Summary
Background
Antigenic drift and shift of influenza viruses require frequent reformulation of influenza vaccines. In addition, seasonal influenza vaccines are often mismatched to the epidemic influenza strains. This stresses the need for a universal influenza vaccine.
Methods
BALB/c mice were vaccinated with the trivalent live attenuated (LAIV; FluMist) or inactivated (TIV; FluZone) influenza vaccines and challenged with PR8 (H1N1), FM/47 (H1N1), or HK/68 (H3N2) influenza virus. Cytokines and antibody responses were tested by ELISA. Furthermore, different LAIV dosages were applied in BALB/c mice. LAIV vaccinated mice were also depleted of T-cells and challenged with PR8 virus.
Results
LAIV induced significant protection against challenge with the non-vaccine strain PR8 influenza virus. Furthermore, protective immunity against PR8 was dose-dependent. Of note, interleukin 2 and interferon gamma cytokine secretion in the lung alveolar fluid were significantly elevated in mice vaccinated with LAIV. Moreover, T-cell depletion of LAIV vaccinated mice compromised protection, indicating that T-cell-mediated immunity is required. In contrast, passive transfer of sera from mice vaccinated with LAIV into naïve mice failed to protect against PR8 challenge. Neutralization assays in vitro confirmed that LAIV did not induce cross-strain neutralizing antibodies against PR8 virus. Finally, we showed that three doses of LAIV also provided protection against challenge with two additional heterologous viruses, FM/47 and HK/68.
Conclusions
These results support the potential use of the LAIV as a universal influenza vaccine under a prime–boost vaccination regimen.
Keywords: Live attenuated influenza vaccine, Trivalent inactivated influenza vaccine, Heterologous influenza viruses, Cross-strain protective immunity, T-cell-mediated immunity, Prime–boost vaccination
Introduction
Influenza viruses are single-stranded, negative-sense RNA viruses with a genome encoding 11–13 viral proteins.1 Influenza viruses are divided into subtypes based on hemagglutinin (HA) and neuraminidase (NA), two main structural surface proteins that induce specific antibodies during influenza virus infection. There are 17 HA and 10 NA subtypes.2–5 The influenza viruses circulating in humans are mainly H1N1, H3N2, and B influenza viruses. Last century, there were three influenza pandemics. The 1918 Spanish influenza pandemic was caused by an H1N1 influenza virus originating from an avian influenza virus,6 while the 1957 Asian influenza (H2N2) and 1968 Hong Kong influenza (H3N2) pandemic viruses were descendants of the 1918 human influenza virus and avian influenza virus, respectively.7 In contrast, a novel H1N1 influenza virus emerged in 2009 to produce the first human influenza pandemic of the twenty-first century; this reassorted virus was initially named swine-origin influenza virus (S-OIV).8 Within 1 year, this new S-OIV had spread to over 214 countries and caused over 18 000 confirmed deaths worldwide.9
The frequent evolution of influenza viruses allows them to escape immunity induced by annual influenza vaccination. This is made possible by point mutations occurring around the conserved receptor binding site of HA protein. Sometimes, reassortment of HA among different influenza virus subtypes, or antigenic shift, results in a new influenza virus subtype for which the population lacks protective immunity and can consequently lead to a new influenza pandemic. Thus, the influenza viruses must be under surveillance and the influenza vaccine must be reformulated each year to keep pace with the mutation of influenza viruses. The pandemic 2009 H1N1 influenza painfully highlighted that the development of a matching vaccine is a time-consuming process, and in many countries, vaccines did not become available until after the peak of the pandemic.10 The rapid dissemination of the 2009 pandemic influenza viruses and the potential for H5N1 virus infection in humans also underscore the urgent need for universal influenza vaccines that elicit cross-immunity against different influenza virus strains.2
Current influenza vaccines rely on trivalent inactivated or live attenuated vaccine (TIV and LAIV, respectively). FluMist (LAIV, nasal spray) and FluZone (TIV, intramuscular injection) are designed for protection against seasonal influenza virus infection. Cross-strain protection in humans as elicited by seasonal influenza vaccination is rare.11,12 Furthermore, two studies showed that prior seasonal influenza vaccination did not have a significant effect on the incidence of 2009 pandemic influenza virus infection.13,14 In contrast, data collected during the 2009 H1N1 influenza pandemic indicated that a large segment of the population previously exposed to an influenza infection needed only one dose of the novel pandemic H1N1 influenza vaccine to sufficiently elicit protective humoral immunity,15 suggesting a priming effect by previous influenza infection.
