Abstract
Seminal proteins are critical for reproductive success in all animals that have been studied. Although seminal proteins have been identified in many taxa, and female reproductive responses to receipt of these proteins have been documented in several, little is understood about the mechanisms by which seminal proteins affect female reproductive physiology. To explore this topic, we investigated how a Drosophila seminal protein, ovulin, increases ovulation rate in mated females. Ovulation is a relatively simple physiological process, with known female regulators: previous studies have shown that ovulation rate is promoted by the neuromodulator octopamine (OA) in D. melanogaster and other insects. We found that ovulin stimulates ovulation by increasing OA signaling in the female. This finding supports a model in which a male seminal protein acts through “hacking” a well-conserved, regulatory system females use to adjust reproductive output, rather than acting downstream of female mechanisms of control or in parallel pathways altogether. We also discuss similarities between 2 forms of intersexual control of behavior through chemical communication: seminal proteins and pheromones.
Keywords: seminal proteins, female post-mating responses, ovulation, octopamine, ovulin, neuromodulators, pheromones
Seminal proteins are important for reproductive success in all sexually reproducing animals that have been investigated.1-5 For example, vertebrate seminal fluid includes proteins that regulate sperm motility, sperm association with and release from females’ storage reservoirs, fertilization, ovulation, semen liquefaction, female immune responses and female reproductive tract physiology (for reviews see refs. 1,2,6-10). Insect seminal proteins have particularly dramatic effects on females, with effects including the modulation of egg production and ovulation, the storage and utilization of sperm, and several female behaviors including attractiveness, receptivity to mating, feeding, and locomotor behaviors (Fig. 1A; for reviews see refs. 3,4,11). While males induce such changes in female reproductive physiology through their transferred seminal proteins, these proteins must act within the context of the female’s physiology and its regulatory mechanisms (earlier work reviewed in ref. 12; see also recent refs. 13,14). The physiological mechanisms by which seminal proteins from the male affect the reproductive biology of the female are only now becoming amenable to study.
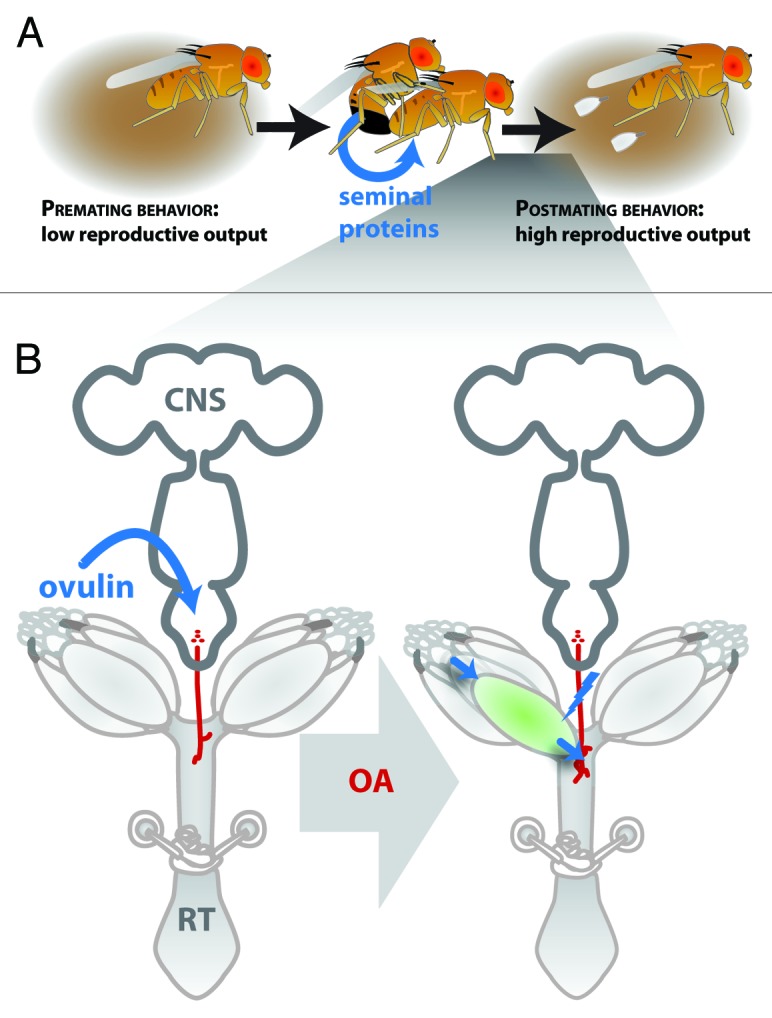
Figure 1. Seminal proteins are important regulators of female reproductive behavior. (A) Before mating, Drosophila females exhibit minimal reproductive behaviors, including laying few eggs. After mating and receiving seminal proteins, female reproductive behavior is dramatically changed; for example mated females increase their egg production as illustrated here. As discussed in the article, one mechanism by which seminal proteins could increase or induce reproductive behavior is by acting on conserved, regulatory systems that females use to adjust reproductive output. Another possibility is that seminal proteins act downstream of female mechanisms of control or in parallel pathways altogether. (B) Consistent with the first possibility noted in A, the seminal protein ovulin increases ovulation rates in mated females by increasing signaling of octopamine (OA) neurons (shown in red), a canonical female regulator of ovulation rates. Ovulin acts to increase OA neuronal signaling (blue lightning bolt). The increased OA neuronal signaling acts on the female’s reproductive tract to stimulate the process of ovulation, the movement (arrows) of an egg (green) out of the ovary. Ovulin’s effects on OA neurons are also seen by the increased synaptic growth of those neurons (indicated here by a larger synaptic arborization of the neurons). RT, reproductive tract; CNS, central nervous system. Sperm storage organs and ovaries are included in the figure, but are not labeled.
