Abstract
Autophagy is the main cellular catabolic process responsible for degrading organelles and large protein aggregates. It is initiated by the formation of a unique membrane structure, the phagophore, which engulfs part of the cytoplasm and forms a double-membrane vesicle termed the autophagosome. Fusion of the outer autophagosomal membrane with the lysosome and degradation of the inner membrane contents complete the process. The extent of autophagy must be tightly regulated to avoid destruction of proteins and organelles essential for cell survival. Autophagic activity is thus regulated by external and internal cues, which initiate the formation of well-defined autophagy-related protein complexes that mediate autophagosome formation and selective cargo recruitment into these organelles. Autophagosome formation and the signaling pathways that regulate it have recently attracted substantial attention. In this review, we analyze the different signaling pathways that regulate autophagy and discuss recent progress in our understanding of autophagosome biogenesis.
Keywords: Atgs, autophagosome biogenesis, autophagy, mTOR, signaling
Introduction
Autophagy is a catabolic process by which proteins and organelles are delivered to the lysosome for degradation. As a self-degrading process that is conserved from yeast to man, autophagy is well established as a survival mechanism that maintains cellular homeostasis under normal growth conditions and enables adaptation under stress 1. It has also been implicated in disease, development, and cell differentiation 2–4. Although beneficial under normal conditions, autophagy can be detrimental in diseases such as cancer and neurodegenerative disorders. Therefore, cells have developed control mechanisms that tightly regulate their autophagic activity. More than 30 proteins have been identified as related to autophagy (Atgs), most of them directly associated with autophagosome biogenesis 5,6. According to the current view, autophagy is a progressive process initiated by the elongation of a membrane to form a cup-shaped phagophore into which autophagic cargo is sequestered, a process that initially seemed random but now is mostly regarded as selective. The autophagic membrane is further expanded to its final size and, once sealed, results in a mature double-membrane autophagosome, the outer membrane of which subsequently fuses with the lysosome to create an autolysosome. The autophagosomal content is then degraded and recycled.
This review covers recent progress made in understanding the early stages of autophagy. The external signals and environmental conditions that regulate autophagy—such as growth factors and nutrient deprivation—and the signal transduction pathways involved in such regulation will be discussed, as well as changes in intracellular homeostasis that trigger the autophagic process, such as oxidative stress and internal energy levels. Finally, we will analyze new mechanistic insights into autophagosome biogenesis.
Regulation of autophagy
Eukaryotes have developed signaling networks that control transcription, translation, and protein modification to adapt to changing environmental conditions. At times of shortage, cells need to save energy and nutrients by maintaining basal and essential activities. As part of the cellular response to such conditions, autophagy—a major cellular catabolic process—is subjected to tight regulation by a network of canonical and unique signaling cascades. It is therefore important to examine not only cues originating within the cells, but also signaling initiated in response to external changes (Figs 1 and 2). Autophagy is known to be mainly under the control of the key regulator of cell homeostasis, the Ser/Thr kinase TOR (target of rapamycin), in yeast, or mTOR in mammals 7. TOR is found in two distinct protein complexes, TORC1 or TORC2 8,9. Although both TOR complexes regulate cell metabolism, only TORC1 is directly linked to the regulation of autophagy.
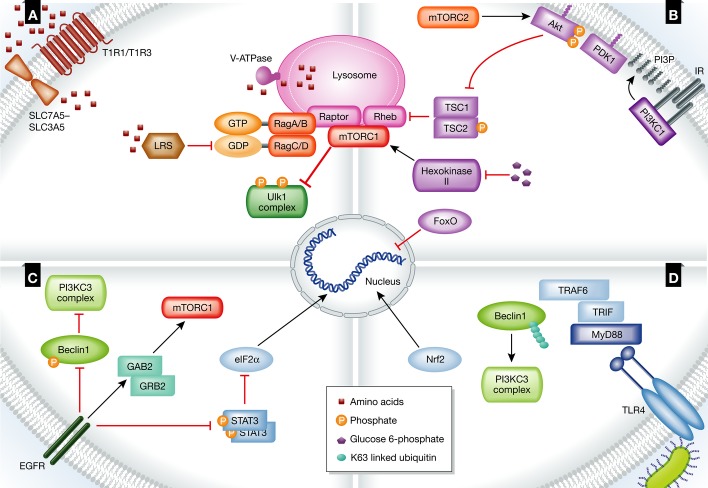
Figure 1. Regulation of autophagy by extracellular cues.
(A) Amino acids are key regulators of autophagy. When they are in excess, mTORC1 is targeted to the lysosomal membrane, where it is activated by Rheb and inhibits autophagy through phosphorylation of Ulk1 complex subunits. (B) Binding of insulin to its receptor (IR) activates mTOR via the PI3KC1/Akt/TSC pathway, inhibiting autophagy. The expression of autophagy-related proteins is inhibited after the inhibition of FoxO transcription factors by Akt. Glucose 6-phosphate inhibits the activity of hexokinase-II, an mTOR activator, inhibiting autophagy. (C) Activation of EGFR by its ligand inhibits autophagy directly by the phosphorylation of Beclin1 or indirectly via GRB2 and GAB2, as well as via the phosphorylation of STAT3, which releases eIF2α to induce the expression of autophagy-related proteins. (D) TLR4 is activated upon binding of LPS, leading to the recruitment of adaptor proteins to the plasma membrane. As a consequence, TRAF6 is recruited, resulting in the Lys63-linked ubiquitination of Beclin1, allowing it to bind PI3KC3 and induce autophagy. Nrf2 is activated, up-regulating the expression of p62.
See Glossary for definitions and the text for details.
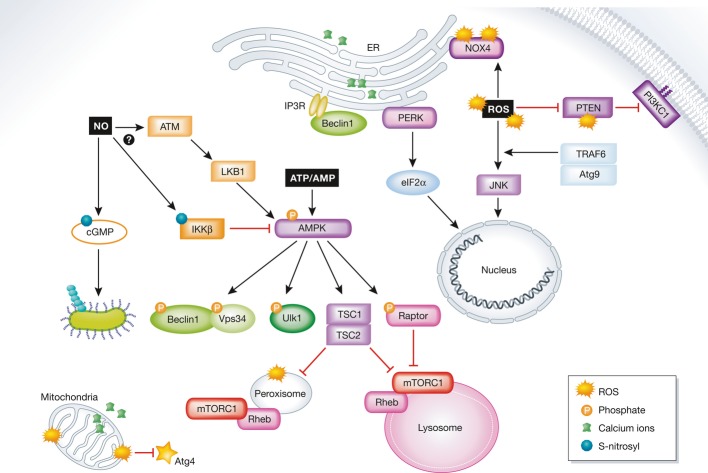
Figure 2. Regulation of autophagy by intracellular cues.
Internal cues regulate autophagy on different levels from many intracellular locations. The activity of mTORC1 is regulated at the lysosome and the peroxisome through AMPK. Active AMPK indirectly inhibits autophagy by activating the TSC1/2 complex and via inhibition of raptor by phosphorylation, both of which lead to the inhibition of mTORC1. Autophagy is inhibited directly by Ulk1, Vps34, and Beclin1 phosphorylation. ROS molecules activate autophagy at the plasma membrane, the ER, and mitochondria, as well as by up-regulating the expression of autophagy-related proteins. Ca2+ signaling is mediated from its intracellular storages in the mitochondria and the ER. The regulation of autophagy through AMPK induced by NO remains poorly understood. NO regulates mitophagy through cGMP.
See Glossary for definitions and the text for details.
Extracellular cues
Amino acid starvation
The most extensively characterized inducer of autophagy is amino acid deprivation (Fig 1A). The absence of specific amino acids such as leucine and glutamine strongly induces autophagy, whereas others have a lesser effect. Several mechanisms have been proposed as part of the amino acid sensing system, all of which finally mediate autophagic activity through TOR. A decline in amino acid content is initially sensed at the plasma membrane, and, in yeast, amino acid transporters were suggested to sense extracellular amino acid concentration to regulate TOR activity 10. However, most studies in yeast induce autophagy by nitrogen starvation, and it has been recently demonstrated that sensing of nitrogen differs from that of amino acids 11. A reduction of extracellular amino acids levels was shown to induce autophagy in a GCN2-GCN4-dependent manner, whereas how the lack of external nitrogen is sensed remains unknown.