Similar priming effects have been observed in mice. Mice that were infected with seasonal H1N1 influenza, or vaccinated with seasonal LAIV (s-LAIV) or pandemic H1N1 LAIV (p-LAIV) prior to p-LAIV had increased antibody titers as measured by ELISA.16 Furthermore, seasonal H1N1 infection followed by p-LAIV vaccination, or two doses of p-LAIV, completely protected the mouse respiratory tract from challenge with pandemic H1N1. However, while two-dose vaccination with p-LAIV leads to robust neutralizing antibody titers against pandemic H1N1, infection with seasonal H1N1 influenza followed by p-LAIV vaccination lacks a significant neutralizing antibody response, but elicits strong cellular responses.16 Thus, because cross-reactive neutralizing antibodies may be lacking, an effective way to decrease the severity of influenza A virus infection may be through cross-strain T-cell immunity.
In this study, we evaluated protection against lethal heterologous influenza virus challenge in mice vaccinated with LAIV (FluMist) and TIV (FluZone) using varying prime–boost regimens. In addition, we evaluated if T-cell immunity induced by LAIV is crucial for cross-protection against heterologous influenza infection. Insight into protective, heterologous immunity will be crucial in designing and implementing new methods to control future influenza epidemics and pandemics.
Methods
Cell culture, vaccines, influenza viruses, and mice
Madin–Darby canine kidney epithelial cells (MDCK) were cultivated in modified Eagle's medium (MEM) with 10% fetal calf serum, 1 mM sodium pyruvate, 1× nonessential amino acids, 5 μg/ml gentamicin sulfate, and 4 mM l-glutamine. Seasonal (2011–2012) influenza vaccines FluMist (lot number 501096P) and FluZone (lot number UH467AA) were manufactured by MedImmune (Gaithersburg, MD, USA) and Sanofi Pasteur (Swiftwater, PA, USA), respectively. PR8 influenza virus (H1N1) was produced and rescued using the influenza viral plasmid system consisting of pHW-PB2, pHW-PB1, pHW-PA, pHW-HA, pHW-NP, pHW-NA, pHW-M, pHWNS and propagated in 10-day-old embryonated eggs. The LD50 (median lethal dose) of influenza viruses in mice were determined using the Reed–Muench method. Influenza virus FM/47 (H1N1) was a gift from Dr Richard J. Webby, and HK/68 (H3N2) was provided by the Biodefense and Emerging Infections Research Resources Repository (BEI Resources). Embryonated eggs and BALB/c mice were purchased from Charles Rivers Laboratories (Wilmington, MA, USA). BALB/c mice were housed under specific pathogen-free conditions in the Laboratory Animal Resources Center facilities at Texas Tech University Health Sciences Center.