Drosophila melanogaster is a particularly effective model to understand the nature and actions of seminal proteins. First, the Drosophila seminal proteome is known4,15,16 and shares general similarities with seminal proteomes across numerous species. For example, it is rich in proteases and their regulators, in apparent peptide hormones and their precursors, in sperm-binding proteins, in antioxidants and antimicrobial molecules, and in other classes of proteins found in seminal proteomes of other animals. In addition, as in other animals, the Drosophila seminal proteome includes a substantial number of proteins that have evolved under positive selection and exhibit rapid sequence evolution. Second, many post-mating effects are known in Drosophila, and have been associated with the actions of specific seminal proteins. For example, the changes in feeding, receptivity, and locomotor behaviors are the consequences of the female’s receipt of a “sex peptide” in the seminal fluid; the female’s ovulation rate is increased by receipt of the seminal protein ovulin; and, the seminal protein Acp36DE promotes uterine contractions that lead to the storage of sperm by female flies. Third, in the case of sex peptide, several studies have identified the female receptor and the populations of neurons that are important for its function.17-21 However, exactly how seminal proteins act to regulate female physiology or behavior is not well understood.
Broadly speaking, there are 2 mechanisms by which seminal proteins might induce female post-mating responses. First, seminal proteins might act on female pathways that are direct and dedicated to detecting and responding to seminal proteins (for example, on dedicated neural circuitry or signal transduction pathways). Alternatively, male seminal proteins might co-opt conserved female signaling pathways that predate the origin of the seminal protein. For most post-mating responses it has been difficult to distinguish these 2 options. Little detail is known about the physiology that governs many post-mating responses, like re-mating receptivity and post-mating changes in feeding and locomotion.
Among post-mating responses, ovulation is a particularly good system with which to dissect how seminal proteins can induce female post-mating responses. First, a seminal protein that increases ovulation by mated females is known. This protein, ovulin, is a 264-amino acid glycoprotein22,23 that is cleaved within the reproductive tract of mated female flies,22 and ectopic expression assays suggest that this processing generates more-active sub-regions of ovulin.24 Second, the physiology of ovulation has been studied in insects. In several insects, ovulation is promoted by the neuromodulator octopamine (OA), a tyrosine-derived molecule that affects a wide range of behaviors, including flight, aggression, and locomotion.25-28 In particular in D. melanogaster, females lacking OA (or its receptor OAMB) exhibit severe ovulation defects.29-33
The knowledge that ovulation requires OA in females, and that the male-derived protein ovulin increases ovulation rate, led to a simple, testable hypothesis: perhaps ovulin acts by modulating the level of activity of the pre-existing OA pathway in females. Unmated females ovulate at a low rate, so the OA pathway is already acting for ovulation, but at a low level in unmated females. Ovulin from the male would simply need to turn up the activity of this pathway to increase ovulation rate.