In mammalian cells, the G protein-coupled receptors (GPCRs) T1R1/T1R3 have been implicated in the extracellular sensing of amino acid availability, which leads to autophagy induction mediated by a decrease in mTOR activity 12. Leucyl-tRNA synthetase (LRS) was recently shown to sense increases in intracellular leucine levels and to mediate TOR activation by slightly different mechanisms in yeast and mammalian cells 13,14. Moreover, leucine also regulates mTOR activity through glutamate dehydrogenase (GLUD) 15. In addition to its leucine-mediated regulation, mTOR is regulated by α-ketoglutarate, which is produced during glutamine metabolism 16. Notably, glutamine plays a key role in the internalization of essential amino acids by the bidirectional transporter SLC7A5-SLC3A5 17.
Upon starvation, the low amino acid concentration is sensed by Rag GTPases on the lysosomal surface 18,19, which then form inactive heterodimers of RagA/RagB bound to RagC/RagD 19. Under amino acid-rich conditions, the Rag complex, together with a multi-protein signaling complex known as the Ragulator and the vacuolar protein pump v-ATPase, targets mTOR to the lysosome for its activation 18,20. It is tempting to speculate that the localization of active mTOR on the lysosomal membrane allows it to be directly regulated by free amino acids produced by protein degradation within the lysosome. Indeed, the v-ATPase was suggested to serve as a link between mTOR and the amino acids generated by the lysosome 20. Active mTORC1 is localized mainly on the lysosomal membrane 21,22; under amino acid deprivation, inactivation of the Rag complex causes its detachment from raptor (part of mTORC1) and separation of mTOR from the lysosome and its activator Rheb, resulting in autophagic stimulation 23. An important connection between the Rag complex and tumorigenesis was recently suggested, as this complex is negatively regulated by the tumor suppressor SH3BP4 24. Sabatini and co-workers also identified a protein complex termed GATOR, which is comprised of the subunits GATOR-1 and GATOR-2. GATOR-1 is a GAP for RagA/RagB, mediating mTORC1 sensitivity to amino acids 25. Importantly, mutations in the GAP activity of GATOR-1 are associated with human cancer.
A more direct mechanistic connection of TOR to autophagy is illustrated by its ability to phosphorylate the Atg1 complex (ULK1 complex in mammals) 5. In yeast, TOR binds and phosphorylates Atg13, detaching it from the Atg1 complex, whereas in mammals, it regulates the constantly assembled Ulk1 complex—Atg13, ULK1, and FIP200—through direct binding and phosphorylation of Atg13 and Ulk1 26–29. Under unfavorable conditions, the Atg1 complex in yeast is activated by the release of Atg13 from inactive TOR, whereas in mammals, the whole Ulk1 complex is activated by its detachment from mTOR 30. Ulk1 can be then auto-phosphorylated and phosphorylate Atg13 and FIP200, triggering complex activity in the initial steps of autophagosome biogenesis 29.
An important link exists between mTORC1, the Rag GTPase complex, and the scaffold protein p62, which is also an autophagic cargo receptor 31. p62 recruits TRAF6, an E3 ubiquitin ligase that is essential for activation of mTOR through Lys63 ubiquitination 32. It would therefore be interesting to determine whether p62 serves as a molecular switch modulating mTOR activity during changes in growth conditions. If this is indeed the case, it could explain why p62 mediates mTOR activity and autophagic inhibition under normal conditions, yet sequesters cargo for lysosomal degradation upon autophagic induction. Phosphorylation of Bcl2 by JNK-1 was recently shown to induce its dissociation from Beclin1, enabling it to form the PI3KC3 essential for autophagosome formation 33,34, thereby providing another important insight into the mechanism by which starvation induces autophagy.
Insulin and glucose starvation
At high glucose concentrations, autophagy is down-regulated through insulin receptor signaling 35. Binding of insulin to its receptor activates PI3KC1 to generate PtdIns3P at the plasma membrane 36, thereby recruiting and activating both PDK1 and Akt/PKB (Fig 1B). Upon insulin signaling, Akt is activated by two pathways, PDK1 and mTORC2, thus mediating an indirect regulation of the non-autophagic mTOR complex on autophagy 37,38. Akt activation leads to the inhibition of TSC1/2, an mTOR inhibitor, resulting in the inhibition of autophagy 8. Interestingly, loss of the TSC1/2 complex induces constitutive mTOR activity at the plasma membrane due to the enhanced activity of RalA/RalB through the exocyst complex 39. The long-term regulation of autophagy by Akt includes down-regulation of the expression of many autophagic proteins through a process mediated by the FoxO transcription factor family 40–42.
Autophagy was recently directly linked to glucose deprivation in cardiomyocytes 43. In low glucose conditions, hexokinase-II—the first enzyme in the glycolysis pathway—was postulated to directly bind and thereby inhibit mTOR. This binding would be inhibited by glucose 6-phosphate, the substrate of hexokinase-II (Fig 1B). Furthermore, glucose deprivation in neonatal mice was shown to induce the lysosomal targeting of mTOR through the Rag GTPase pathway, previously implicated only in amino acid starvation 44. This issue has not yet been fully resolved, however, as another study in mice indicated that long periods of glucose deprivation do not inhibit autophagy 45.
Epidermal growth factor
Autophagy plays a crucial metabolic role, especially when supplies are limited, implying that it is tightly linked to growth factors. Recent studies have indeed demonstrated that the EGFR system inhibits autophagy, either indirectly through GRB2 and GAB2 46, or directly by phosphorylating Beclin1, prompting its dimerization to prevent its activity 47 (Fig 1C). Alternatively, autophagy can be induced through EGF-dependent phosphorylation and dimerization of STAT3, releasing its binding of PKR, the catalytic domain of eIF2α 48,49. This up-regulates the transcription and translation of the core autophagic proteins LC3 and Atg5 50. Interestingly, expression of the constitutively active EGFR mutant EGFRvIII results in extensive autophagic activity, promoting cell survival in tumor cells especially under stressful conditions, such as uncontrolled Ras signaling and oxidative stress 51. It would thus be interesting to further elucidate the molecular switches dictating the regulation of autophagy by the EGFR family upon changes in growth conditions. Accordingly, serum starvation was recently reported to induce autophagy through GSK3, which phosphorylates and activates the acetyltransferase TIP60, leading to acetylation and activation of Ulk1 52.
Toll-like receptors
Autophagy has been widely implicated in immunity through its support of immune-cell activity. The toll-like receptor (TLR) family, an essential part of the innate immune system, has been implicated in the regulation of autophagy, and the mechanism governing this process was recently elucidated 53 (Fig 1D). Two adaptor proteins, MyD88 and TRIF 54, recruit the E3 ligase TRAF6 to the autophagic regulator Beclin1 via its two TRAF6-binding domains 55. Beclin1 is polyubiquitinated with a Lys63-linked ubiquitin chain on Lys117, which is located within its BH3 domain. This induces Beclin1 detachment from Bcl2 to induce autophagy in a process regulated by the deubiquitinating enzyme A20 55. Beclin1 is then free to form, together with Atg14, Vps34, and Vps15, the PI3KC3 complex essential in the initial stages of autophagosome biogenesis 5. Moreover, the up-regulation of p62 expression by the TLR4 pathway, which is mediated by the transcription factor Nrf2, further extends the induction of autophagy in a MyD88- and p38-dependent manner 56. Activation of TLR4 thus leads to lysosomal elimination of invading bacteria, a process termed xenophagy, which is beyond the scope of the present review 57.
Cytokines, a large group of immune signaling molecules that are secreted to promote differentiation, recruitment, and activation of immune cells, have also been implicated in the regulation of autophagy. The proinflammatory cytokines IL-1β, IFN-γ, and TNF were found to induce autophagy to protect macrophages from bacterial infection 58. In contrast, the cytokines IL-4, IL-13, IL-10, and IL-6 signal for autophagic inhibition, each via a different signaling pathway. IL-10 inhibits autophagy through the Akt signaling pathway, whereas IL-4 and IL-13 are inhibitory only when autophagy is induced by starvation. IL-6 inhibits autophagy by down-regulating the expression of autophagic proteins mediated by STAT3 regulation 58.
Intracellular cues
Autophagy is the main intracellular process responsible for the clearance of defective organelles and protein aggregates caused by aging, cellular malfunction, or both. This is particularly important in long-lived cells such as neurons. The internal state of the cell is monitored to maintain homeostasis under different growth conditions (Fig 2).