Vaccination and challenge of mice with PR8 (H1N1) influenza virus
Sixty-four 6–8-week-old BALB/c mice were divided into groups of eight. The following control groups were included: (1) negative control, administered with 50 μl phosphate-buffered saline (PBS) intranasally, and (2) positive control, inoculated intranasally with 25 plaque-forming units (PFU) of PR8 influenza viruses in 50 μl PBS. In the remaining groups, mice were primed with 10 μl FluMist in 40 μl PBS (in a total volume of 50 μl) or 55 μl FluZone on day 0, and boosted with the same dose of FluZone or FluMist on day 28. To test whether intranasal vaccination with FluZone and intramuscular vaccination with FluMist induce a potent humoral immune response, two groups were set. In one group, mice were primed and boosted with 10 μl FluMist in 40 μl PBS intramuscularly. In the other group, mice were primed and boosted with 55 μl FluZone intranasally. Serum was collected and stored at −80 °C until further testing. On day 42 post-vaccination, all mice were challenged with 100 × LD50 PR8 virus and observed for clinical symptoms. When one mouse died, the weight calculation for this group ceased. At the end of the experiments, animals used in the experiments were euthanatized in a CO2 chamber in a manner consistent with the AVMA Guidelines for the Euthanasia of Animals (2013 edition).17
Lung alveolar fluid collection
Forty-eight 6–8-week-old BALB/c mice were divided into eight groups. Two negative control groups were administered with 50 μl PBS intranasally for each mouse, two positive control groups were vaccinated intranasally with 25 PFU PR8 influenza viruses in 50 μl PBS for each mouse, and two groups were vaccinated intranasally with 10 μl FluMist in 40 μl PBS for each mouse. Boost vaccination was performed on day 28. On day 34, one mice group [Au?1] from each of the negative control, positive control, and FluMist groups was sacrificed to collect lung alveolar fluid for the analysis of cytokines. On day 42, the remaining mice were sacrificed to collect lung alveolar fluid to test IgA.
Enzyme-linked immunosorbent assay (ELISA)
Immune sera were tested for influenza-specific IgG and antibodies by quantitative ELISA methods, as described previously,18,19 using FluZone as the plate-coating antigen. Similar ELISA methods were used to measure influenza-specific IgA antibodies, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin 2 (IL-2), and interleukin 4 (IL-4) cytokine levels in the lung alveolar fluid. All antibodies used for cytokine ELISA were purchased from BD Biosciences (San Jose, CA, USA).
Protection experiment with different dosages of LAIV
Thirty-two 6–8-week-old BALB/c mice were divided into four groups. One group was set as the negative control and inoculated intranasally with 50 μl PBS. Mice in the second group were vaccinated with 10 μl FluMist in 40 μl PBS. Mice in the third group were vaccinated with FluMist and boosted at day 15 after primary vaccination, and the fourth group was vaccinated with FluMist and boosted twice at day 15 and day 30 after primary vaccination, respectively. At 15 days after second boost, all mice were challenged with 100 × LD50 PR8 influenza virus. After being challenged, mice were observed and recorded for clinical symptoms and survival.
Anti-influenza virus cross-strain neutralization assay
Cross-strain neutralization antibody titers in vaccinated mouse sera, reactive with PR8 influenza virus, were detected using a microneutralization assay, as described previously.20
Passive serum protection
Twenty-four BALB/c mice were divided into three groups: (1) negative control mice were injected with serum from mice previously treated with PBS; (2) mice were injected with serum from mice vaccinated three times with FluMist; and (3) mice were injected with serum from mice vaccinated with PR8 influenza virus. Serum samples were pooled and incubated at 56 °C for 30 min before injection. Two hundred microliters of inactivated mouse serum was injected into the tail vein on days 0, 1, and 3 post-challenge. Mice were challenged with 100 × LD50 PR8 influenza virus and monitored daily after challenge.
Depletion of CD4+ and CD8+ T-cells in vivo
To test the role of T-cells in the cross-protection induced by FluMist vaccine, mice were vaccinated with FluMist on days 0, 14, and 28. Mice were challenged with 100 × LD50 PR8 influenza virus on day 42 post-vaccination. On days 1 and 3 before challenge, and day 1 after challenge, groups of mice were injected intraperitoneally with rat anti-mouse antibodies: 100 μg anti-CD4+ antibody, 100 μg anti-CD8a+, 100 μg CD4+ and 100 μg CD8+ antibodies, or 100 μg rat IgG2a isotype control (BD Pharmingen). After viral challenge, mice were monitored daily for clinical symptoms and survival.