We tested this hypothesis by genetically increasing the activity of OA neurons by expressing within them a dominant negative potassium channel.34 Consistent with the idea that ovulin modulates the level of OA activity, females with genetically enhanced OA neuronal activity showed high rates of ovulation, whether or not they received ovulin from their mates.35 Since artificially increasing OA neuronal signaling compensated for lack of ovulin receipt during mating, this result suggested that ovulin increases ovulation rates by stimulating the OA pathway.
Ovulation is mediated by coordination of female reproductive tract muscle activity, including the ovaries and the oviducts. OA induces contraction of ovarian muscles and blocks contraction of oviduct muscle.36,37 These differential effects on contraction and relaxation were suggested to push the egg from the ovary into a relaxed oviduct.37 Consistent with this view of OA action, measures of oviduct sarcomere length showed that oviduct muscles do relax after mating. This post-mating muscle relaxation depends on the female’s OA signaling35: only females that could produce OA responded to mating by relaxing their oviduct muscles. Further, artificially and selectively increasing the activity of OA neurons using the thermally activated TrpA channel38,39 was also able to induce the relaxation in oviduct muscle.35
The findings that ovulin acted through OA to increase ovulation rate and that OA affects muscle contraction in the reproductive tract suggested that ovulin and OA might together act to increase ovulation rates by regulating muscle contraction in the female reproductive tract, such as relaxing oviduct muscles or stimulating ovarian muscle contraction (although the latter is speculative). Consistent with this, the post-mating relaxation of oviduct muscles noted above did not occur if females did not receive ovulin from their mates. Moreover, ectopically expressing ovulin in unmated females also relaxed oviduct muscles—in an OA-dependent manner.35
There were several ways by which ovulin could regulate OA signaling. Ovulin could act upstream of OA release to increase the amount of OA neuronal activity or the number of OA synaptic release sites. Alternatively, ovulin might sensitize the female’s reproductive tract to her own OA signaling, without affecting the amount of OA release (or release sites). To test the first possibility, we examined whether ovulin affected the number of synaptic sites between OA neurons and oviduct musculature. We did this by counting synaptic boutons, which are sites of synaptic release whose increase in number can be indicative of increased neuronal activity40-42 (reviewed in ref. 43). We found that ovulin increased the number of synaptic release sites of neurons that innervated oviduct muscles, thus supporting the conclusion that ovulin acts to regulate the production of OA or the amount of OA signaling in the female. Although future experimentation will be needed to determine whether ovulin causes the synaptic effect by increasing OA neuronal activity or by upregulating cell signaling pathways involved in activity-dependent synaptic plasticity, these results are the first to demonstrate that seminal proteins can affect synaptic remodeling. Further, a more detailed examination of the female postmating neural circuit may illuminate upon which neurons ovulin acts, with a subset of OA/dsx+ neurons as a strong candidate (Rézaval et al. (2014) recently reported that these neurons and octopamine mediate post-mating behavioral responses).44 Additionally, other seminal proteins may work via additional neuromodulators that are dynamically regulated within the reproductive tract after mating.14 Together, these findings indicated that Drosophila ovulin acts through a conserved regulator of ovulation to affect female reproductive physiology (Fig. 1B).
