Abstract
Courtship is pivotal to successful reproduction throughout the animal kingdom. Sexual differences in the nervous system are thought to underlie courtship behavior. Male courtship behavior in Drosophila is in large part regulated by the gene fruitless (fru). fru has been reported to encode at least three putative BTB-zinc-finger transcription factors predicted to have different DNA-binding specificities. Although a large number of previous studies have demonstrated that fru plays essential roles in male courtship behavior, we know little about the function of Fru isoforms at the molecular level. Our recent study revealed that male-specific Fru isoforms are expressed in highly overlapping subsets of neurons in the male brain and ventral nerve cord. Fru isoforms play both distinct and redundant roles in male courtship behavior. Importantly, we have identified for the first time, by means of the DamID technique, direct Fru transcriptional target genes. Fru target genes overwhelmingly represent genes previously reported to be involved in the nervous system development, such as CadN, lola and pdm2. Our study provides important insight into how the sexually dimorphic neural circuits underlying reproductive behavior are established.
Keywords: sexual behavior, courtship, sexual dimorphism, fruitless, transcription factor, DamID, nervous system development
fru is a Masculinization Factor Required for Male Sexual Behavior
Male fruit flies carrying mutations in the fru locus show little, if any, courtship toward virgin females, resulting in limited reproductive success. In addition, fru mutants can also display elevated levels of courtship toward males, suggesting they have lost their ability to discriminate between the sexes.1-5 Importantly, when fru is artificially expressed in the female nervous system, these females are able to display a range of, although not all, male-typical courtship behaviors.6,7 In addition to these behavioral phenotypes, fru also plays a role in the induction of the male-specific muscle of Lawrence (MOL), formed in the fifth segment of the adult abdomen.2,8-12 fru is expressed in a subset of neurons exclusively in the male nervous system, where it has been shown to contribute to neuronal dimorphisms between the sexes.10,13,14 One of the most striking examples of this dimorphism in the brain is seen in the male-specific P1 neural cluster15 (also known as pMP-e16 or pMP417), which consists of approximately 20 posteriorly located neurons that extend their neurites toward the anterior region of the superior protocerebrum. The male-specific P1 neurons are established through the action of fru, along with another sex determination gene doublesex (dsx).15 The P1 neurons are thought to be courtship-triggering neurons, because, when these 20 neurons are ectopically induced in females, these females display male-typical courtship behaviors, even if other cells throughout the nervous system and body maintain a female identity.15 In addition, when the P1 neurons are artificially activated, males display courtship behavior even in the absence of a potential mate.18,19 Another well-characterized fru-expressing neuron is the MOL-inducing (Mind) motoneuron. The Mind neuron is a single glutamatergic motoneuron located in the abdominal ganglion. The Mind motoneuron innervates the MOL and supplies an inductive cell-non-autonomously by dynamin-dependent exocytosis during metamorphosis. The Mind motoneuron is inferred to be male-specific, and Fru may prevent this neuron from the programmed cell death, because artificial expression of either fru or a cell death inhibitor, p35, in motoneurons ectopically induces the MOL in females, which otherwise lack it.5,12
The fru gene encodes multiple putative transcription factors.2,3 The fru locus has at least four promoters, however only the proteins produced from the most distal promoter, P1, are male-specific (FruM).3,10,13,20,21 Through alternative splicing, three protein isoforms are produced from the P1 promoter: FruMA, FruMB, and FruMC 3, 11 (also known as FruAM, FruEM, and FruBM, respectively10). Each isoform has a shared N-terminal BTB dimerization domain and distinct C-terminal zinc-finger motif containing domains.2,3,10,11 What are the functional differences between the isoforms? A previous study began to unravel these diferences with the identification of a mutant specific to the FruC isoform (fru∆C).11 This study showed that the formation of the MOL depends solely on the expression of FruMC in the motoneurons. FruMC was also shown to play roles in male sexual behavior: males lacking the FruMC isoform show decreased mating success and fertility. Isoform complexity was further expanded by the analysis of male-specific serotonergic neurons in the abdominal ganglion, where both FruMB and FruMC, but not FruMA, were shown to be required for their sexually dimorphic innervations to the male reproductive system.11,22,23