Virus isolation in mice challenged with FM/47 (H1N1) and HK/68 (H3N2)
Mice were vaccinated with FluMist three times. At 14 days after the last vaccination, mice were challenged with 105 PFU FM/47 (H1N1) or HK/68 (H3N2) influenza virus. At 4 days post-challenge, mice were euthanized. Each lung was collected and homogenized in 0.5 ml PBS. After centrifugation, the viral burden in supernatants was measured by plaque assay on MDCK cell monolayers.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5. The data were expressed as the mean ± standard deviation. Analysis of variance (ANOVA) and/or the Student's t-test were used for comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
Seasonal LAIV (FluMist) vaccination induces protection against lethal challenge with influenza H1N1 PR8 virus
The 2011–2012 seasonal FluMist and FluZone vaccines are designed to protect against three viral strains: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), and B/Brisbane/60/2008. To determine whether vaccination with FluZone and FluMist could induce broad protection against heterologous influenza infection, BALB/c mice were initially vaccinated with FluZone or FluMist and given boosts of FluMist or FluZone 28 days later. The results showed that primary vaccination with FluMist induced some protection against lethal PR8 influenza virus challenge and a boost of FluMist increased protection (Figure 1). Animals primed and boosted with FluMist were protected at a survival rate of 75%; those primed with FluMist and boosted with FluZone were protected at 50%; the group primed with FluZone and boosted with FluMist was protected at 25% (Figure 1B). In contrast, the group vaccinated with only FluZone had a survival rate of 12.5%. After challenge, the weights of mice in the 2 × FluMist group decreased less than weights in the other groups (p < 0.001).
Figure 1.
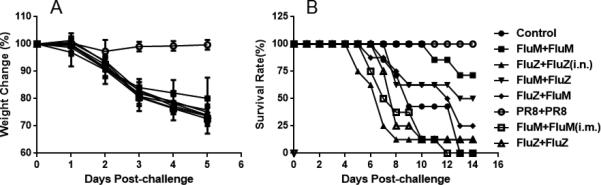
Intranasal vaccination with FluMist induced heterologous protection. Groups of mice were vaccinated with 10 μl FluMist in 40 μl PBS (in a total volume of 50 μl) or 55 μl FluZone, and then boosted at day 28 post-vaccination with the same dose of FluMist or FluZone. Unless otherwise noted, FluMist and FluZone were administered are per manufacturer's indication, intranasally and intramuscularly, respectively. In one group (marked with ‘i.m.’), mice were primed and boosted via intramuscular injections of 10 μl FluMist in 40 μl PBS; in another group, mice (marked with ‘i.n.’) were primed and boosted with 55 μl FluZone intranasally. Mice in the negative control group were inoculated intranasally with 50 μl PBS. At 42 days post-vaccination, mice were lethally challenged with 100 × LD50 PR8 (H1N1) influenza virus. (A) Mouse weight change; the weights of mice in the FluMist + FluMist group decreased less than weights in other groups (p < 0.001). (B) Mouse survival rates by different vaccination regimens. (n = 8 in each group.)
Next, specific antibody concentrations were determined for each group and the results showed that primary vaccination with either FluMist or FluZone induced high systemic levels of IgG when boosted with FluMist, but intranasal vaccinations with FluZone and intramuscular vaccinations with FluMist failed to induce a strong humoral immune response (Figure 2A). Furthermore, prime and boost with FluMist also induced high levels of mucosal IgA (Figure 2B). We also measured the levels of IFN-γ, TNF-α, IL-2, and IL-4 cytokines in mouse lung alveolar fluid. Prime and boost with FluMist induced significant levels of IL-2 and IFN-γ (Figure 3A and 3B), while induction of TNF-α and IL-4 in lung alveolar fluid were low (data not shown).
Figure 2.
