Abstract
The Hippo-YAP pathway is altered and implicated as an oncogenic signaling pathway in many human cancers. Hypoxia is an important microenvironmental factor that promotes tumorigenesis. However, the effects of hypoxia on the two most important Hippo-YAP effectors, YAP (Yes-associated protein) and TAZ (transcriptional co-activator with PDZ-binding motif), have not been reported. In this work, we demonstrated that TAZ was functionally involved in cell proliferation and/or migration in epithelial ovarian cancer (EOC) or human ovarian surface epithelial (HOSE) cells. Hypoxic conditions (1% O2 or hypoxia mimics) induced a reduction of YAP phosphorylation (S127) and total YAP expression in EOC cell lines OVCAR5 and SKOV3. However, these conditions up-regulated levels of S69 phosphorylated TAZ in EOC cells. The known TAZ kinases, Lats1 and Akt, were unlikely to be involved in up-regulated pTAZ by hypoxic conditions. Together, our data revealed new and differential regulating mechanisms of TAZ and YAP in cancer cells by hypoxia conditions.
Keywords: Hypoxia, Hippo-YAP pathway, YAP (Yes-associated protein), TAZ (transcriptional co-activator with PDZ-binding motif)
Introduction
The Hippo-YAP pathway controls organ size in diverse species (1, 2). Deregulation of this pathway can induce tumors and/or promote tumor progression in model organisms and occurs in a broad range of human carcinomas, including lung, colorectal, ovarian, prostate, and liver cancers (2-5).
The studies of deregulation of the Hippo-YAP pathway have been focused mostly at the post-transcriptional and/or post-translational levels (5, 6). In particular, the two most important downstream factors of this pathway, Yes-associated protein (YAP1) and its paralog transcriptional coactivator with PDZ-binding motif (TAZ) are mainly regulated via phosphorylation-dephosphorylation, with the dephosphorylated forms being active and nucleus-targeted to induce tumor promoting genes. Their functions are involved in cell proliferation, apoptosis, epithelialmesenchymal transition (EMT), the loss of contact inhibition observed in cancer cells, migration, and invasion (5, 6).
The functional involvement of YAP in EOC cells has been reported, including in one of our recent papers (7-9). However, the role of TAZ in EOC had not been reported until very recently. Jeong et al showed that TAZ mediates lysophosphatidic acid (LPA)-induced migration and proliferation of human R182 EOC cells (10). TAZ shares ~ 50% sequence identity and very similar topology with YAP (2). Published data up to date show that TAZ is regulated by the Hippo pathway in a fashion similar to YAP (1, 2, 6, 11). TAZ can be phosphorylated by Lats on serine residues in four HXRXXS motifs, including S89, the counterpart of YAP S127 (2). Phosphorylation on TAZ S89 resulted in TAZ sequestration in the cytoplasm and inactivation (2). In addition, TAZ functions as a transcriptional co-activator, similar to YAP and has similar function in promoting cell proliferation, EMT, migration, and invasion (12). Both TAZ and YAP have been implicated in stemness (2). In spite of these similarities, however, existing evidence suggests that YAP and TAZ do not compensate each other, since YAP and TAZ knockout mice show different phenotypes and the phenotype of YAP or TAZ knockdown cannot be compensated by the other gene (12). Whether YAP and TAZ can be differentially regulated by up-stream factors and whether YAP and TAZ regulate different downstream targets and biological functions remain to be further investigated.
Hypoxia is an important micro-environmental factor that induces cancer progression and metastasis, contributing to angiogenesis, metastasis (including EMT), resistance to apoptosis, chemotherapy and radiotherapy (13). Hypoxia-inducible factor-1alpha (HIF-1α) is the key transcription factor induced under hypoxic conditions. We have shown that the human EOC ascites environment is hypoxic in vivo and hypoxia conditions enhance lysophosphatidic acid (LPA)-induced cell invasion in HEY EOC cells (14). In an ovarian orthotopic xenograft mouse model, we showed that LPA enhanced tumor metastasis (but not primary tumor growth) and that 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; an inhibitor of the heat shock protein 90 and HIF-1α) effectively blocked LPA-induced tumor metastasis (14). Thus hypoxia is likely to be a tumor-promoting micro-environmental factor of EOC. There are two previous reports showing that TAZ activates HIF-1α in breast cancer MDA-MB231 and 1833-bone metastatic clone cells (15, 16). However, the potential effect of hypoxia on YAP and TAZ regulation has yet to be shown.