Energy level
The energy status of the cell is typically sensed by the ATP/AMP ratio and regulated by AMPK binding 59. When AMP is in excess, indicating low energy levels, it binds AMPK, leading to phosphorylation and activation by LKB1 60. This consequently activates autophagy via two main signaling pathways: mTOR inhibition through the TSC1/2 complex 61,62 or the phosphorylation of raptor and binding to 14-3-3 proteins, which also inhibits mTOR 63. An alternative mechanism was recently described whereby upon glucose deprivation, AMPK directly phosphorylates Ulk1 64, Vps34, and Beclin1 (members of the PI3KC3 complex), leading to PI3KC3 stabilization and activation of autophagy, which ensures cell survival 65.
Oxidative cues
Intracellular pathways lead to the production of ROS, which serve as signaling molecules at low concentrations yet are highly hazardous and must be eliminated. The main ROS molecules that participate in autophagic signaling are H2O2 and O2−. ROS regulate autophagy at various intracellular locations. At the plasma membrane, H2O2 directly modifies and inactivates PTEN, which inhibits PI3KC1 activity, thus eventually activating mTOR 66. ROS have also been implicated in the activation of JNK, in a process regulated through the interaction of Atg9 with TRAF6 67 that induces autophagy by up-regulating the expression of different Atg proteins 68–70. In the ER of cardiomyocytes, ROS levels are intentionally up-regulated by NOX4 upon glucose deprivation to induce autophagy through the PERK and eIF2α pathway, thus preventing cell death 71. TSC1 and TSC2 are targeted to the peroxisomal membrane by pex19 and pex5, respectively, where they inhibit mTOR activity by hydrolyzing GTP-Rheb upon exposure to ROS 72, suggesting that peroxisomes may induce autophagy in response to oxidative stress.
In mammalian systems, ROS production at the mitochondria is elevated upon starvation and was found to directly regulate the activity of Atg4, the priming and delipidating enzyme of Atg8 73. Upon oxidation, Atg4 is transiently and locally inactivated to stabilize Atg8s in their lipidated active form. Moreover, ROS production by mitochondria could serve as a signal for their elimination by mitophagy 74. In this regard, GLUD activity at the mitochondria inhibits autophagy, probably through the generation of NADPH, which prevents ROS accumulation 15.
Nitric oxide
NO is produced in cells by NOS and acts as a signaling molecule in different immune response pathways and the cardiovascular system, among other contexts 75. NO was initially shown to inhibit autophagosome biogenesis in HeLa cells by inducing mTOR activation via the AMPK-TSC pathway, through S-nitrosylation of IKKβ 76. In MCF-7 breast cancer cells, however, NO was reported to lead to the induction of autophagy in an ATM- and mTOR-dependent manner 77. The difference observed between the two cell lines may be explained by the lack of LKB1 in HeLa cells, which is essential in the autophagic regulation of the IKKβ signaling pathway by NO.
NO regulates immune responses and was recently shown to be implicated in xenophagy 78. NO was shown to nitrosylate cGMP following cell stimulation by LPS and IFN-γ, forming 8-nitro-cGMP, which can subsequently modify cysteine residues in target proteins. Proteins on the bacterial surface of group A Streptococcus (GAS) were modified by S-guanylation after cellular invasion, which marked bacteria for K63-linked polyubiquitination, inducing their engulfment by autophagosomes and lysosomal targeting. It would be interesting to investigate whether S-guanylation by 8-nitro-cGMP serves as a broad modification marking substrates in additional forms of selective autophagy.
Ca2+ ions
Ca2+ is a well-established signaling molecule implicated in numerous cellular processes, and its cytosolic concentration is tightly regulated. The ER and the mitochondria serve as the primary Ca2+ storage organelles. ER stress, for example, leads to the release of Ca2+ from internal ER pools into the cytosol to regulate different stages along the autophagy pathway, yet this process remains poorly understood at a mechanistic level 79–81.
The inositol 1,4,5-trisphosphate receptor, IP3R, is a Ca2+ channel activated by IP3 binding 82. It is located on various membranes and regulates Ca2+ levels in organelles, and consequently in the cytosol. The N-terminal region of the receptor interacts with Beclin1 through a non-Bcl2-interacting region to regulate autophagy and was shown to sensitize the receptor to IP3 binding during autophagy 83. Interestingly, the IP3R also regulates low Ca2+ levels in the mitochondria, impairing ATP production and increasing the AMP/ATP ratio, thereby inducing autophagy in an AMPK-dependent manner 84.
Autophagosome biogenesis
According to the current view, autophagosomes originate from a membrane that elongates until it is finally sealed as a mature double-membrane autophagosome, which subsequently fuses with the lysosome, where its content is degraded. The search for the membrane origin of the phagophore has been an enticing quest for many years. The introduction of autophagy-specific molecular tools and sophisticated imaging techniques led the way to the identification of multiple cellular membranes as possible sources of the isolation membrane. The first report in this regard utilized a GFP-tagged FYVE zinc finger domain of DFCP1, an ER resident protein that does not participate in autophagy but has high affinity for PtdIns3P on membranes 85. The induction of autophagy apparently leads to the recruitment of this artificial reporter protein into an ER subdomain that contains autophagic factors such as Atg14 and WIPI and to the formation of a cup-shaped membrane termed omegasome 85–87. However, additional membrane sources for phagophore formation have been suggested, such as plasma membrane 88,89, mitochondria 90, Golgi 91, ERGIC 92, ER–mitochondria contact sites 93, ER exit sites 94, and recycling endosomes 95. This issue has been extensively reviewed and is therefore not discussed here 5,6,96.
Nucleation
The initial step in membrane nucleation for phagophore formation is the recruitment of autophagic proteins to a membrane in the cell designated by the presence of PtdIns3P. Yeast phagophores are initiated at one location termed the pre-autophagosomal structure (PAS), whereas in mammals, they are synthesized throughout the cell. Microscopic analysis of the recruitment order of the autophagy-related proteins in both yeast and mammalian systems suggested well-defined hierarchies for the order of incorporation of complexes into the site of autophagosome formation 97,98. Regulation of the stability of PI3KC3, which is composed of Vps34, Vps15, Atg14, and Beclin1, and that of the Ulk1 complex, is essential for the nucleation process in mammalian cells and is regulated by post-translational modifications (Fig 3). Vps34 is a class III PI3K that phosphorylates phosphatidylinositol at the designated membrane, generating PtdIns3P 99. EM analysis utilizing quick-freezing and freeze-fracture replica labeling revealed differences in the dispersion of PtdIns3P in yeast and mammalian autophagosomes 100. In yeast, PtdIns3P was found mostly in the inner membrane leaflets facing the luminal barrier within the double membrane, whereas in mammals, this lipid was mostly localized to the outer autophagosomal membrane leaflets, suggesting differences in the autophagosome formation process in the different organisms. The site of PtdIns3P formation dictates the location of phagophore formation, as it leads to the recruitment of early autophagosome biogenesis factors, such as the WD-40-repeat-domain containing proteins WIPI1 and WIPI2 101,102. In addition, the FYVE-domain containing protein ALFY is recruited 103 and was recently defined as an adaptor protein able to concentrate several factors that are essential in the different stages of autophagosome biogenesis to the phagophore 104. E3 ubiquitin ligases, such as TRAF6, ubiquitinated protein aggregates, the core autophagic protein Atg5, and the cargo recruiters p62 and NBR1, have all been shown to bind ALFY 105,106. Following protein aggregation in cells, ALFY is exported from the nucleus and targets the protein aggregates to the phagophore via p62. The implication of ALFY in neurodegenerative diseases is consistent with its importance in aggregate clearance 107. However, the signaling pathways that dictate the cellular location of ALFY and its targeting to the membrane remain unknown. Importantly, starvation leads to a decrease in ALFY level, suggesting that this protein is important for the clearance of protein aggregates yet may be toxic under stressful conditions 108. It would be interesting to determine whether ALFY is implicated in additional forms of selective autophagy and whether the budding of the phagophore occurs in parallel to ALFY recruitment or sequentially. The order by which proteins are recruited by ALFY and the time point of membrane binding is likely to shed new light on the early stages of autophagosome biogenesis.
Figure 3. Post-translational modifications regulate PI3KC3 and the Ulk1 complex.