Respective Roles of Fru Isoforms in Male Sexual Behavior
Why does fru encode multiple isoform variants and how do they individually contribute to the observed behavioral phenotypes in males? Other closely related BTB-zinc-finger genes also encode multiple isoforms, which often vary in the developmental and tissue specificity of their expression, suggesting variation in expression is fundamental to their individual functions. To determine if FruM isoforms display unique expression patterns in the central nervous system (CNS), contributing to their individual functions, we examined the distribution of the three FruM isoforms in the CNS and found, perhaps surprisingly, that FruM isoforms have highly overlapping patterns, with only the FruA distribution being in a much smaller subset of FruM neurons24 (see schematic in Figure 1). These observations are consistent with a recently published complementary study that examined the distributions of the respective Fru isoforms in the CNS,25 however this study found broader expression of the FruA isoform (a difference that could be a result of the unique antibodies used between studies, compounded by the apparent low level expression of FruA observed in both studies). Therefore broad differences in expression do not underlie FruM isoform-specific functions.
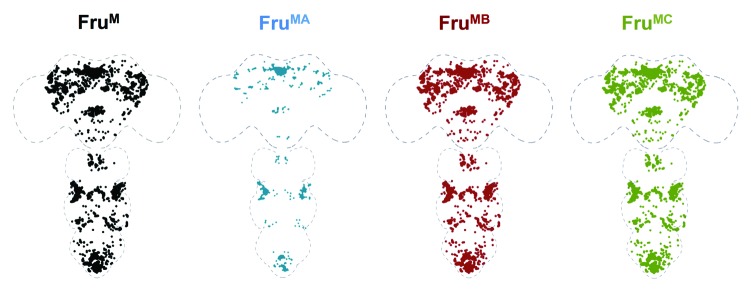
Figure 1. Schematic drawings showing the distributions of FruM isoforms in the CNS. All three FruM isoform distributions as detected with an anti-FruM antibody are shown in black. The distributions of FruMA, FruMB and FruMC as detected with isoform specific antibodies are shown in blue, red and green, respectively. FruM isoforms show a highly overlapping expression pattern.
To directly compare the roles of all Fru male-specific isoforms, we set out to generate a full complement of FruM isoform-specific mutants for behavioral analyses. We established strains of flies carrying mutations in either FruA- or FruB-encoding exons, generating the novel mutants fru∆A and fru∆B, respectively. We confirmed that fru∆A and fru∆B mutants specifically lack the FruA or FruB isoforms, respectively, in the adult CNS based on immunohistochemical staining with isoform-specific Fru antibodies. Consistent with previous studies,5,10,12 we found that fru∆C mutant males lack the MOL, whereas neither fru∆A nor fru∆B mutant males do, again confirming that only the FruMC isoform is indispensable for the MOL formation.
Isoform-specific fru mutant males were examined in detail for their sexual behavior (summarized in Table 1). First, we performed single-pair mating assays, in which a male and a virgin female are put into the small observation chamber and the male’s mating performance toward the female is recorded and analyzed afterwards. Under these experimental conditions, fru∆B mutant males less vigorously court females and show significantly decreased copulation success, as compared with control or other isoform-specific mutants. Even those that successfully copulate with females, take a significantly longer time getting there. fru∆C mutant males, in contrast, do not show any defects in courtship latency or courtship index. However, they never manage to copulate within the one-hour observation period, which may be, at least in part, due to significantly reduced levels of unilateral wing extension used to generate courtship song. We additionally conducted a behavioral assay in which multiple males are grouped together in the same observation chamber. It was previously reported that, when fru mutant males are grouped, they form so-called “courtship chains,” in which a courting male is courted by another male which in turn is courted by another male, resulting in the long chain-like formations.2 In our assays, courtship chains were observed in both fru∆B and fru∆C mutant groups, suggesting both mutants have, at least partially, lost their ability to discriminate between the sexes. Interestingly, no detectable behavioral defects were found in our fru∆A mutant males under our experimental conditions, although another recently published study using an independently generated mutation in the FruA-encoding exon reported that FruA appears to play a role in copulation success (Table 1).25
Table 1. Summary of the behavioral profiles of isoform-specific fru mutants.