There are several hypoxia mimics, including the divalent metals (such as CoCl2), iron chelators (such as deferoxamine, DFO), and the prolyl 4-hydroxylase inhibitor dimethyloxaloylglycine (DMOG), that induce HIF-1α expression, HIF-1 DNA-binding activity, and trans-activation of genes containing HIF-1 binding sites (17, 18).
In the current work, we employed gain-of- and loss-of function studies to investigate the role of TAZ in EOC and HOSE cells. The effects of hypoxia conditions (1% O2 or hypoxic mimics) on expression and phosphorylation of TAZ and YAP in EOC cells were examined. The mechanisms by which hypoxic conditions differentially regulate TAZ and YAZ were reported.
Materials and Methods
Materials
Oleoyl-LPA was purchased from Avanti Polar Lipids (Birmingham, AL). The following inhibitors or reagents were used in this study: MK2206 (Biovision, Milpitas, CA), dimethyloxalylglycine (DMOG), deferoxamine (DFO), cobalt(II) chloride (CoCl2), actinomycin D (ActD) and cyclohexamide (CHX) were from Sigma-Aldrich (St. Louis, MO). YAP, phospho-YAP (Ser127) and phospho-Lats1 (Ser909) antibodies were from Cell Signaling (Boston, MA). p-TAZ (Ser89) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-TAZ antibody was from Abcam (Cambridge, MA). Alexa fluor secondary antibodies were from Life Technologies (Grand Island, NY). HIF1-a siRNA and TAZ shRNA, were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and culture
All cell lines were maintained in a humidified atmosphere at 37°C with 5% CO2. T-29 (an immortalized human epithelial ovarian surface cell line; a kind gift from Dr. Jinsong Liu (MD Anderson), SKOV3, OVCAR5 and PC3 cells were cultured in RPMI 1640 with glutamine, FBS (10%) (ATCC, Manassas, VA), and penicillin/streptomycin (P/S, 100 μg/ml). SW480 and MDAMB-231 cells were cultured in DMEM with glutamine, FBS (10%), and penicillin/streptomycin. OVCAR5 cells were transfected with TAZ shRNA constructs and selected by puromycin (2 μg/ml) to create TAZ shRNA stable cell lines. OVCAR5 cells were transfected with constitutively active TAZ construct and selected by G418 (200 μg/ml).
Hypoxia and hypoxic mimics treatment
Cells were starved in 6-well plate for 24 hr and then incubated in either atmosphere O2 pressure (21% O2) or hypoxic (1% O2) conditions for the indicated times. Alternatively, the cells were treated with DMOG, DFO or CoCl2 for the indicated times after FBS starvation,
Western blot analyses
Western blot analyses were conducted using standard procedures and proteins were detected using primary and fluorescent secondary antibodies (IRDye800CW-conjugated or IRDye680-conjugated anti-species IgG, Li-Cor Biosciences, Lincoln, NE). The fluorescent signals were captured on an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE) with both 700- and 800-nm channels. Boxes were manually placed around each band of interest, and the software returned near-infrared fluorescent values of raw intensity with background subtraction (Odyssey 3.0 analytical software, Li-Cor Biosciences, Lincoln, NE).
MTT proliferation assay
5,000 cells in 200 μl RPMI160 medium/well were seeded into 96 well plates and starved overnight. The medium was replaced with fresh RPMI1640 with or without 1% FBS and cultured for 72 hr. 10 μl of 3-(4,5-Dimethylthiazol-2-yl)-2,5-dipheny-ltetrazoliumbromide (MTT, 5 mg/ml) was added into the cells for 4 hr; media were removed and the blue formazan crystals trapped in cells were dissolved in sterile DMSO (100 μl) by incubating at 37 °C for 30 min. The absorbance of the solution at 550 nm was determined by a Vector3 spectrophotometer (PerkinElmer, Waltham, MA).