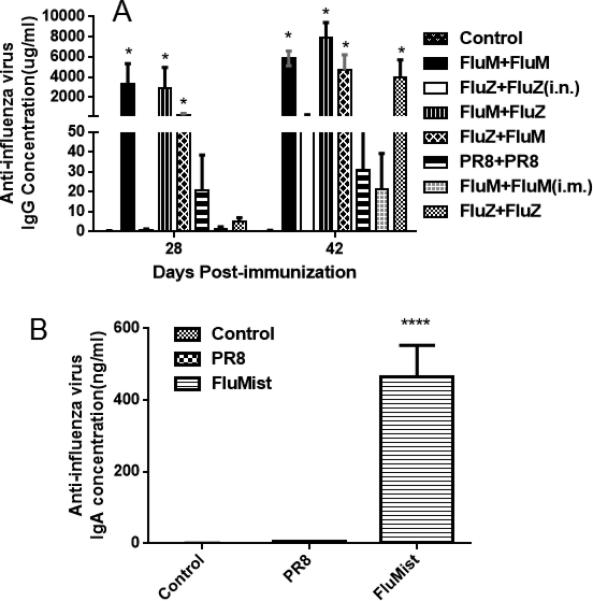
Measurement of anti-influenza IgG in sera and IgA in lung alveolar fluid by ELISA. (A) Mice were primed with 10 μl FluMist in 40 μl PBS or 55 μl FluZone, and then boosted on day 28 with FluZone or FluMist, as indicated. Mouse blood was collected and the anti-influenza IgG concentration in sera was measured (*p < 0.01 compared with the negative PBS control group). (B) Mice were administered with PBS, PR8, or FluMist intranasally, and lung alveolar fluid was collected on day 14 post-boost. Anti-influenza IgA in lung alveolar fluid was measured (****p < 0.0001 compared with the negative PBS control and PR8 groups). (n = 8 in each group.)
Figure 3.
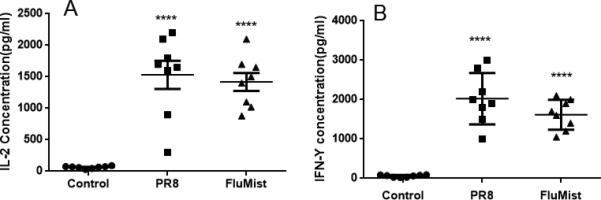
Cytokine levels in lung alveolar fluid of vaccinated mice as determined by ELISA. Mice were primed on day 0, and then boosted on day 28 with PBS, PR8, or FluMist in a 50-μl volume per animal, and then lung alveolar fluid was collected at day 5 post-boost. (A) Interleukin 2 (IL-2) concentration in lung alveolar fluid. (B) Interferon gamma (IFN-γ) level in lung alveolar fluid. (****p < 0.0001 compared with the negative PBS control group; n = 8 in each group.)
Boost with FluMist enhances cross-strain protective immunity
As indicated in Figure 1, prime–boost with FluMist provided better cross-protection against PR8 influenza virus than other experimental groups. To determine if protection by FluMist is dose-dependent, mice were vaccinated one to three times with FluMist, then challenged with lethal PR8 influenza virus. As shown in Figure 4, primary vaccination with FluMist followed by two boosts improved cross-protection to 100% against heterologous fatal PR8 influenza virus challenge in mice, while one vaccination yielded a 37.5% survival rate. Furthermore, these mice recovered their weight by 16 days post-challenge. These results suggest that multiple vaccinations with FluMist could provide complete protection against heterologous PR8 influenza virus challenge in mice.
Figure 4.
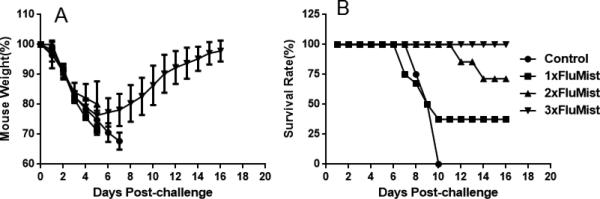
Protection conferred by FluMist is dose-dependent. Thirty-two BALB/c mice were divided into four groups randomly. The first group was set as a negative control group and was inoculated intranasally with 50 μl PBS one time at day 0. The second group was vaccinated intranasally with three doses of 10 μl FluMist in 40 μl PBS (total volume of 50 μl per mouse) on day 0, day 15, and day 29. The third group was vaccinated with two doses of FluMist on day 15 and day 29. The fourth group was treated with one dose of FluMist on day 29. All mice were challenged intranasally with 100 × LD50 PR8 influenza virus on day 42. (A) Weight change caused by PR8 influenza virus challenge. (B) Mouse survival rate following the challenge with PR8 influenza virus. (n = 8 in each group.)
