation of this neuron promotes proboscis extension in the absence of hunger suggesting that dopamine release correlates with starvation.
Figure 3. A hypothetical model that summarizes the neural mechanisms involved in context-dependent CO2 avoidance modification in fruit flies. In fed flies, low dopamine (DA) levels promote a strong innate avoidance reaction toward CO2 mediated through a neuronal circuit in which the MB is redundant. In fed flies, the output of the LH is sufficient to initiate avoidance. Once the animal is starved, high DA levels allow integration of context and shift the behavior toward reduced CO2 avoidance that depends on the pathway involving the biVPN (in blue) and the MB. Thus, in starved flies, the neural pathway involving other VPNs (in red) and the LH is not sufficient to initiate avoidance. AN, antenna; V, V-glomerulus; AL, antennal lobe; MB, mushroom body; LH, lateral horn; DA, dopamine.
Summary and Conclusions
The CO2 sensory system, from the periphery up to the AL with the involvement of a heteromeric receptor and a single CO2-responsive glomerulus is more streamlined than most other olfactory pathways. Because of this the finding that the information flow from the periphery to the higher brain centers is mediated via 2 parallel pathways according to the feeding state of the fly is surprising. This suggests that pathways underpinning innate behaviors that are thought to be hardwired are, in fact, more flexible than expected. Furthermore, we showed that the MB, a brain center required for olfactory memory, is critical for context-dependent CO2 avoidance. Why does the fruit fly employ 2 alternative pathways to detect a single sensory cue? And what advantage does the fly glean from employing these 2 pathways? A labeled line circuit, such as CO2 sensory system of fruit fly, has the advantage that it will always elicit a stereotyped behavior upon contact with a certain sensory cue; this arrangement is also energy efficient and may allow for a more prompt behavioral response. Arguably most sensory cues need to be interpreted in a context-specific manner. Here, a parallel processing pathway required only according to a specific context, such as starvation, allows for integration of internal state and environmental context, including the scent or presence of a potential food source. Thus, parallel processing presents an advantage to the fly in a situation, where they are pressed for limited resources for survival, and allows them to adjust their behavior according to their particular situation. On the other hand, when food is abundant, flies might more readily, without the need of additional processing, react to a potential danger source with a quick flight response. Processing via additional brain centers could be more expensive to the animal in terms of energy and time required to induce a behavioral output. But because the MB also receives signals from other sensory, including additional olfactory, modalities and neuromodulatory neurons, this arrangement makes the MB a convenient center for processing and fine-tuning state-dependent choice behaviors. Therefore, even with a potential energy or time disadvantage, recruiting the MB into context-dependent CO2 avoidance facilitates the integration of internal state, together with other olfactory stimuli, especially those conveying food related information.
Currently, we do not know whether other insects use similar parallel pathways for innate odor processing in a context-dependent manner. Given that other insects, such as blood-feeding mosquitoes use CO2 as a sensory cue to locate potential hosts to blood feed, understanding the modulatory aspects of the CO2 sensory system in a context dependent manner might help in the design of effective vector control strategies. Previous research suggested that modulation in the sensory neuron changes olfactory sensitivity of mosquitoes before and after a blood meal.33 Thus, it is possible that similar pathway modifications as we have described for Drosophila melanogaster apply to mosquitoes.
But even beyond insects, mammals constantly face sensory cues that need to be integrated with internal stage and external context to make appropriate choices. At this point, our understanding of how, for instance, foraging is regulated and processed on the level of neural circuits is extremely limited in mammals.7 It is clear that neuromodulators, including dopamine influence decision-making and modify behavioral outcomes. Also, similar to the insect, it has been suggested that distinct brain structures process innate olfaction as supposed to learned olfactory behaviors.34 For instance, the amygdala might represent a structure that could be compared with the insect LH, while the piriform cortex and other cortical areas process and store olfactory associations. Therefore, as demonstrated for other behaviors, studies in the fly like ours might prove useful in understanding context-dependent processing of chemosensory cues also in higher animals.
Experimental Protocols
Behavior, calcium imaging, and data analysis were performed according to the method previously described (Bräcker et al., 2013).
Behavior
For behavior experiments we used 6–8 d old flies of the following genotypes: TH-GAL4:dTRPA1, TH-GAL4:Shits1, TH-GAL4/w1118. Flies were either kept on food (fed condition) or starved for 42 h (starved condition). Flies were tested in groups of ~60 in a standard T-maze by giving a choice between CO2 and air. After the test, flies were counted and the performance index (PI) was calculated by subtracting the number of flies on the air side from the number of flies on the CO2 side and normalizing this result with the total number of flies.
Calcium imaging
For dopamine bath application experiments, in vivo preparation of flies expressing MB186B:GCaMP5.0 that were either fed or starved for 42 h were used. In these preparations, at first the calcium response to 1% CO2 stimulation was measured. Thereafter, 5 µl of 1 M dopamine hydrochloride (Sigma) dissolved in imaging saline were added to 500 µl of imaging bath to attain a final dopamine concentration of 10 mM. Five min after adding the dopamine to the imaging bath, we again measured the calcium response to 1% CO2 stimulation. For control experiments we repeated the same procedure in which dopamine addition was replaced by 5 µl of imaging saline.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 2.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–8. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–50. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–6. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 5.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–12. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–57. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci. 2013;16:1717–24. doi: 10.1038/nn.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerenstein PG, Hildebrand JG. Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol. 2008;53:161–78. doi: 10.1146/annurev.ento.53.103106.093402. [DOI] [PubMed] [Google Scholar]
- 9.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–9. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 10.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–81. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 11.Bräcker LB, Siju KP, Varela N, Aso Y, Zhang M, Hein I, Vasconcelos ML, Grunwald Kadow IC. Essential role of the mushroom body in context-dependent CO₂ avoidance in Drosophila. Curr Biol. 2013;23:1228–34. doi: 10.1016/j.cub.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–27. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–5. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–55. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 15.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–15. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–13. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HH, Chu LA, Fu TF, Dickson BJ, Chiang AS. Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science. 2013;340:1338–41. doi: 10.1126/science.1236693. [DOI] [PubMed] [Google Scholar]
- 18.Gruber F, Knapek S, Fujita M, Matsuo K, Bräcker L, Shinzato N, Siwanowicz I, Tanimura T, Tanimoto H. Suppression of conditioned odor approach by feeding is independent of taste and nutritional value in Drosophila. Curr Biol. 2013;23:507–14. doi: 10.1016/j.cub.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–44. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nässel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Van Swinderen B, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc Biol Sci. 2011;278:906–13. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther AM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci U S A. 2009;106:13070–5. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friggi-Grelin F, Iché M, Birman S. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis. 2003;35:175–84. doi: 10.1002/gene.10178. [DOI] [PubMed] [Google Scholar]
- 24.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–20. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 26.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaillard D, Passilly-Degrace P, Besnard P. Molecular mechanisms of fat preference and overeating. Ann N Y Acad Sci. 2008;1141:163–75. doi: 10.1196/annals.1441.028. [DOI] [PubMed] [Google Scholar]
- 28.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–64. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Plaçais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–6. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 30.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–7. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Swinderen B, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc Biol Sci. 2011;278:906–13. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marella S, Mann K, Scott K. Dopaminergic modulation of sucrose acceptance behavior in Drosophila. Neuron. 2012;73:941–50. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siju KP, Hill SR, Hansson BS, Ignell R. Influence of blood meal on the responsiveness of olfactory receptor neurons in antennal sensilla trichodea of the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2010;56:659–65. doi: 10.1016/j.jinsphys.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–6. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]