DNA and RNA transfection
Six-well plates were seeded with 5×105cell/well in 2 ml media 24 hr before transfection. Cells were transfected with siRNA (10 μM/well) or plasmid DNA (4 μg/well) using Lipofectamine 2000 Reagent (Life Technologies, Grand Island, NY) according to manufacturer's instruction.
Cell migration assays
Migration and invasion assays were conducted using Transwell plates with 8 μm pore size membranes (Corning Inc., Corning, NY) as described previously (9). After incubation for 4 hr, cells remaining in the upper side of the filter were removed with cotton swabs. The cells attached on the lower surface were fixed and stained using crystal violet and washed with water. Results were presented as the mean number of cells migrated per well. All experiments were conducted in triplicate.
Statistical Analyses
The Student's t-test was utilized to assess the statistical significance of the difference between two treatments. A P value of less than 0.05 was considered significant.
Results
TAZ was functionally involved in cell proliferation in OC cells
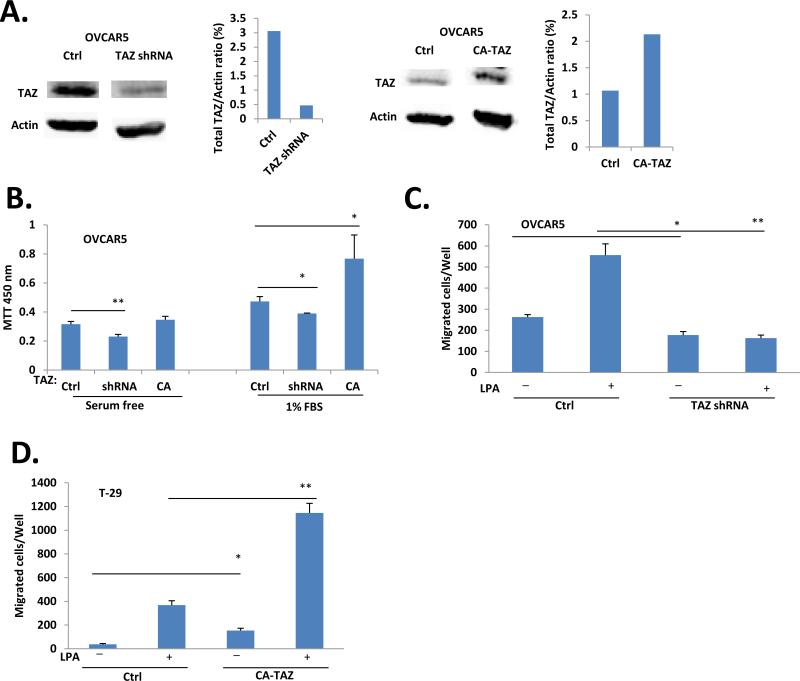
We tested the potential role of TAZ in proliferation in EOC cells. As shown in Fig. 1A, shRNA efficiently down-regulated TAZ and the constitutive-active (CA)-TAZ increased total TAZ expression in OVCAR5 cells. Under these conditions, FBS-induced cell proliferation was approximately 18% down-regulated and 62% up-regulated, respectively (Fig. 1B). TAZ was also involved in LPA-induced cell migration, as down-regulation of TAZ by shRNA completely blocked LPA-induced migration of OVCAR5 cells (Fig. 1C). In addition, we tested the effect of TAZ and YAP in HOSE T29 cells and found that overexpression of either the wild type (WT) or the CA form of YAP and TAZ did not increased their proliferation potential (MTT and colony assays; data not shown), but increased their migratory abilities (Fig. 1D).
Figure 1. TAZ was involved in proliferation and migration of ovarian cancer cells.