| Neville et al., 2014 | von Philipsborn et al., 2014 | ||
|---|---|---|---|
| Courtship latency | A | Normal | |
| B | Increased * | ||
| C | Normal | ||
| Courtship index | A | Normal | |
| B | Decreased *** | ||
| C | Normal | ||
| Wing extension index | A | Normal | |
| B | Normal | ||
| C | Decreased *** | ||
| Copulation success in a short experimental period | A | Normal | Decreased *** |
| B | Decreased *** | Decreased *** | |
| C | Lost **** | Decreased *** | |
| Copulation latency | A | Normal | |
| B | Increased ** | ||
| C | Not applicable | ||
| Fertility | A | Normal | Normal |
| B | Normal | Decreased | |
| C | Decreased *** | Decreased | |
| Chaining index | A | None | |
| B | Increased * | ||
| C | Increased *** | ||
| Interpulse interval | A | Normal | Increased *** |
| B | Increased ** | Increased *** | |
| C | Increased *** | Increased *** | |
| Pulse frequency | A | Normal | Decreased *** |
| B | Normal | Normal | |
| C | Increased * | Increased ** | |
| Sine song | A | Normal | Normal |
| B | Normal | Normal | |
| C | Lost **** | Lost **** | |
| Sine frequency | A | Normal | Increased *** |
| B | Normal | Normal | |
| C | Normal | Normal |
During courtship, males extend their wings unilaterally to generate species-specific courtship song.26 Courtship song consists of two discrete elements: alternating continuous oscillations called “sine song” and trains of pulses called “pulse song.” The time between pulses (interpulse interval or IPI) varies among different species. The IPI of D. melanogaster is approximately 34 ms on average,27 and that of a closely related species, D. simulans, is approximately 48 ms.28 Courtship song contributes to species recognition and renders conspecific females sexually receptive.27,29-32 We recorded and analyzed the sine and pulse songs generated by our isoform-specific fru mutants. fru∆B males show a slight but significantly longer IPI of nearly 40 ms, while fru∆C males showed an even longer IPI of 45 ms, which is much closer to the IPI of D. simulans than that of D. melanogaster.28 Another striking song deficit observed in fru∆C males is the consistent and complete absence of sine song. Although we did not find any abnormalities in courtship song generated by males lacking FruMA, von Philipsborn et al., 201425 using their fruA mutant, reported mild song defects including a longer IPI (Table 1). The differences in the behavioral profiles of the isoform-specific fru mutants between these studies may be at least in part due to the different techniques used to establish the mutant strains, the differing allelic combinations used, along with differing experimental conditions. Based on these observations, courtship song appears to be specified in large part by the FruMC isoform.