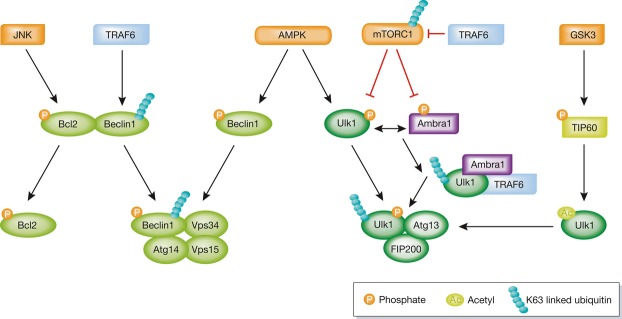
Ubiquitination, phosphorylation, and acetylation of Ulk1 and Beclin1 regulate autophagy by promoting or preventing the formation of the Ulk1 complex or PI3KC3.
See Glossary for definitions and the text for details.
The formation of the PI3KC3 is supported by UVRAG 109 and by Ambra1, a Beclin1-interacting protein 110. Ambra1 was recently shown to be a target of mTOR and to be inhibited by its phosphorylation at Ser52 under normal growth conditions 111. Upon autophagic induction, Ambra1 is phosphorylated by Ulk1, which detaches it from dynein on microtubules and targets it to the ER 112. Ambra1 then binds Ulk1 and TRAF6, promoting the Ulk1 Lys63-linked polyubiquitination that is essential for creation of the Ulk1 complex 111. Interestingly, WASH—an endosome-associated protein—was shown to compete with Ulk1 ubiquitination and with Beclin1 binding by Ambra1 113. Ambra1 therefore appears to act as a novel link between PI3KC3 and the Ulk1 complex, both of which are essential in the initial steps of autophagosome biogenesis.
Notably, although its activity is crucial, only a limited number of Ulk1 effectors have been identified. A recent study in yeast shows that Atg9 is a direct substrate of Atg1 114, the yeast homolog of Ulk1. As active mTORC1 resides on the lysosomal membrane, the inhibited Ulk1 complex could share the same location. To become active, Ulk1 needs to be shuttled from the lysosomal membrane by a mechanism yet to be resolved. A key factor in this process might be Ambra1, owing to its location along microtubules.
Connexins, a family of multispan transmembrane proteins that form plasma membrane gap junctions, were recently suggested to negatively regulate autophagosome formation by direct interaction with the PI3KC3 at the plasma membrane. According to the proposed model, under starvation conditions, Atg14 is incorporated into the plasma membrane, where it releases connexin-induced inhibition by directing these proteins to lysosomal degradation 115.
Phagophore formation and elongation
Downstream of the recruitment of WIPI1/2 are two ubiquitin-like (UBL) systems specific to the autophagic process. The first is the conjugation of Atg12, a UBL protein, to Atg5 by the E1 enzyme Atg7 and the E2 enzyme Atg10 116. Atg5 binds the N-terminal region of Atg16 through a non-covalent bond, independently of its interaction with Atg12 117. Atg16 creates homodimers, each capable of binding an Atg12–Atg5 conjugate resulting in a heterohexamer 118,119. The Atg12–Atg5–Atg16 complex is known to dictate the site of autophagosome formation by acting as an E3 ligase in the second UBL conjugation system, that of Atg8 to phosphatidylethanolamine (PE) 117. The conjugation process is mediated by Atg7 as the E1-conjugating enzyme, and Atg3 as the E2-conjugating enzyme. The mammalian Atg8 is a UBL protein family consisting of eight family members grouped into the LC3 and GABARAP subfamilies 120. WIPI2 was recently reported to recruit the Atg12–Atg5–Atg16 complex to the site of autophagosome formation by directly interacting with Atg16 121.
The exact mechanism whereby the Atg12–Atg5–Atg16 complex induces phagophore elongation is still unclear. Using GUVs and purified recombinant proteins, it was initially suggested that the complex participates in vesicle tethering 122. Atg12 has also been shown to bind Atg3 and carry it to the membrane, promoting Atg8 lipidation, which supports its role as an E3 in Atg8 conjugation. Interestingly, conjugation of Atg8 to PE is promoted by the Atg12–Atg5–Atg16 complex on SUVs but not on GUVs, indicating that membrane curvature is a significant factor in the activity of this complex. In agreement with this hypothesis, Atg3 was shown to be targeted to highly curved membranes, where it promotes Atg8–PE conjugation 123. A more recent study in GUVs suggested a slightly different scenario, in which Atg12–5–16 initially catalyzes the lipidation of Atg8 to the membrane, which in turn acts to stabilize the association of the Atg12–5–16 complex on the membrane 124. Thus, Atg8 is suggested to play a structural role and Atg12–5–16 to function as a coat. Both of these in vitro studies need further clarification. The observed differences might be associated with alterations in membrane curvature (Fig 4). During the initial stages of autophagosome biogenesis, when the phagophore is still relatively small and highly curved, the Atg12–Atg5–Atg16 complex might promote lipidation. As the phagophore continues to grow, its elongation points exhibit high curvature, whereas the sites already built are less curved and need to be stabilized. At these locations, the Atg12–Atg5–Atg16 complex might be essential in maintaining membrane structure and stability.
Figure 4. Model of the Atg12–Atg5–Atg16 complex function in autophagy.
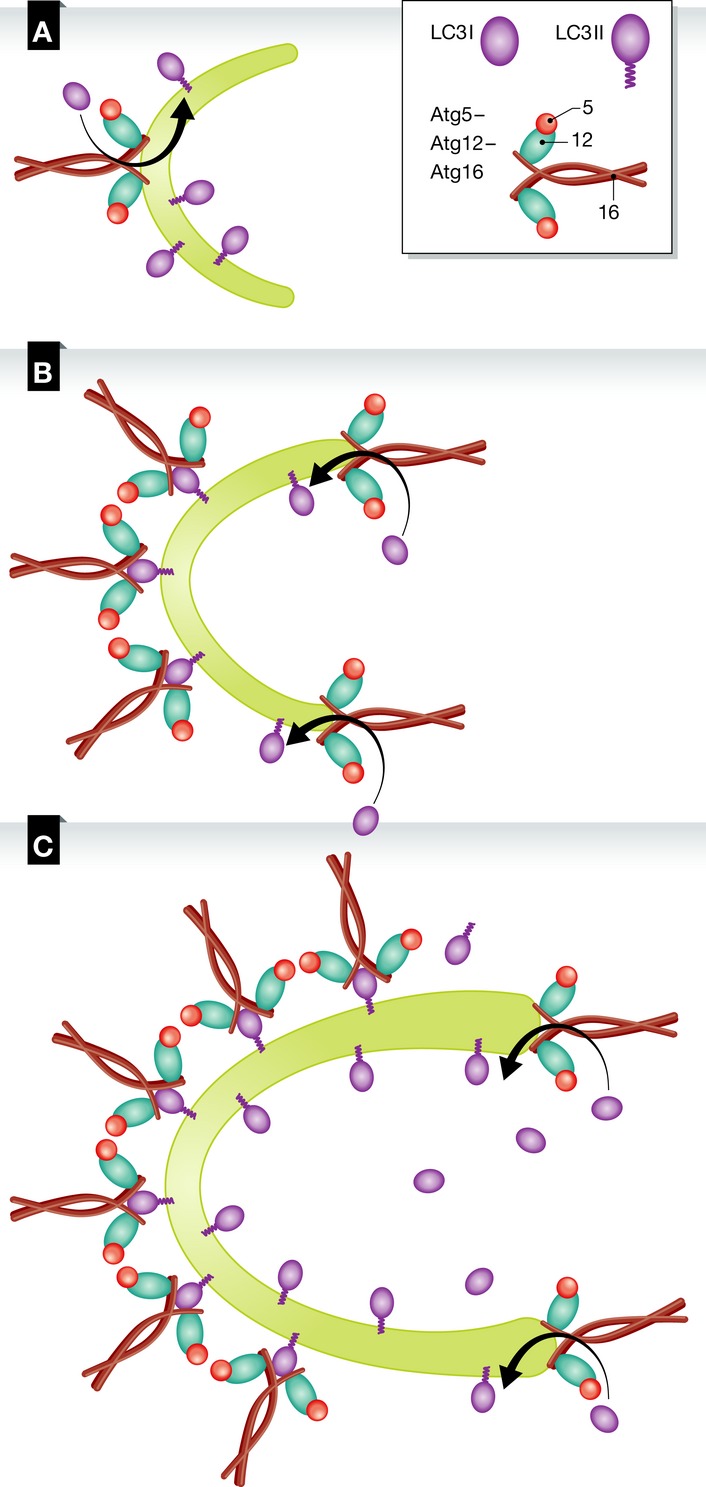
(A) The Atg12–Atg5–Atg16 complex is recruited to the phagophore after its initial nucleation. At this stage, the membrane is curved and the complex promotes the lipidation of LC3 with PE. (B) Once the membrane elongates, the complex remains associated with the membrane through LC3 on membranes with low curvature for their stabilization and continues to promote LC3 lipidation at the highly curved edges of the phagophore. (C) As the elongation continues, the Atg12–Atg5–Atg16 complex, together with the lipid-conjugated LC3, forms a coat-like structure that stabilizes the structure of the phagophore.