A. OVCAR5 stable cell lines generated by transfection with TAZ shRNA, or constitutively active (CA)-TAZ constructs as described in ‘Materials and methods’. The down- or up- TAZ expression by shRNA and overexpression of TAZ were confirmed by Western blot analysis. B. Cell proliferation by MTT assays in down- or up-regulated TAZ OVCAR5 cells. C. Cell migration was reduced in of TAZ-down-regulated OVCAR5 cells. D. Overexpression of CATAZ increased migration of T29 cells.
Hypoxia differentially regulated YAP and TAZ in EOC cells
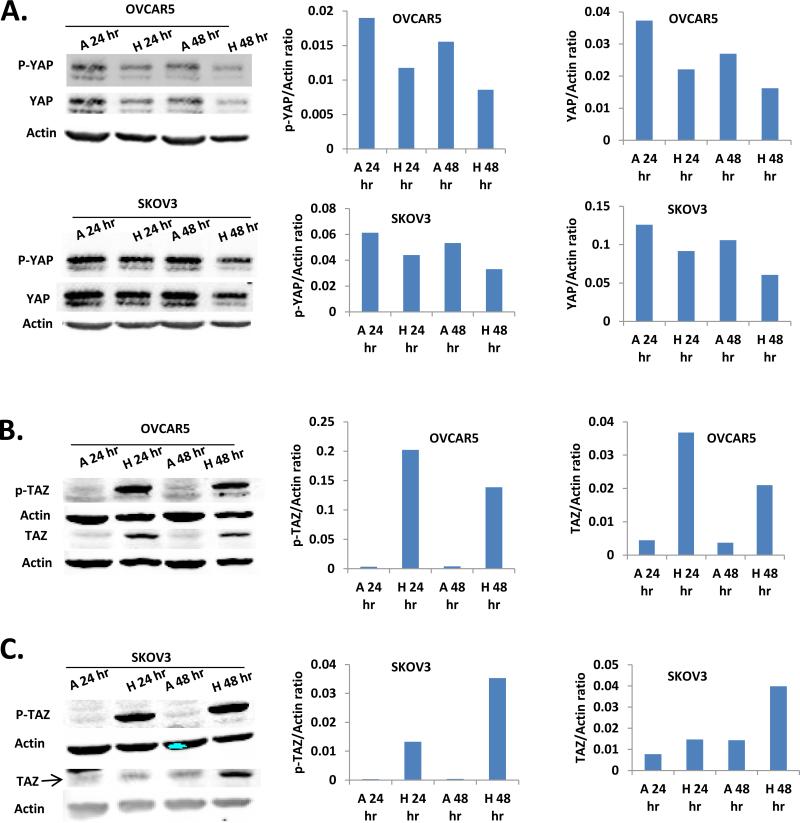
We tested the effect of hypoxia (1% oxygen) on YAP and TAZ phosphorylation in OVCAR5 and SKOV3 EOC cell lines. Both OVCAR5 and SKOV3 cell lines responded to hypoxia (1%, 24 and 48 hr) to have reduced pYAP. Hypoxia also induced a reduction of total YAP (Fig. 2A).
Figure 2. The effect of hypoxia on YAP and TAZ phosphorylation.
A. Both p-YAP and total YAP in OVCAR5 and SKOV3 EOC cell lines were reduced under hypoxia condition (1% oxygen, 24 and 48 hr). B. Hypoxia condition (1% oxygen, 24 and 48 hr) induced strong increase of p-TAZ level and modest increase of total TAZ in OVCAR5 cells. C. Low oxygen (1% O2) up-regulated pTAZ and total TAZ in SKOV3 cells. A: Atmosphere O2 pressure, 21% oxygen; H: Hypoxia, 1% oxygen.
To our surprise, hypoxia induced a very potent S69-phosphoarylation of TAZ in OVCAR5 cells in both 24 and 48 hr. This was accompanied by a modest, but significant increase in total TAZ expression levels (Fig. 2B). Low O2 (1% O2) also up-regulated pTAZ in another EOC cell line SKOV3 and several other cells lines, including PC3, SW480, and MDA-MB-231 cells (Fig. 2C and data not shown).