OA signaling is known to regulate ovulation in insect species.25-27,29,31,45 Interestingly, the origins of a role of an OA-like molecule in ovulation may be even more ancient. The vertebrate analog of invertebrate OA is noradrenaline (NA), whose dysregulation has been implicated in polycystic ovarian syndrome (PCOS),46-53 a common cause of infertility in women involving disrupted ovulation and follicular growth. In contrast to the conservation of OA’s role in ovulation in insects (at least), the Drosophila seminal regulator of ovulation, ovulin, is a recently evolved component of the pathway, specific to the reproductive system of some species of Drosophila.54-56 Thus, this new regulator has been able to acquire a function by “hacking” the activity of a conserved, core regulator module. In thinking about how a molecule from one animal (in this case, the male) could act on a pathway in another (in this case, the female), it is simplest to imagine this evolving if the molecule were to act either at the “top” or the “bottom” of a pathway, thus preserving the internal workings of the pathway in their most efficient form. Moreover, if a male molecule acts at the “top” of the physiological pathway, evolutionary forces that might interfere with or alter the male molecule (or the female’s response or resistance to it) will not impact the core of the pathway, only the efficiency with which it is regulated. Since a significant number of seminal proteins (including ovulin) have been found to evolve rapidly,54-58 it will be interesting to see whether action at the “top” of conserved female pathways is a general property of seminal protein function.
The male’s impact on female ovulation behavior via ovulin is reminiscent of pheromonal communication, whereby volatile or contact pheromones released from one animal and detected by sensory neurons of a conspecific animal regulate aspects of the recipient’s physiology and behavior. Indeed, there are several additional parallels between pheromonal communication and seminal fluid action.
First, in pheromonal communication, the recipient has at least 3 components to receive and process the pheromones: olfactory or gustatory receptors to detect the presence of the chemical and transduce that signal into a neuronal response,59,60 odorant degrading enzymes which reduce the quantity of the pheromone over time,61,62 and odorant binding proteins,63,64 which are believed to bind and chaperone olfactory cues to facilitate detection by receptors (for review see refs. 60,65). Interestingly, seminal protein response pathways may contain comparable components. For example a G-protein coupled receptor for 1 Drosophila seminal protein (the sex peptide) has been identified; this receptor is expressed in neurons.18 Further, there is at least 1 case in which a female protease appears to degrade a seminal protein (the sex peptide again) thus limiting the window in which it can act through the circulatory system.66 Finally, seminal fluid contains,16 and female sperm storage organs express, odorant binding proteins,67 which could potentially assist in presenting molecules in seminal fluid to the female receptors. (We note, however, that this hypothesis is entirely conjectural at this point).
A second parallel between pheromones and seminal fluid proteins involves a common feature of insect pheromone communication: the prevalence of chemical blends (for review see refs. 68,69). Interactive pheromone blends typically result in an effect distinct from the responses to the component chemicals,70 often transmitting species-specific information (for review see refs. 68,69). In this light, it is interesting that seminal fluid also contains a large suite of proteins (> 200 in Drosophila), with a significant number under positive selection.16,54,55 It is tempting to speculate that the blend of seminal fluid molecules might be particularly effective in eliciting post-mating responses in its own species, perhaps serving as one of the several barriers to interspecific reproduction. Thus, there are analogies between pheromones’ facilitating pre-coital communication to affect social interactions, and seminal proteins mediating post-coital social and reproductive interactions. Furthermore, just as the study of olfactory reception of pheromones and their behavioral responses (for example, moths and Drosophila; refs. 69,71) has informed how circuits underlie behavior, studying the circuits that induce profound post-mating changes (like ovulin regulating OA signaling to increase ovulation in Drosophila35) could be a powerful tool in understanding how neural circuits turn sensory signals in to behavioral responses.
Due to the ease of Drosophila genetics, efficient screening, a detailed annotated seminal proteome, and a developing efficient targeted genome engineering strategy72-80 for review see refs. 81,82), Drosophila remains a powerful model to dissect seminal protein function and, hopefully, identify seminal protein receptors and the cell populations in which they function. This will lead to a better understanding of how mating experience can lead to sweeping changes in female physiology and behavior.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank B Rattner for the invitation to write this article, present and former members of the Wolfner lab for helpful discussions, and F Avila, G Findlay, J Sitnik, and anonymous reviewers for helpful comments on this manuscript. We thank NIH grant R01-HD038921 (to M.F.W.) for supporting our work on the function of Drosophila seminal proteins, including ovulin.