Further support for the notion that curvature is important for the recruitment and activity of the biogenesis machinery comes from the association of SNX18 with autophagic induction 125. SNX18 contains a PX domain that targets it to PIP2 on membranes, as well as SH3 and a BAR domains known to sense and endorse membrane curvature. It was shown to target Atg16 to perinuclear recycling endosomes and interact directly with LC3 125, suggesting that Atg16 mediates LC3 lipidation on highly curved membranes. SNX18 was further suggested to induce the tubulation of recycling endosomes that supply membranes for the elongating phagophore. Interestingly, endosomal tubulation driven by the overexpression of TBC1D14, a Rab11 binding protein, was found to be inhibitory for autophagosome formation, suggesting an antagonist role for SNX18 126. Another pathway thought to target Atg16 to the phagophore is through interaction with FIP200, a subunit of the Ulk1 complex, as it relieves Atg16 auto-inhibition 127. The discovery of multiple Atg16 targeting pathways to membranes reflects the vital role of Atg16 in autophagosome biogenesis and strengthens the hypothesis that it plays multiple roles in this process.
The phagophore membrane is subsequently elongated through a process that is not fully characterized. A given membrane source could continue to elongate until the autophagosome is completed or, alternatively, small vesicles could fuse with the phagophore to expand its membrane 128. In mammals, the VAMP7 SNARE complex—which includes VAMP7, syntaxin 7, syntaxin 8, and Vti1b—was the first reported to mediate autophagosome biogenesis 88. In a parallel yeast study, in which trafficking of ectopically expressed Atg9 was studied, the t-SNARE Tlg2 and the v-SNAREs Sec22 and Ykt6 were shown to be essential for autophagosome biogenesis through mediation of Atg9 trafficking 89. Notably, Atg9 overexpression results in the formation of tubular structures suggested to serve as Atg9 reservoirs and as a membrane source for the PAS 129, a finding recapitulated also in mammalian cells 130. When Atg9 expression was regulated by its endogenous promoter, however, this phenomenon was observed only on small unilaminar vesicles synthesized de novo from the Golgi membrane 128. These vesicles were shown to fuse with autophagosomes that contain Atg9 on the outer membrane, which is detached only after autophagosome maturation. Furthermore, relatively few Atg9-tagged vesicles were shown to fuse with the phagophore, and therefore, additional membranes sources are required. Atg9 trafficking is regulated by a complex machinery, as became recently apparent 131. TRAPPIII, which is part of the general trafficking machinery, was implicated in Atg9 trafficking under normal growth conditions, but shown to be less necessary under starvation conditions, in which the GARP pathway is essential. In mammalian cells, Atg9 was initially found in the Golgi and is transported to endosomal compartments upon autophagic induction 132, yet it was recently detected on additional intracellular compartments 130. It was also shown to be essential in the early stages of autophagosome formation by transiently interacting with the expanding phagophore 130. In addition, Atg9 has been recently reported to localize to the plasma membrane, from which it is internalized and fused with Atg16-tagged vesicles, a finding consistent with the suggested involvement of both the plasma membrane and the ER–Golgi system in autophagosome formation 95.
Atg8 and its mammalian orthologs have been implicated in the elongation of the phagophore 5,133–135. In yeast, phagophore elongation and autophagosome size are controlled by Atg8 134,135. Notably, an in vitro system using liposomes containing different concentrations of PE yielded contradictory results regarding the fusogenic activity of Atg8s 89. LC3, a mammalian homolog of Atg8, is also involved in phagophore expansion 136. Interestingly, the N-terminal region of LC3 and GATE-16 promotes vesicle tethering and fusion in vitro, suggesting their involvement in elongation of the phagophore membrane 137. It would therefore be interesting to study the significance of Atg8 in the fusion of Atg9-containing vesicles to the phagophore.
Although typically described as a progressive process whereby the edges of a crescent-shaped phagophore elongate continuously to form autophagosomes, it may be also worth considering an alternative view whereby formation of the phagophore membrane is initiated at several sites, possibly by different membrane sources (Fig 5). Different pieces of membranes could then be tied together by membrane fusion events. This would require many autophagy biogenesis complexes acting on several sites at different stages of biogenesis, and attempting to reconstitute such a process in the test tube would be extremely challenging. The available cell-free systems aimed at reconstituting autophagosome formation can reflect only fragments of the complex process. Accordingly, GUVs could represent only events that take place on membranes with relatively low curvature, whereas small liposomes may mimic highly curved membrane. If multiple events occur in parallel on different sites, it is possible that several biogenesis pathways occur simultaneously in vivo. Therefore, various membrane sources can contribute to biogenesis, since within the cell’s third dimension, there are many organelles and membranes in close proximity to the phagophore.
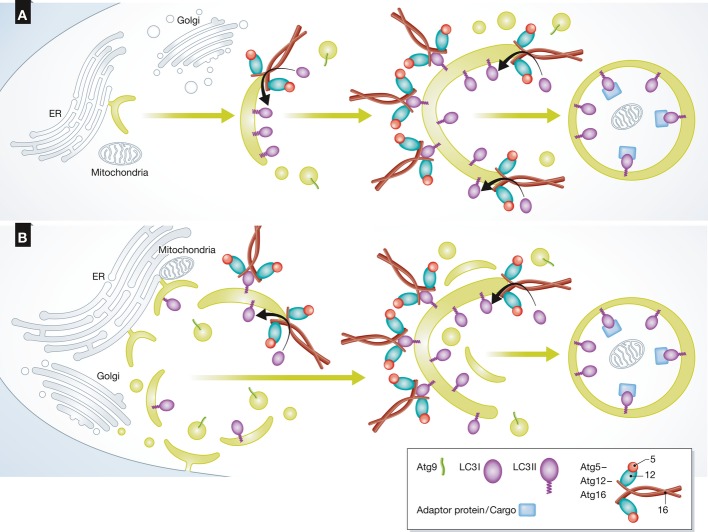
Figure 5. Models of autophagosome biogenesis.
(A) The current view of autophagosome biogenesis is a continuous process, where an initial membrane for autophagosome formation buds out from an existing organelle and is further elongated by the fusion of vesicles, some containing Atg9. The Atg12–Atg5–Atg16 complex promotes LC3 lipidation on the highly curved membranes while supporting the membrane’s structure as it elongates. Once the autophagosome is sealed, it encapsulates cargo for degradation and the external biogenesis machinery is removed. (B) A new model for autophagosome biogenesis. Multiple nucleation membranes bud from several organelles to contribute to the formation of the initial membrane of the autophagosome. Each membrane elongates individually until all are fused to create an autophagosome that encapsulates cargo for lysosomal degradation.
Sealing and maturation
The final step of autophagosome biogenesis is the sealing of the phagophore to form a double-membrane vesicle. This rather under-studied process dictates the final size of the autophagic organelle. Regulation of this process also serves as part of the regulation of fusion with the lysosomes. The mammalian homologs of Atg8 have been suggested to promote this stage of autophagosome biogenesis. Consistently, studies in Caenorhabditis elegans showed that the GABARAP homolog LGG-1 and the LC3 homolog LGG-2 act consecutively 138. LGG-2 activity downstream of LGG-1 promotes autophagosome maturation through a pathway that also involves the HOPS subunit VPS39.