Hypoxia mimics up-regulated pTAZ and total TAZ via a HIF1-α-dependent manner
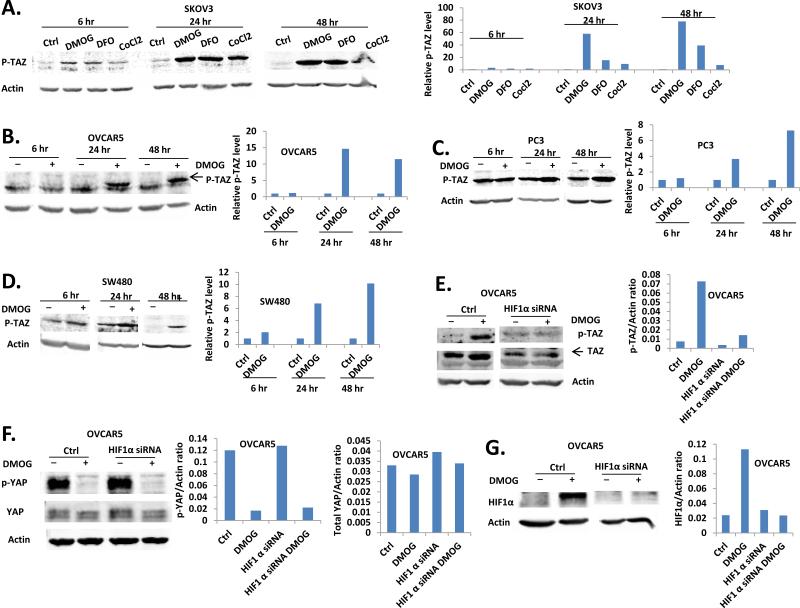
Since HIF1-α is a critical transcriptional factor under hypoxia conditions, we also tested several of HIF1-α activators, including CoCl2 (19), DMOG, and DFO (20) in EOC and/or other pTAZ up-regulation, cancer cell lines. In SKOV3 cells, all three hypoxia mimics tested induced a time-dependent with the strongest effects observed in 24 hr (Fig. 3A). Similarly, DMOG induced pTAZ in OVCAR5 cells was also time-dependent, although the effect was strongest at 48 hr. (Fig. 3B). This induction was also observed in PC3 and SW480 cells (Fig. 3C, D).
Figure 3. HIF1-α mediated hypoxia mimics induced up-regulation of pTAZ and total TAZ.
A. DMOG (500 μM), DFO (500 μM) and CoCl2 (300 μM) induced p-TAZ up-regulation in SKOV3 cells at 6, 24 and 48 hr. B. DMOG (500 μM) induced p-TAZ up-regulation in OVCAR5 cells at 6, 24 and 48 hr. C. The level of p-TAZ in PC3 cells were increased by DMOG (500 μM) treatment. D. DMOG (500 μM) treatment up-regulated p-TAZ in SW480 cells. E. DMOG induced p-TAZ and total TAZ up-regulation at 24 hr was inhibited by HIF-1α knockdown in OVCAR5 cells. F. DMOG induced OVCAR5 p-YAP and total YAP down-regulation was not affected by HIF-1α knockdown. G. Confirmation of HIF-1α knockdown by western blot analysis.
Since the common target of these hypoxia mimics is HIF1-α , we tested the role of HIF1-α in pTAZ induction. As shown in Fig. 3E, knocking-down HIF1-α significantly reduced DMOG-inducted pTAZ and total TAZ. On the other hand, down-regulation of HIF1-α did not affect DMOG-regulated dpYAP and total YAP expression (Fig. 3F, G).