References
- 1.Poiani A. Complexity of seminal fluid: a review. Behav Ecol Sociobiol. 2006;60:289–310. doi: 10.1007/s00265-006-0178-0. [DOI] [Google Scholar]
- 2.Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 3.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirot LK, LaFlamme BA, Sitnik JL, Rubinstein CD, Avila FW, Chow CY, Wolfner MF. Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet. 2009;68:23–56. doi: 10.1016/S0065-2660(09)68002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA, Adams GP. The nerve of ovulation-inducing factor in semen. Proc Natl Acad Sci U S A. 2012;109:15042–7. doi: 10.1073/pnas.1206273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proceedings of the National Academy of Sciences 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52:455–62. doi: 10.1387/ijdb.072527ss. [DOI] [PubMed] [Google Scholar]
- 8.Adams GP, Ratto MH. Ovulation-inducing factor in seminal plasma: a review. Anim Reprod Sci. 2013;136:148–56. doi: 10.1016/j.anireprosci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, Robertson SA. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci U S A. 2014;111:2200–5. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean MD. Genetic disruption of the copulatory plug in mice leads to severely reduced fertility. PLoS Genet. 2013;9:e1003185. doi: 10.1371/journal.pgen.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman T, Davies SJ. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides. 2004;25:1477–90. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Wolfner MF. Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered. 2009;100:399–410. doi: 10.1093/jhered/esp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laflamme BA, Wolfner MF. Identification and function of proteolysis regulators in seminal fluid. Mol Reprod Dev. 2013;80:80–101. doi: 10.1002/mrd.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heifetz Y, Lindner M, Garini Y, Wolfner MF. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr Biol. 2014;24:731–7. doi: 10.1016/j.cub.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009;19:886–96. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Häsemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–8. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Yapici N, Kim Y-J, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–7. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 19.Haussmann IU, Hemani Y, Wijesekera T, Dauwalder B, Soller M. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc Biol Sci. 2013;280:20131938. doi: 10.1098/rspb.2013.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol. 2012;22:1155–65. doi: 10.1016/j.cub.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–26. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monsma SA, Wolfner MF. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 1988;2:1063–73. doi: 10.1101/gad.2.9.1063. [DOI] [PubMed] [Google Scholar]
- 23.Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci U S A. 1995;92:10114–8. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci U S A. 2005;102:743–8. doi: 10.1073/pnas.0407692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange AB. Neural mechanisms coordinating the female reproductive system in the locust. Front Biosci (Landmark Ed) 2009;14:4401–15. doi: 10.2741/3536. [DOI] [PubMed] [Google Scholar]
- 26.Cook BJ, Wagner RM. Some pharmacological properties of the oviduct muscularis of the stable fly Stomoxys calcitrans. Comp Biochem Physiol C. 1992;102:273–80. doi: 10.1016/0742-8413(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 27.Sefiani M. Regulation of egg-laying and in vitro oviductal contractions in Gryllus bimaculatus. J Insect Physiol. 1987;33:215–22. doi: 10.1016/0022-1910(87)90040-0. [DOI] [Google Scholar]
- 28.Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–77. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 29.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Monastirioti M, Linn CE, Jr., White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–11. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–55. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 32.Lee H-G, Rohila S, Han K-A. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 2009;4:e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H-G, Seong C-S, Kim Y-C, Davis RL, Han K-A. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264:179–90. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Broughton SJ, Kitamoto T, Greenspan RJ. Excitatory and inhibitory switches for courtship in the brain of Drosophila melanogaster. Curr Biol. 2004;14:538–47. doi: 10.1016/j.cub.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein CD, Wolfner MF. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc Natl Acad Sci U S A. 2013;110:17420–5. doi: 10.1073/pnas.1220018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodríguez-Valentín R, López-González I, Jorquera R, Labarca P, Zurita M, Reynaud E. Oviduct contraction in Drosophila is modulated by a neural network that is both, octopaminergic and glutamatergic. J Cell Physiol. 2006;209:183–98. doi: 10.1002/jcp.20722. [DOI] [PubMed] [Google Scholar]
- 37.Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJH. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–20. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–88. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–68. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosca TJ, Carrillo RA, White BH, Keshishian H. Dissection of synaptic excitability phenotypes by using a dominant-negative Shaker K+ channel subunit. Proc Natl Acad Sci U S A. 2005;102:3477–82. doi: 10.1073/pnas.0406164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, Budnik V. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–18. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menon KP, Carrillo RA, Zinn K. Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip Rev Dev Biol. 2013;2:647–70. doi: 10.1002/wdev.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr Biol. 2014;24:725–30. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monastirioti M, Gorczyca M, Rapus J, Eckert M, White K, Budnik V. Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J Comp Neurol. 1995;356:275–87. doi: 10.1002/cne.903560210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalro BN, Loucks TL, Berga SL. Neuromodulation in polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:35–62. doi: 10.1016/S0889-8545(05)70184-4. [DOI] [PubMed] [Google Scholar]
- 47.Paredes AH, Salvetti NR, Diaz AE, Dallard BE, Ortega HH, Lara HE. Sympathetic nerve activity in normal and cystic follicles from isolated bovine ovary: local effect of beta-adrenergic stimulation on steroid secretion. Reprod Biol Endocrinol. 2011;9:66. doi: 10.1186/1477-7827-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acuña E, Fornes R, Fernandois D, Garrido MP, Greiner M, Lara HE, Paredes AH. Increases in norepinephrine release and ovarian cyst formation during ageing in the rat. Reprod Biol Endocrinol. 2009;7:64. doi: 10.1186/1477-7827-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greiner M, Paredes A, Araya V, Lara HE. Role of stress and sympathetic innervation in the development of polycystic ovary syndrome. Endocrine. 2005;28:319–24. doi: 10.1385/ENDO:28:3:319. [DOI] [PubMed] [Google Scholar]
- 50.Greiner M, Paredes A, Rey-Ares V, Saller S, Mayerhofer A, Lara HE. Catecholamine uptake, storage, and regulated release by ovarian granulosa cells. Endocrinology. 2008;149:4988–96. doi: 10.1210/en.2007-1661. [DOI] [PubMed] [Google Scholar]
- 51.Lara HE, Dorfman M, Venegas M, Luza SM, Luna SL, Mayerhofer A, Guimaraes MA, Rosa E Silva AA, Ramírez VD. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc Res Tech. 2002;59:495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 52.Urman B, Yakin K. Ovulatory disorders and infertility. J Reprod Med. 2006;51:267–82. [PubMed] [Google Scholar]
- 53.Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf) 2012;77:791–801. doi: 10.1111/cen.12003. [DOI] [PubMed] [Google Scholar]
- 54.Tsaur SC, Wu CI. Positive selection and the molecular evolution of a gene of male reproduction, Acp26Aa of Drosophila. Mol Biol Evol. 1997;14:544–9. doi: 10.1093/oxfordjournals.molbev.a025791. [DOI] [PubMed] [Google Scholar]
- 55.Wagstaff BJ, Begun DJ. Comparative genomics of accessory gland protein genes in Drosophila melanogaster and D. pseudoobscura. Mol Biol Evol. 2005;22:818–32. doi: 10.1093/molbev/msi067. [DOI] [PubMed] [Google Scholar]
- 56.Aguadé M, Miyashita N, Langley CH. Polymorphism and divergence in the Mst26A male accessory gland gene region in Drosophila. Genetics. 1992;132:755–70. doi: 10.1093/genetics/132.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panhuis TM, Clark NL, Swanson WJ. Rapid evolution of reproductive proteins in abalone and Drosophila. Philos Trans R Soc Lond B Biol Sci. 2006;361:261–8. doi: 10.1098/rstb.2005.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc Natl Acad Sci U S A. 2001;98:7375–9. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–87. doi: 10.1016/0092-8674(91)90418-X. [DOI] [PubMed] [Google Scholar]