Atg4 plays a dual role by priming Atg8 both for its lipid conjugation and for its removal from membranes 139. Interestingly, deconjugation of Atg8 by Atg4 was shown to be important in both early and late stages of biogenesis, allowing the fusion of autophagosomes with lysosomes. There are four members of the Atg4 mammalian family, each able to specifically modify different Atg8 family members. In erythrocytes, mammalian Atg4B participates in the regulation of autophagosome maturation that is necessary for cell differentiation 140. The protein FYCO1, previously shown to bind LC3 and mediate autophagosome trafficking, was recently implicated in autophagosome maturation as well 141.
Sidebar A: In need of answers —
How are amino acids sensed by TORC1?
How are various internal signals integrated to regulate autophagy?
How does the lysosomal location of mTORC1 affect its downstream signaling on the Ulk1 complex?
How do Atg9-containing vesicles contribute to autophagosome biogenesis?
How are the SNARE molecules needed for fusion of the autophagosome with the lysosome incorporated into the outer autophagosomal membrane?
How are the inner and outer membranes of the phagophore sorted into two sub-domains?
How are the cup-shaped phagophores formed and stabilized?
What dictates the final sealing of the autophagosome?
The participation of trafficking factors in the sealing and maturation of autophagosomes is well established. Several Rab proteins are known to play a role in autophagosome maturation 142, a process in which SNARE molecules have also been implicated, as discussed above. In a recent study, immuno-electron microscopy was used to show that syntaxin 13 localizes with LC3 on immature autophagosomes 143. Depletion of syntaxin 13 leads to the accumulation of immature autophagosomes, indicating that it participates in the maturation process. In addition, syntaxin 17 was suggested to promote autophagosomal sealing by trafficking through a still unknown mechanism to phagophores at late stages of biogenesis, enabling them to fuse with the lysosome 144. Recent studies in Drosophila and mammalian cells suggested that syntaxin 17 binds the HOPS complex, thereby promoting the fusion between autophagosomal and lysosomal membranes 145,146. Syntaxin 17 was also shown to induce, together with Atg14, budding of the source membrane at ER contact sites 93. Additional studies are clearly needed to clarify the exact role of syntaxin 17 along the autophagic pathway.
Interestingly, the lysosome-located protein TECPR1 was implicated in fusion between the autophagosome and the lysosome by binding the Atg12–Atg5 conjugate 147. Depletion of TECPR1 resulted in the accumulation of autophagosomes that do not fuse with lysosomes. As the Atg12–Atg5 conjugate is removed from autophagosomes prior to their final sealing, these results suggest a direct link between autophagosome sealing and removal of this complex. It is possible that autophagosomes only reach their final maturation, cued by the removal of all biogenesis machinery, immediately before their fusion with the lysosomes. Importantly, Atg5 binding by TECPR1 was also suggested to promote the initial stages of autophagosome formation and to target bacteria to the phagophore during xenophagy, but not upon starvation 148. The exact role of TECPR1 in autophagy thus remains to be elucidated.
Concluding remarks
Autophagy lies at a fundamental junction in cell fate, determining death or survival. The integration between stress-induced pathways and autophagic proteins is rather complex, given that different cues are funneled to activate a relatively small pool of autophagic proteins. The continuing discovery of connections between well-established signaling pathways and autophagy-related proteins, as well as of new regulators of both processes, contributes substantially to our understanding of the regulation of autophagy in response to changes in the extracellular and intracellular environment.
The mechanism of autophagosome biogenesis is still elusive. Our knowledge concerning the order of incorporation of the different factors into sites of autophagosome formation, as well as on the functional complexes involved in this process, has increased substantially in recent years. However, the exact details concerning the way in which all of these factors act in concert are still missing. The reconstitution of such a step in the test tube will clearly be very challenging, but if successful, it will provide invaluable information on this process. There are several open questions remaining in this field (Sidebar A). One of them is how the membrane of the phagophore is sorted into two sub-domains, leading to the formation of distinct inner and outer membranes. The former membrane will be degraded in the lysosome, whereas the latter fuses with the lysosome-limiting membrane to become an integral part of it. Understanding this process in molecular terms is of broad interest, as it may shed light on the mechanism by which the lysosome-limiting membrane is protected from degradation.
Acknowledgments
ZE is the incumbent of the Harold Korda Chair of Biology. We are grateful for funding from the Israeli Science Foundation (ISF) (Grant Number 535/11), the German-Israeli Foundation (GIF) (Grant Number 1129/157), and the Legacy Heritage Fund (Grant Number 1309/13).
Glossary
- ALFY
autophagy-linked FYVE protein
- Ambra1
activating molecule in Beclin1-regulated autophagy
- AMPK
5′ AMP-activated protein kinase
- ATM
ataxia-telangiectasia mutated
- BAR
Bin1/amphiphysin/Rvs167
- Bcl2
B-cell lymphoma 2
- DFCP1
Double FYVE-containing protein 1
- EGFR
epidermal growth factor receptor
- eIF2α
eukaryotic initiation factor 2α
- ER
endoplasmic reticulum
- ERGIC
ER Golgi intermediate compartment
- FIP200
200-kDa focal adhesion kinase family-interacting protein
- FoxO
forkhead-box transcription factor class O
- FYCO1
FYVE and coiled-coil domain containing 1
- FYVE
Fab1, YOTB, Vac 1, EEA1
- GAB2
GRB2-associated binding protein 2
- GABARAP
GABA receptor-associated
- GAP
GTPase activating protein
- GARP
Golgi-associated retrograde protein
- GATE16
Golgi-associated ADPase enhancer of 16 kDa
- GCN
general control non-derepressible
- GLUD
glutamate dehydrogenase
- GRB2
growth factor receptor-bound protein 2
- GSK3
glycogen synthase kinase 3
- GUVs
giant unilamellar vesicles
- HOPS
homotypic fusion and protein sorting complex
- IKKβ
inhibitor of nuclear factor κB kinase
- IL-1β
interleukin 1β
- IFN-γ
interferon γ
- IP3R
IP3 receptor
- JNK
c-JUN NH2-terminal kinase
- LC3
light chain 3
- LKB1
liver kinase B1
- LPS
liposaccharide
- LRS
Leucyl-tRNA synthetase
- MyD88
myeloid differentiation factor 88
- NBR1
neighbor of BRCA1 gene
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX4
NADPH oxidase 4
- Nrf2
nuclear factor erythroid 2-related factor 2
- PDK1
phosphoinositide-dependent kinase 1
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- pex19
peroxisomal membrane protein
- PI3KC1/3
phosphoinositide 3-kinase complex 1/3
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKB
protein kinase B
- PKR
protein kinase R
- PtdIns3P/PI3P
phosphatidylinositol 3-phosphate
- PTEN
phosphatase and tensin homolog
- Rag
Ras-related GTP-binding protein
- RalA
Ras-like protein
- ROS
reactive oxygen species
- SH3BP4
SH3-domain binding protein 4
- SNARE
soluble NSF attachment protein receptor protein
- SNX18
sorting nexin 18
- STAT3
signal transducer and activator of transcription 3
- SUVs
small unilamellar vesicles
- TECPR1
tectonin β-propeller repeat containing 1
- TIR
toll/interleukin-1 receptor
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TRAF6
tumor necrosis factor receptor-associated factor 6
- TRAPPIII
transport protein particle
- TRIF
toll/interleukin-1 receptor homology domain-containing adaptor inducing interferon-β
- TSC1/2
tuberous sclerosis protein 1 and 2
- Ulk1
UNC-51-like kinase 1
- UVRAG
UV irradiation resistance-associated gene
- VAMP7
vesicle-associated membrane
- Vps34
vacuolar protein sorting 34
- WASH
Wiskott–Aldrich syndrome protein WASP and SCAR homolog
- WIPI1/2
WD repeat proteins interacting with phosphoinositides 1/2
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K. Dynamic regulation of autophagy and endocytosis for cell remodeling during early development. Traffic. 2013;14:479–486. doi: 10.1111/tra.12050. [DOI] [PubMed] [Google Scholar]
- Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, Wang C, Wolvetang E, Vazquez-Martin A, Zhang J. Autophagy in stem cells. Autophagy. 2013;9:830–849. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Shvets E, Elazar Z. Biogenesis and cargo selectivity of autophagosomes. Annu Rev Biochem. 2011;80:125–156. doi: 10.1146/annurev-biochem-052709-094552. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22:R29–R34. doi: 10.1016/j.cub.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41:1103–1130. doi: 10.1042/BST20130134. [DOI] [PubMed] [Google Scholar]
- Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab. 2009;296:E603–E613. doi: 10.1152/ajpendo.91002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker N, Mor A, Journo D, Abeliovich H. Induction of autophagic flux by amino acid deprivation is distinct from nitrogen starvation-induced macroautophagy. Autophagy. 2010;6:879–890. doi: 10.4161/auto.6.7.12753. [DOI] [PubMed] [Google Scholar]
- Wauson EM, Zaganjor E, Lee AY, Guerra ML, Ghosh AB, Bookout AL, Chambers CP, Jivan A, McGlynn K, Hutchison MR, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell. 2012;47:851–862. doi: 10.1016/j.molcel.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Lorin S, Tol MJ, Bauvy C, Strijland A, Pous C, Verhoeven AJ, Codogno P, Meijer AJ. Glutamate dehydrogenase contributes to leucine sensing in the regulation of autophagy. Autophagy. 2013;9:850–860. doi: 10.4161/auto.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Khambu B, Zhang H, Kang JH, Chen X, Chen D, Vollmer L, Liu PQ, Vogt A, Yin XM. Suppression of lysosome function induces autophagy via a feedback down-regulation of MTOR complex 1 (MTORC1) activity. J Biol Chem. 2013;288:35769–35780. doi: 10.1074/jbc.M113.511212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Stone M, Hwang TH, Kim YG, Dunlevy JR, Griffin TJ, Kim DH. SH3BP4 is a negative regulator of amino acid-Rag GTPase-mTORC1 signaling. Mol Cell. 2012;46:833–846. doi: 10.1016/j.molcel.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–979. doi: 10.4161/auto.5.7.9296. [DOI] [PubMed] [Google Scholar]
- Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5:649–662. doi: 10.4161/auto.5.5.8249. [DOI] [PubMed] [Google Scholar]
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, du Lam H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruno F, Perez-Jimenez E, Knecht E. Regulation of autophagy by glucose in Mammalian cells. Cells. 2012;1:372–395. doi: 10.3390/cells1030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Birnbaum MJ. Insulin, but not contraction, activates Akt/PKB in isolated rat skeletal muscle. J Biol Chem. 1998;273:14679–14682. doi: 10.1074/jbc.273.24.14679. [DOI] [PubMed] [Google Scholar]
- Martin TD, Chen XW, Kaplan RE, Saltiel AR, Walker CL, Reiner DJ, Der CJ. Ral and Rheb GTPase activating proteins integrate mTOR and GTPase signaling in aging, autophagy, and tumor cell invasion. Mol Cell. 2014;53:209–220. doi: 10.1016/j.molcel.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Tan SH, Shui G, Zhou J, Shi Y, Huang J, Xia D, Wenk MR, Shen HM. Critical role of SCD1 in autophagy regulation via lipogenesis and lipid rafts-coupled AKT-FOXO1 signaling pathway. Autophagy. 2014;10:226–242. doi: 10.4161/auto.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Tan-Sah VP, Ding EY, Smith JM, Miyamoto S. Hexokinase-II positively regulates glucose starvation-induced autophagy through TORC1 inhibition. Mol Cell. 2014;53:521–533. doi: 10.1016/j.molcel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T, Kuma A, Mizushima N. Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. J Biol Chem. 2013;288:21074–21081. doi: 10.1074/jbc.M113.456228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M, Domin J. Recruitment of the class II phosphoinositide 3-kinase C2beta to the epidermal growth factor receptor: role of Grb2. Mol Cell Biol. 2001;21:6660–6667. doi: 10.1128/MCB.21.19.6660-6667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, Kinch L, Koduru P, Christudass CS, Veltri RW, et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–1284. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutten B, Rouschop KM. EGFR signaling and autophagy dependence for growth, survival, and therapy resistance. Cell Cycle. 2014;13:42–51. doi: 10.4161/cc.27518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Niso-Santano M, Adjemian S, Takehara T, Malik SA, Minoux H, Souquere S, Mariño G, Lachkar S, Senovilla L, et al. Cytoplasmic STAT3 represses autophagy by inhibiting PKR activity. Mol Cell. 2012;48:667–680. doi: 10.1016/j.molcel.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Li TY, Liu Q, Zhang C, Li X, Chen Y, Zhang SM, Lian G, Liu Q, Ruan K, et al. GSK3-TIP60-ULK1 signaling pathway links growth factor deprivation to autophagy. Science. 2012;336:477–481. doi: 10.1126/science.1217032. [DOI] [PubMed] [Google Scholar]
- Into T, Inomata M, Takayama E, Takigawa T. Autophagy in regulation of Toll-like receptor signaling. Cell Signal. 2012;24:1150–1162. doi: 10.1016/j.cellsig.2012.01.020. [DOI] [PubMed] [Google Scholar]
- O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3:ra42. doi: 10.1126/scisignal.2000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Maeda D, Xiao Q, Srinivasula SM. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc Natl Acad Sci U S A. 2011;108:1427–1432. doi: 10.1073/pnas.1014156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell. 2014;54:224–233. doi: 10.1016/j.molcel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013;13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290–303. doi: 10.1016/j.cell.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Tang HW, Liao HM, Peng WH, Lin HR, Chen CH, Chen GC. Atg9 interacts with dTRAF2/TRAF6 to regulate oxidative stress-induced JNK activation and autophagy induction. Dev Cell. 2013;27:489–503. doi: 10.1016/j.devcel.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- Meng Y, Yong Y, Yang G, Ding H, Fan Z, Tang Y, Luo J, Ke ZJ. Autophagy alleviates neurodegeneration caused by mild impairment of oxidative metabolism. J Neurochem. 2013;126:805–818. doi: 10.1111/jnc.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2 alpha/activating transcription factor 4 pathway. Circ Res. 2013;113:1253–1264. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kim J, Alexander A, Cai S, Tripathi DN, Dere R, Tee AR, Tait-Mulder J, Di Nardo A, Han JM, et al. A tuberous sclerosis complex signalling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat Cell Biol. 2013;15:1186–1196. doi: 10.1038/ncb2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Korolchuk VI, Renna M, Imarisio S, Fleming A, Williams A, Garcia-Arencibia M, Rose C, Luo S, Underwood BR, et al. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi DN, Chowdhury R, Trudel LJ, Tee AR, Slack RS, Walker CL, Wogan GN. Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc Natl Acad Sci U S A. 2013;110:E2950–E2957. doi: 10.1073/pnas.1307736110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito C, Saito Y, Nozawa T, Fujii S, Sawa T, Inoue H, Matsunaga T, Khan S, Akashi S, Hashimoto R, et al. Endogenous nitrated nucleotide is a key mediator of autophagy and innate defense against bacteria. Mol Cell. 2013;52:794–804. doi: 10.1016/j.molcel.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, et al. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, Wong PM, Gammoh N, Jiang X. Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol Cell. 2011;42:731–743. doi: 10.1016/j.molcel.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East DA, Campanella M. Ca2+ in quality control: an unresolved riddle critical to autophagy and mitophagy. Autophagy. 2013;9:1710–1719. doi: 10.4161/auto.25367. [DOI] [PubMed] [Google Scholar]
- Kiviluoto S, Vervliet T, Ivanova H, Decuypere JP, De Smedt H, Missiaen L, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim Biophys Acta. 2013;1833:1612–1624. doi: 10.1016/j.bbamcr.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgó J, Agostinis P, Missiaen L, De Smedt H, Parys JB, et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Nishino M, Fujita N, Noda T, Yamaguchi A, Yoshimori T, Yamamoto A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11:1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy. 2009;5:1180–1185. doi: 10.4161/auto.5.8.10274. [DOI] [PubMed] [Google Scholar]
- Moreau K, Ravikumar B, Renna M, Puri C, Rubinsztein DC. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146:303–317. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair U, Jotwani A, Geng J, Gammoh N, Richerson D, Yen WL, Griffith J, Nag S, Wang K, Moss T, et al. SNARE proteins are required for macroautophagy. Cell. 2011;146:290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart A, Griffith J, Reggiori F. Exit from the Golgi is required for the expansion of the autophagosomal phagophore in yeast Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:2270–2284. doi: 10.1091/mbc.E09-04-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Melville D, Zhang M, Schekman R. The ER-Golgi intermediate compartment is a key membrane source for the LC3 lipidation step of autophagosome biogenesis. Elife. 2013;2:e00947. doi: 10.7554/eLife.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- Graef M, Friedman JR, Graham C, Babu M, Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol Biol Cell. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri C, Renna M, Bento CF, Moreau K, Rubinsztein DC. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154:1285–1299. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9:1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Kihara A, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Fujita A, Yamamoto H, Tatematsu T, Kakuta S, Obara K, Ohsumi Y, Fujimoto T. Yeast and mammalian autophagosomes exhibit distinct phosphatidylinositol 3-phosphate asymmetries. Nat Commun. 2014;5:3207. doi: 10.1038/ncomms4207. [DOI] [PubMed] [Google Scholar]