LATS was unlikely to be involved in regulation of pTAZ
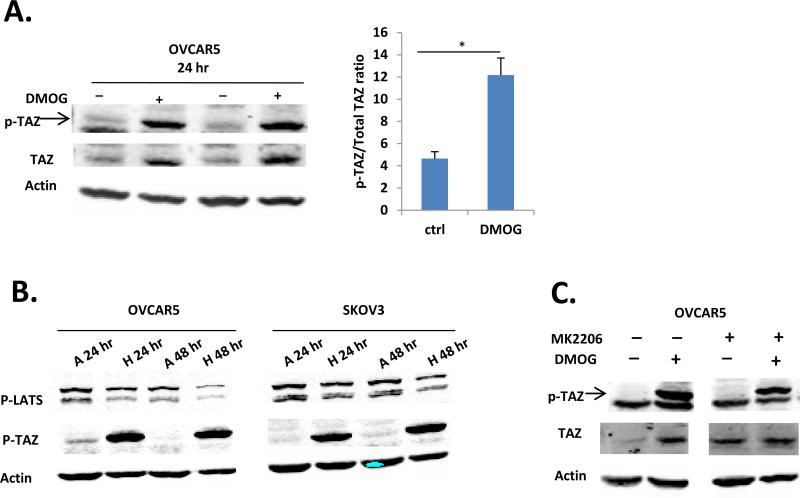
While both pTAZ and total TAZ were up-regulated at the protein level, the ratio of pTAZ/total TAZ was increased by DMOG-treatment (Fig. 4A), suggesting an additional regulation on phosphorylation of TAZ. We tested the YAP/TAZ canonic kinase Lats. In multiple cell lines, including OVCAR5, SKOV3, PC3, and SW480, the conditions when pTAZ was up-regulated by hypoxia, pLATS1 (the active form) was down-regulated (Fig. 4B and data not shown). We also tested the potential involvement of Akt, which has been previously shown to phosphorylate YAP in Cos-7 cells (21). No effect of ATK inhibitor MK2206 on DMOG-induced pTAZ was detected (Fig. 4C). These data suggest that Lats1 and Akt are unlikely to be the main regulators of the up-regulated pTAZ under these conditions.
Figure 4. LATS and AKT were unlikely to be involved in regulation of p-TAZ under hypoxia condition.
A. Quantitative analysis of DMOG (500 μM for 24 hr) induced p-TAZ and total TAZ expression in OVCAR5 cells by western blot. B. Hypoxia condition (1% oxygen, 24 and 48 hr) down-regulated p-LAT1 level in OVCAR5 and SKOV3 cells. C. DMOG-induced p-TAZ was not affected by ATK inhibitor MK2206 treatment (1 μM) in OVCAR5 cells. A: Atmosphere O2 pressure, 21% oxygen; H: Hypoxia, 1% oxygen.
Discussion
Both YAP and TAZ are TEAD-interacting transcriptional co-activators. Although TAZ and YAP have been implicated as oncogenes in several cancers (22-25), the potential roles of TAZ in EOC have not been reported until very recently. Jeong et at have shown that in R182 human EOC cells, TAZ is mediating LPA-induced cell migration (10). We showed here that TAZ was also involved in cell proliferation of EOC cells and report the first line of evidence that TAZ also had functional effect on cell migration in human HOSE cells. Although YAP and TAZ are the two most important down-stream factors of the Hippo pathway and they share a high-level homology, evidence derived from YAP and TAZ deficient mice suggests that YAP and TAZ do not compensate each other. YAP knockout animals are embryonic lethal, but TAZ knockout mice are viable withrenal deficiencies (2). In addition, in several reports, the phenotypes of YAP or TAZ knockdown are not masked by the presence of the other (2). Moreover, co-targeting YAP and TAZ appears to be advantageous in restoring anoikis in cells (12). Together with our data shown in this work, they support the notion to target both YAP and TAZ to suppress their oncogenic effects in cancer cells.