Collectively our phenotypic analyses using isoform-specific fru mutants show that the overall performance of courtship behavior can occur in the absence of any one isoform, although some phenotypes, such as the production of sine song, clearly depend on only one isoform. Clearly the FruMB and FruMC isoforms are the major players in most aspects of male sexual behavior, while the FruMA isoform plays a minor role, perhaps functioning more specifically in subtle phenotypes associated with the broad range of sensory inputs a male experiences in a natural setting. Interestingly, these findings may be supported by a previous study examining evolutionary changes and conservation of the fru locus33 (Fig. 2). In D. melanogaster, fru has three C2H2 zinc-finger motif-encoding exons: A, B, and C; however in some non-drosophilid holometabolous insect species, two other exons are known to exist in the fru locus: F and G. Malaria mosquitoes, Anopheles gambiae, have the F exon in the fru locus, in addition to the A, B and C exons. In silkworm moths, Bombyx mori, the fru locus carries the G exon but lacks the A exon. In parasitoid wasps, Nasonia vitripennis, all five exons (A, B, C, F, and G) are included in the fru locus. Although the presence or absence of the respective C2H2 zinc-finger motif-encoding exons in the fru locus varies dramatically between species, only exons B and C are conserved in all species shown in Figure 2. Based on these observations, isoforms FruMB and FruMC likely play essential roles in various insect species, which is consistent with our findings that these isoforms are the major players in male-specific behaviors. The appearance and disappearance of fru C2H2 zinc-finger domains through presumably a combination of exon duplication and/or loss, enables a single gene to diversify its functions, while ensuring its essential functions are maintained. Interestingly, a recent finding by Parker et al., 201434 showed that the fruA C2H2 zinc-finger containing exon is under strong positive selection within the Drosophila species, suggesting changes in this exon may contribute to speciation, while the other zinc-finger exons fruB and fruC are highly conserved.
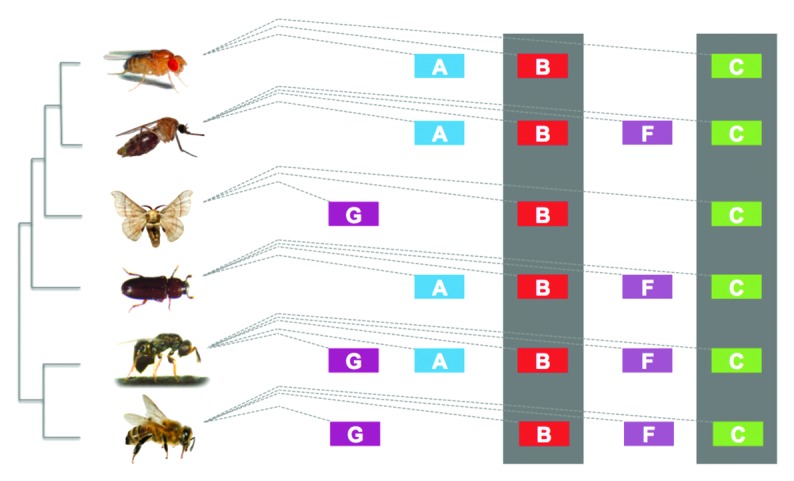
Figure 2. Comparison of Fru isoform-specific C2H2 zinc-finger containing exons between holometabolous insect species. From top to bottom, the Fru isoforms of D. melanogaster, A. gambiae, B. mori, C. castaneum, N. vitripennis and A. melifera are shown, respectively. In A. melifera, the A exon is either highly evolved or represents a novel exon. The conserved exons B and C are highlighted in gray. Figure modified from Bertossa et al., 2009.33 ©Photographer name/Dreamstime.com for the images of D. melanogaster, A. gambiae, B. mori, C. castaneum and A. melifera. Image of N. vitripennis courtesy of Dr. Oliver Niehuis, University of Bonn.
fru Regulates the Transcription of Genes Required for the Development of the Nervous System, Thereby Specifying Sexually Dimorphic Neural Circuits
Although fru has long been postulated to encode transcription factors, its direct target genes were yet to be identified. Our recent study took advantage of the DNA adenine methyltransferase identification (DamID) technique to identify genes directly regulated by Fru.35 We generated functional Dam-fru fusion constructs coding for all three FruM isoforms, as well as a control with a mutation in C2H2 zinc-finger domain of FruMB rendering it unable to bind DNA. These Dam-fru fusions were expressed in flies and the CNSs were subsequently dissected out and then genomic DNA was isolated for analyses. As a result of the DamID experiments, we were able to show for the first time FruM isoform-specific interactions with the genome throughout development in the CNS. Interestingly, we found that many of the same genes were targeted by all three of the FruM isoforms throughout development, a highly significant proportion of which have previously been reported to play important roles in the development of the nervous system. This suggests potential cooperativity and/or redundancy in the targeting of these loci by multiple FruM isoforms, this complements our evolutionary understanding of Fru isoforms as well as the behavioral analysis of isoform-specific mutants.