- Proikas-Cezanne T, Waddell S, Gaugel A, Frickey T, Lupas A, Nordheim A. WIPI-1 alpha (WIPI49), a member of the novel 7-bladed WIPI protein family, is aberrantly expressed in human cancer and is linked to starvation-induced autophagy. Oncogene. 2004;23:9314–9325. doi: 10.1038/sj.onc.1208331. [DOI] [PubMed] [Google Scholar]
- Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, Tooze SA. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Birkeland HC, Gillooly DJ, Mizushima N, Kuma A, Yoshimori T, Slagsvold T, Brech A, Stenmark H. Alfy, a novel FYVE-domain-containing protein associated with protein granules and autophagic membranes. J Cell Sci. 2004;117:4239–4251. doi: 10.1242/jcs.01287. [DOI] [PubMed] [Google Scholar]
- Filimonenko M, Isakson P, Finley KD, Anderson M, Jeong H, Melia TJ, Bartlett BJ, Myers KM, Birkeland HC, Lamark T, et al. The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol Cell. 2010;38:265–279. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, Brech A, Øvervatn A, Stenmark H, Bjørkøy G, Simonsen A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6:330–344. doi: 10.4161/auto.6.3.11226. [DOI] [PubMed] [Google Scholar]
- Isakson P, Lystad AH, Breen K, Koster G, Stenmark H, Simonsen A. TRAF6 mediates ubiquitination of KIF23/MKLP1 and is required for midbody ring degradation by selective autophagy. Autophagy. 2013;9:1955–1964. doi: 10.4161/auto.26085. [DOI] [PubMed] [Google Scholar]
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson P, Holland P, Simonsen A. The role of ALFY in selective autophagy. Cell Death Differ. 2013;20:12–20. doi: 10.1038/cdd.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, Corazzari M, Fuoco C, Ucar A, Schwartz P, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007;447:1121–1125. doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–168. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Wang S, Du Y, Zhao Z, Shi L, Sun L, Huang G, Ye B, Li C, Dai Z, et al. WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. EMBO J. 2013;32:2685–2696. doi: 10.1038/emboj.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papinski D, Schuschnig M, Reiter W, Wilhelm L, Barnes CA, Maiolica A, Hansmann I, Pfaffenwimmer T, Kijanska M, Stoffel I, et al. Early steps in autophagy depend on direct phosphorylation of atg9 by the atg1 kinase. Mol Cell. 2014;53:471–483. doi: 10.1016/j.molcel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano E, Yuste A, Patel B, Stout RF, Jr, Spray DC, Cuervo AM. Connexins modulate autophagosome biogenesis. Nat Cell Biol. 2014;16:401–414. doi: 10.1038/ncb2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser SE, Mao K, Taherbhoy AM, Yu S, Olszewski JL, Duda DM, Kurinov I, Deng A, Fenn TD, Klionsky DJ, et al. Noncanonical E2 recruitment by the autophagy E1 revealed by Atg7-Atg3 and Atg7-Atg10 structures. Nat Struct Mol Biol. 2012;19:1242–1249. doi: 10.1038/nsmb.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J. 1999;18:3888–3896. doi: 10.1093/emboj/18.14.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277:18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12:226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley HC, Razi M, Polson HE, Girardin SE, Wilson MI, Tooze SA. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12–5-16L1. Mol Cell. 2014 doi: 10.1016/j.molcel.2014.05.021. DOI 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov J, Walczak M, Ibiricu I, Schuchner S, Ogris E, Kraft C, Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31:4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Dancourt J, Shteyn V, Puente G, Fong WM, Nag S, Bewersdorf J, Yamamoto A, Antonny B, Melia TJ. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol. 2014;16:415–424. doi: 10.1038/ncb2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Beier V, Franquelim HG, Wollert T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell. 2014;156:469–481. doi: 10.1016/j.cell.2013.12.022. [DOI] [PubMed] [Google Scholar]
- Knævelsrud H, Søreng K, Raiborg C, Håberg K, Rasmuson F, Brech A, Liestøl K, Rusten TE, Stenmark H, Neufeld TP, et al. Membrane remodeling by the PX-BAR protein SNX18 promotes autophagosome formation. J Cell Biol. 2013;202:331–349. doi: 10.1083/jcb.201205129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longatti A, Lamb CA, Razi M, Yoshimura S, Barr FA, Tooze SA. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J Cell Biol. 2012;197:659–675. doi: 10.1083/jcb.201111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Kaizuka T, Cadwell K, Sahani MH, Saitoh T, Akira S, Virgin HW, Mizushima N. FIP200 regulates targeting of Atg16L1 to the isolation membrane. EMBO Rep. 2013;14:284–291. doi: 10.1038/embor.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, Ichikawa R, Kinjo M, Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198:219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM, Tooze SA. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol Biol Cell. 2012;23:1860–1873. doi: 10.1091/mbc.E11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama-Noda K, Kira S, Yoshimori T, Noda T. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J Cell Sci. 2013;126:4963–4973. doi: 10.1242/jcs.131318. [DOI] [PubMed] [Google Scholar]
- Young AR, Chan EY, Hu XW, Kochl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006;119:3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- Yang A, Li Y, Pantoom S, Triola G, Wu YW. Semisynthetic lipidated LC3 protein mediates membrane fusion. ChemBioChem. 2013;14:1296–1300. doi: 10.1002/cbic.201300344. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130:165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29:1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20:444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Manil-Segalen M, Lefebvre C, Jenzer C, Trichet M, Boulogne C, Satiat-Jeunemaitre B, Legouis R. The C. elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Dev Cell. 2014;28:43–55. doi: 10.1016/j.devcel.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Ishii J, Asai E, Ohsumi Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy. 2012;8:177–186. doi: 10.4161/auto.8.2.18373. [DOI] [PubMed] [Google Scholar]
- Betin VM, Singleton BK, Parsons SF, Anstee DJ, Lane JD. Autophagy facilitates organelle clearance during differentiation of human erythroblasts: evidence for a role for ATG4 paralogs during autophagosome maturation. Autophagy. 2013;9:881–893. doi: 10.4161/auto.24172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Becker C, Reyes C, Underhill DM. Cutting edge: FYCO1 recruitment to dectin-1 phagosomes is accelerated by light chain 3 protein and regulates phagosome maturation and reactive oxygen production. J Immunol. 2014;192:1356–1360. doi: 10.4049/jimmunol.1302835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao X, Zou L, Wu Y. Regulation of autophagy by the Rab GTPase network. Cell Death Differ. 2014;21:348–358. doi: 10.1038/cdd.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang Z, Sun D, Sweeney ST, Gao FB. Syntaxin 13, a genetic modifier of mutant CHMP2B in frontotemporal dementia, is required for autophagosome maturation. Mol Cell. 2013;52:264–271. doi: 10.1016/j.molcel.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Jiang P, Nishimura T, Sakamaki Y, Itakura E, Hatta T, Natsume T, Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol Biol Cell. 2014;25:1327–1337. doi: 10.1091/mbc.E13-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáts S, Pircs K, Nagy P, Varga Á, Kárpáti M, Hegedus K, Kramer H, Kovács AL, Sass M, Juhász G. Interaction of the HOPS complex with Syntaxin 17 mediates autophagosome clearance in Drosophila. Mol Biol Cell. 2014;25:1338–1354. doi: 10.1091/mbc.E13-08-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Fan W, Lu Y, Ding X, Chen S, Zhong Q. A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Mol Cell. 2012;45:629–641. doi: 10.1016/j.molcel.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Yoshikawa Y, Kobayashi T, Mimuro H, Fukumatsu M, Kiga K, Piao Z, Ashida H, Yoshida M, Kakuta S, et al. A Tecpr1-dependent selective autophagy pathway targets bacterial pathogens. Cell Host Microbe. 2011;9:376–389. doi: 10.1016/j.chom.2011.04.010. [DOI] [PubMed] [Google Scholar]