- 60.Wilson RI. Early olfactory processing in Drosophila: mechanisms and principles. Annu Rev Neurosci. 2013;36:217–41. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nef P, Heldman J, Lazard D, Margalit T, Jaye M, Hanukoglu I, Lancet D. Olfactory-specific cytochrome P-450. cDNA cloning of a novel neuroepithelial enzyme possibly involved in chemoreception. J Biol Chem. 1989;264:6780–5. doi: 10.1016/S0021-9258(18)83497-4. [DOI] [PubMed] [Google Scholar]
- 62.Ding XX, Porter TD, Peng HM, Coon MJ. cDNA and derived amino acid sequence of rabbit nasal cytochrome P450NMb (P450IIG1), a unique isozyme possibly involved in olfaction. Arch Biochem Biophys. 1991;285:120–5. doi: 10.1016/0003-9861(91)90337-I. [DOI] [PubMed] [Google Scholar]
- 63.Pelosi P, Baldaccini NE, Pisanelli AM. Identification of a specific olfactory receptor for 2-isobutyl-3-methoxypyrazine. Biochem J. 1982;201:245–8. doi: 10.1042/bj2010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–3. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- 65.Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373–91. doi: 10.1146/annurev-ento-120811-153635. [DOI] [PubMed] [Google Scholar]
- 66.Pilpel N, Nezer I, Applebaum SW, Heifetz Y. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem Mol Biol. 2008;38:320–30. doi: 10.1016/j.ibmb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Prokupek AM, Eyun SI, Ko L, Moriyama EN, Harshman LG. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster. J Evol Biol. 2010;23:1386–98. doi: 10.1111/j.1420-9101.2010.01998.x. [DOI] [PubMed] [Google Scholar]
- 68.Smadja C, Butlin RK. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity (Edinb) 2009;102:77–97. doi: 10.1038/hdy.2008.55. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Diaz C, Benton R. The joy of sex pheromones. EMBO Rep. 2013;14:874–83. doi: 10.1038/embor.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Connell RJ. Responses to pheromone blends in insect olfactory receptor neurons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;156:747–61. doi: 10.1007/BF00610828. [DOI] [Google Scholar]
- 71.Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–23. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O’Connor-Giles KM. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–35. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–8. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sebo ZL, Lee HB, Peng Y, Guo Y. A simplified and efficient germline-specific CRISPR/Cas9 system for Drosophila genomic engineering. Fly (Austin) 2013;8 doi: 10.4161/fly.26828. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu Z, Ren M, Wang Z, Zhang B, Rong YS, Jiao R, Gao G. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics. 2013;195:289–91. doi: 10.1534/genetics.113.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren X, Sun J, Housden BE, Hu Y, Roesel C, Lin S, Liu LP, Yang Z, Mao D, Sun L, et al. Optimized gene editing technology for Drosophila melanogaster using germ line-specific Cas9. Proc Natl Acad Sci U S A. 2013;110:19012–7. doi: 10.1073/pnas.1318481110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195:715–21. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O’Connor-Giles KM. Highly Specific and Efficient CRISPR/Cas9-Catalyzed Homology-Directed Repair in Drosophila. Genetics. 2014 doi: 10.1534/genetics.113.160713. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bassett AR, Tibbit C, Ponting CP, Liu JL. Mutagenesis and homologous recombination in Drosophila cell lines using CRISPR/Cas9. Biol Open. 2014;3:42–9. doi: 10.1242/bio.20137120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP. Accelerated homologous recombination and subsequent genome modification in Drosophila. Development. 2013;140:4818–25. doi: 10.1242/dev.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gratz SJ, Wildonger J, Harrison MM, O’Connor-Giles KM. CRISPR/Cas9-mediated genome engineering and the promise of designer flies on demand. Fly (Austin) 2013;7 doi: 10.4161/fly.26566. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beumer KJ, Carroll D. Targeted genome engineering techniques in Drosophila. Methods. 2014 doi: 10.1016/j.ymeth.2013.12.002. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]