We next looked for DNA motifs that were enriched in our FruM-genomic binding data and found that each isoform was associated with distinct motifs throughout development, suggesting unique DNA binding specificities. This complements the finding of Dalton et al., 201336 who used SELEX to show that Fru isoform-specific zinc-fingers indeed confer different DNA-binding specificities in vitro.37 We found that the FruMB-enriched DNA motif was the most robust and consistent throughout development; in addition it closely resembles the in vitro binding site identified by Dalton et al., 2013.36 As FruM is male-specific, we next tested whether FruMB-bound genomic regions containing the putative FruMB-binding sites exhibit sexually dimorphic expression patterns in fru-expressing neurons. To do this, we made use of available FlyLight Gal4 lines38,39 carrying the genomic fragments of interest, in combination with fruFLP to restrict GFP reporter expression (UAS > stop > mCD8::GFP) to fru-expressing neurons.17 Of the 14 Gal4 lines where we observed expression in fru-expressing neurons, 10 showed sexually dimorphic expression patterns. Some lines, such as lola-Gal4 (GMR44C03) and stan-Gal4 (GMR32B11), show more intense expression in males than in females, and others, such as pdm2-Gal4 (GMR11G05) and Abl-Gal4 (GMR67B05), show female-biased expressions.
When examining the function of the genes associated with our identified FruMB motif we found a highly significant enrichment in genes associated with neuronal projection morphogenesis. Comparing these genes in relation to genes shown to be over- and under-expressed when the FruMB isoform is overexpressed in fruP1-GAL4 neurons,36,40 we found that the majority of the FruMB motif-enriched genes whose expression changes when FruMB is overexpressed appear to be directly upregulated by FruMB (Fig. 3). Interestingly, a functional gene ontology analysis showed that genes directly involved in the sculpting of the nervous system appear to be specifically upregulated by FruMB, including a number of key cell surface molecules, such as Dscam,41 CadN,42 Sema-1a,43 and Fas3.44 These results suggest that these cell surface molecules are likely indispensable for the establishment of the sexually dimorphic nervous system underlying sexual differences in behavior. Indeed, we found that a genomic enhancer associated with CadN (GMR32D06) shows sexually dimorphic expression patterns in the abdominal ganglion. In addition, when CadN expression was abolished in fruGAL4 neurons,45 males display no courtship behavior toward females.24 Cell surface molecules act as guidance cues in the nervous system, mediating the intricate connections between neuronal processes during development. Our data suggest that Fru acts to sculpt a sexually dimorphic nervous system, at least in part through the direct targeting of these cell surface molecules, where it likely changes the ‘cocktail’ of these molecules in a cell (or cluster)-specific manner, leading to the appropriate neuronal connections in males.
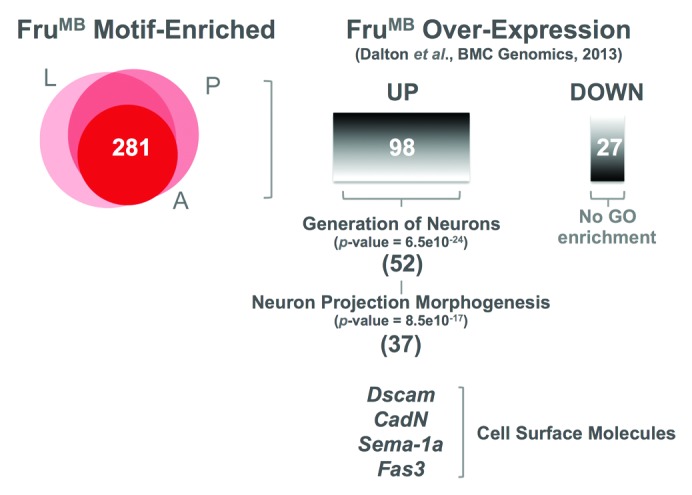
Figure 3. FruMB directly regulates key genes involved in sculpting the nervous system. FruMB-motif enriched genes found in the larvae (L), pupae (P) and adult (A) CNS were examined for expression changes in response to FruMB overexpression in fruP1-GAL4 neurons.36 A global gene ontological (GO) enrichment analysis of biological functions of up and downregulated genes revealed that genes known to play a role in the generation of neurons (GO:0048699) and more specifically in neuron projection morphogenesis (GO:0048812) were significantly enriched in those upregulated by FruMB overexpression, including key cell surface molecules.