The potential involvement of hypoxic condition in regulating YAP and TAZ was not previously reported. We presented the first line of evidence that hypoxic conditions had potent influence in YAP and TAZ regulation. Our results have several surprising and novel aspects. First, whether YAP and TAZ can be differentially regulated by up-stream factors is almost totally unknown (2). We showed that hypoxic conditions had opposing roles in the level of p-YAP and p-TAZ; we showed that p127YAP and p89TAZ (the inactive form of YAP and TAZ, respectively) were regulated in the opposite directions by hypoxic condition in EOC cells. Secondly, DMOG induced a strong reduction of pYAP and a weak reduction of the total YAP levels, indicating YAP activation. However, to our surprise, hypoxic conditions had an overall negative regulatory role in TAZ in EOC cells, since the ratios of pTAZ (an inactive form of TAZ)/total TAZ were increased (Fig. 4). Finally, we showed that HIF1- was differentially involved in in DMOG-induced TAZ and YAP regulation (Fig. 3).
While hypoxia has been identified as an important tumor microenvironment promoting factor for essentially all solid tumors (26), its negative roles in tumorigenesis and positive role in cell death induction have also been noted. Hypoxia and HIF1-α can induce cell death (via autophagy or apoptosis) in various cancer cells, including breast cancer cells (27, 28). Our data suggest that hypoxic conditions might be a negative regulator for TAZ, which warrants further investigation. Hypoxia, TAZ, and YAP haven been all been considered as targets for cancers, including EOC, but the potential negative effects of these genes need to be taking into consideration.
The highly up-regulated pTAZ can be the result from activation of its kinase(s) or down-regulation of its phosphatase. We tested two of its known kinases, Lats and Akt, but found that they were unlikely to be involved in hypoxia-induced pTAZ. We also tested the role of protein phosphatase 1 and our data (not shown) did not support its involvement. Hence, the potential mechanism involved in post-translational regulation remains to be determined. It is difficult to exam S69pTAZ's function directly, since the reagents/methods to specifically delete this pTAZ are not available. However the following lines of evidence suggest its negative roles and the negative regulation of hypoxic conditions in cell proliferation. shRNA of total TAZ reduced cell proliferation. In addition, under the conditions when potent increase in pTAZ was induced by DMOG, cell proliferation was reduced (data not shown). Although it is possible that DMOG-induced growth inhibition was related to its effect on other effectors, increased pTAZ may also contribute to the negative effect of proliferation.
We found that the hypoxic condition induced up-regulation of pTAZ and down-regulation of pYAP in several cell lines derived from different cancer types, including prostate, colon and breast cancers. Taken together, these data suggest that we need to take a closer look and pay more attention when hypoxia is viewed as a target for cancer treatment, since it may be a double-edged sword.
Conclusions
New and differential regulating mechanisms of TAZ and YAP in cancer cells by hypoxia conditions have been revealed. These data indicate that although regulations of YAP and TAZ share many commonalities, they can also be differentially regulated under certain conditions. In addition, these data imply that a closer look and more attention need to be taken when hypoxia and/or HIF1-α are targeted for cancer treatment, since it may be a double-edged sword in tumor development.
Highlights.
YAP and TAZ was regulated by hypoxic conditions in cancer cells.
TAZ was functionally involved in ovarian cancer cells.
Hypoxic conditions had opposing roles in the level of p-YAP and p-TAZ:
HIF1-α was differentially involved in in DMOG-induced TAZ and YAP regulation.
Acknowledgement
This work is supported in part by the National Institutes of Health (RO1 155145 to YX); and the Mary Fendrich-Hulman Charitable Trust Fund to YX. We would like to thank Dr. Mircea Ivan, Indiana University for his support in hypoxic cell culture. We would like to thank Mr. Kevin J. McClelland for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: 17-DMAG, 17-dimethylaminoethylamino-17-demethoxygeldanamycin; CA, constitutively active; Ctrl, control; DFO, deferoxamine; DMOG, dimethyloxaloylglycine; EMT, epithelial-mesenchymal transition; EOC, epithelial ovarian cancer; HIF-1α, hypoxia-inducible factor-1 alpha; HOSE, human ovarian surface epithelial; LPA, lysophosphatidic acid (LPA); WT, wild type; YAP, Yes-associated protein; TNF-α, tumor necrosis factor-alpha; TAZ, transcriptional co-activator with PDZ-binding motif.