Future Investigation of Individual Fru Isoforms and Target Genes
Our recent study demonstrates that Fru isoforms play both specific and redundant roles in establishing the sexually dimorphic nervous system underlying male sexual behavior.24 However, directly connecting Fru isoform function with the regulation of the specific target genes required for the sexual differentiation of certain subsets of neurons remains an open question. Future work will focus on first refining our studies of Fru-DNA interactions, unequivocally establishing binding-site specificity in vivo, in addition to examining the temporal dynamics of FruM binding at specific loci.
We would like to correlate occupancy with transcriptional control, however as FruM clearly plays key developmental roles in a large number of cell clusters throughout the nervous system, we will focus on small subsets of fru-expressing cells, establishing the specific developmental role FruM plays in establishing a transcriptional regulatory code leading to a male-specific nervous system.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work discussed here was supported in part by grants from the Wellcome Trust to S.F.G. (WT085521MA and WT082987MF) and the Natural Environment Research Council to S.F.G. (NE/J023647/1).
References
- 1.Hall JC. Courtship among males due to a male-sterile mutation in Drosophila melanogaster. Behav Genet. 1978;8:125–41. doi: 10.1007/BF01066870. [DOI] [PubMed] [Google Scholar]
- 2.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A. 1996;93:9687–92. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–89. doi: 10.1016/S0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 4.Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, Song HJ, Gailey DA, Morales A, Hall JC, Baker BS, et al. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics. 2001;158:1569–95. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billeter J-C, Rideout EJ, Dornan AJ, Goodwin SF. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr Biol. 2006;16:R766–76. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–94. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Rideout EJ, Billeter J-C, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol. 2007;17:1473–8. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence PA, Johnston P. The genetic specification of pattern in a Drosophila muscle. Cell. 1984;36:775–82. doi: 10.1016/0092-8674(84)90357-X. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence PA, Johnston P. The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell. 1986;45:505–13. doi: 10.1016/0092-8674(86)90282-5. [DOI] [PubMed] [Google Scholar]
- 10.Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat Cell Biol. 2000;2:500–6. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- 11.Billeter J-C, Villella A, Allendorfer JB, Dornan AJ, Richardson M, Gailey DA, Goodwin SF. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr Biol. 2006;16:1063–76. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Nojima T, Kimura K, Koganezawa M, Yamamoto D. Neuronal synaptic outputs determine the sexual fate of postsynaptic targets. Curr Biol. 2010;20:836–40. doi: 10.1016/j.cub.2010.02.064. [DOI] [PubMed] [Google Scholar]
- 13.Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J Neurobiol. 2000;43:404–26. doi: 10.1002/1097-4695(20000615)43:4<404::AID-NEU8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–33. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–69. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–14. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 19.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–22. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Heinrichs V, Ryner LC, Baker BS. Regulation of sex-specific selection of fruitless 5′ splice sites by transformer and transformer-2. Mol Cell Biol. 1998;18:450–8. doi: 10.1128/mcb.18.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin SF, Taylor BJ, Villella A, Foss M, Ryner LC, Baker BS, Hall JC. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics. 2000;154:725–45. doi: 10.1093/genetics/154.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee G, Hall JC. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. J Neurosci. 2001;21:513–26. doi: 10.1523/JNEUROSCI.21-02-00513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee G, Villella A, Taylor BJ, Hall JC. New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J Neurobiol. 2001;47:121–49. doi: 10.1002/neu.1021. [DOI] [PubMed] [Google Scholar]
- 24.Neville MC, Nojima T, Ashley E, Parker DJ, Walker J, Southall T, Van de Sande B, Marques AC, Fischer B, Brand AH, et al. Male-specific fruitless isoforms target neurodevelopmental genes to specify a sexually dimorphic nervous system. Curr Biol. 2014;24:229–41. doi: 10.1016/j.cub.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Philipsborn AC, Jörchel S, Tirian L, Demir E, Morita T, Stern DL, Dickson BJ. Cellular and behavioral functions of fruitless isoforms in Drosophila courtship. Curr Biol. 2014;24:242–51. doi: 10.1016/j.cub.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennet-Clark HC, Ewing AW. Stimuli provided by Courtship of Male Drosophila melanogaster. Nature. 1967;215:669–71. doi: 10.1038/215669a0. [DOI] [Google Scholar]
- 27.von Schilcher F. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Anim Behav. 1976;24:622–5. doi: 10.1016/S0003-3472(76)80076-0. [DOI] [Google Scholar]
- 28.Ewing AW, Bennet-Clark HC. The courtship songs of Drosophila. Behaviour. 1968;31:288–301. doi: 10.1163/156853968X00298. [DOI] [Google Scholar]
- 29.Kyriacou CP, Hall JC. Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male’s courtship song. Proc Natl Acad Sci U S A. 1980;77:6729–33. doi: 10.1073/pnas.77.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni SJ, Hall JC. Behavioral and cytogenetic analysis of the cacophony courtship song mutant and interacting genetic variants in Drosophila melanogaster. Genetics. 1987;115:461–75. doi: 10.1093/genetics/115.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kulkarni SJ, Steinlauf AF, Hall JC. The dissonance mutant of courtship song in Drosophila melanogaster: isolation, behavior and cytogenetics. Genetics. 1988;118:267–85. doi: 10.1093/genetics/118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yokokura T, Ueda R, Yamamoto D. Phenotypic and molecular characterization of croaker, a new mating behavior mutant of Drosophila melanogaster. Jpn J Genet. 1995;70:103–17. doi: 10.1266/jjg.70.103. [DOI] [PubMed] [Google Scholar]
- 33.Bertossa RC, van de Zande L, Beukeboom LW. The Fruitless gene in Nasonia displays complex sex-specific splicing and contains new zinc finger domains. Mol Biol Evol. 2009;26:1557–69. doi: 10.1093/molbev/msp067. [DOI] [PubMed] [Google Scholar]
- 34.Parker DJ, Gardiner A, Neville MC, Ritchie MG, Goodwin SF. The evolution of novelty in conserved genes; evidence of positive selection in the Drosophila fruitless gene is localised to alternatively spliced exons. Heredity (Edinb) 2014;112:300–6. doi: 10.1038/hdy.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol. 2000;18:424–8. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 36.Dalton JE, Fear JM, Knott S, Baker BS, McIntyre LM, Arbeitman MN. Male-specific Fruitless isoforms have different regulatory roles conferred by distinct zinc finger DNA binding domains. BMC Genomics. 2013;14:659. doi: 10.1186/1471-2164-14-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klug SJ, Famulok M. All you wanted to know about SELEX. Mol Biol Rep. 1994;20:97–107. doi: 10.1007/BF00996358. [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–20. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 41.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–84. doi: 10.1016/S0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 42.Hummel T, Zipursky SL. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron. 2004;42:77–88. doi: 10.1016/S0896-6273(04)00158-8. [DOI] [PubMed] [Google Scholar]
- 43.Yu HH, Huang AS, Kolodkin AL. Semaphorin-1a acts in concert with the cell adhesion molecules fasciclin II and connectin to regulate axon fasciculation in Drosophila. Genetics. 2000;156:723–31. doi: 10.1093/genetics/156.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–88. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- 45.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]