References
- 1.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes & development. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Current opinion in cell biology. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Wang L, Katoh H, Liu R, Zheng P, Liu Y. Identification of a tumor suppressor relay between the FOXP3 and the Hippo pathways in breast and prostate cancers. Cancer Res. 2011;71:2162–2171. doi: 10.1158/0008-5472.CAN-10-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 5.Nishio M, Otsubo K, Maehama T, Mimori K, Suzuki A. Capturing the mammalian Hippo: Elucidating its role in cancer. Cancer Sci. 2013 doi: 10.1111/cas.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes & development. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell communication and signaling : CCS. 2013;11:31. doi: 10.1186/1478-811X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, Hilsenbeck SG, Orsulic S, Goode S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, Bowtell DD, Harvey KF. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–2822. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 10.Jeong GO, Shin SH, Seo EJ, Kwon YW, Heo SC, Kim KH, Yoon MS, Suh DS, Kim JH. TAZ mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2013;32:253–263. doi: 10.1159/000354434. [DOI] [PubMed] [Google Scholar]
- 11.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes & development. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradford DS, Boachie-Adjei O. One-stage anterior and posterior hemivertebral resection and arthrodesis for congenital scoliosis. The Journal of bone and joint surgery. American volume. 1990;72:536–540. [PubMed] [Google Scholar]
- 14.Kim KS, Sengupta S, Berk M, Kwak YG, Escobar PF, Belinson J, Mok SC, Xu Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66:7983–7990. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 15.Matteucci E, Maroni P, Luzzati A, Perrucchini G, Bendinelli P, Desiderio MA. Bone metastatic process of breast cancer involves methylation state affecting E-cadherin expression through TAZ and WWOX nuclear effectors. Eur J Cancer. 2013;49:231–244. doi: 10.1016/j.ejca.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Bendinelli P, Maroni P, Matteucci E, Luzzati A, Perrucchini G, Desiderio MA. Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Yao SY, Soutto M, Sriram S. Preconditioning with cobalt chloride or desferrioxamine protects oligodendrocyte cell line (MO3.13) from tumor necrosis factor-alpha-mediated cell death. Journal of neuroscience research. 2008;86:2403–2413. doi: 10.1002/jnr.21697. [DOI] [PubMed] [Google Scholar]
- 18.Agani F, Semenza GL. Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Molecular pharmacology. 1998;54:749–754. doi: 10.1124/mol.54.5.749. [DOI] [PubMed] [Google Scholar]
- 19.Dai Y, Li W, Zhong M, Chen J, Liu Y, Cheng Q, Li T. Preconditioning and post-treatment with cobalt chloride in rat model of perinatal hypoxic-ischemic encephalopathy. Brain & development. 2013 doi: 10.1016/j.braindev.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free radical biology & medicine. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 22.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert opinion on therapeutic targets. 2010;14:855–868. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- 23.Xie M, Zhang L, He CS, Hou JH, Lin SX, Hu ZH, Xu F, Zhao HY. Prognostic significance of TAZ expression in resected non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7:799–807. doi: 10.1097/JTO.0b013e318248240b. [DOI] [PubMed] [Google Scholar]
- 24.Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, Turski A, Azodi Y, Yang Y, Doucette T, Colman H, Sulman EP, Lang FF, Rao G, Copray S, Vaillant BD, Aldape KD. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes & development. 2011;25:2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Cristofaro T, Di Palma T, Ferraro A, Corrado A, Lucci V, Franco R, Fusco A, Zannini M. TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur J Cancer. 2011;47:926–933. doi: 10.1016/j.ejca.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu S, Eguchi Y, Kamiike W, Itoh Y, Hasegawa J, Yamabe K, Otsuki Y, Matsuda H, Tsujimoto Y. Induction of apoptosis as well as necrosis by hypoxia and predominant prevention of apoptosis by Bcl-2 and Bcl-XL. Cancer Res. 1996;56:2161–2166. [PubMed] [Google Scholar]
- 28.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]