Vaccination with FluMist does not induce cross-reactive neutralizing antibody
To analyze whether the humoral immune response contributes to the cross-protection against heterologous lethal PR8 influenza virus infection, antibody microneutralization assays were performed using MDCK cells. The results demonstrated that vaccination with FluMist did not elicit high neutralizing serum antibody titers against PR8 influenza virus (Table 1). To confirm these results in vivo, passive transfer of serum from vaccinated mice to naïve mice was performed and protection in these mice was evaluated against challenge with PR8 virus. Passive injection of mice with serum containing antibodies induced by FluMist did not provide protection against infection by heterologous PR8 influenza virus (Figure 5). Taken together, these results indicate that vaccination with FluMist or FluZone does not produce neutralizing antibodies against heterologous lethal influenza virus challenge in mice.
Table 1.
Microneutralization (MN) titers against PR8 (H1N1) influenza virus in sera from vaccinated micea
| Vaccination regimen | MN titer in mouse sera |
|---|---|
| Control (PBS) | <8 |
| FluMist + FluMist | <8 |
| FluZone + FluZone (i.n.) | <8 |
| FluMist + FluZone | <8 |
| FluZone + FluMist | <8 |
| PR8 + PR8 | >256 |
| FluMist + FluMist (i.m.) | <8 |
| FluZone + FluZone | <8 |
PBS, phosphate-buffered saline.
Sera were collected from negative control (PBS) mice, or mice vaccinated with FluMist or FluZone and boosted with FluZone or FluMist, or positive control mice vaccinated intranasally with PR8 influenza viruses. There are two special groups: in one group (marked with ‘i.m.’), mice were injected with 10 μl FluMist in 40 μl PBS and boosted with 10 μl FluMist in 40 μl PBS; in another group, mice (marked with ‘i.n.’) were primed and boosted intranasally with 55 μl FluZone.
Figure 5.
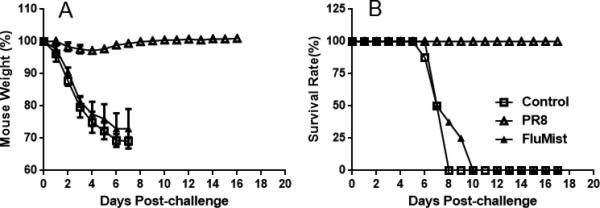
Passive protection provided by injection with sera from mice vaccinated with PR8 influenza virus or FluMist. Mice were injected intraperitoneally with 200 μl sera from negative control, PR8 vaccinated, or FluMist (three doses) vaccinated mice, and were then challenged with 100 × LD50 PR8 influenza virus in 50 μl PBS. (A) Weight change after mice were challenged with PR8 influenza virus. (B) Mouse survival rate after mice were challenged with PR8 influenza virus. (n = 8 in each group.)
T-cells are crucial in the cross-protection induced by FluMist vaccination
To further explore the mechanism of cross-protection against heterologous lethal influenza viruses induced by vaccination with FluMist, T-cell depletion studies were performed in vivo. Successful depletion was confirmed by flow cytometry analysis of peripheral blood mononuclear cells (PBMC) where 98% of CD4 and 96% of CD8 T-cells were depleted (data not shown). T-cell-depleted mice were challenged with 100 × LD50 PR8 influenza virus. As shown in Figure 6, CD4+ T-cell-depleted mice had a 50% survival rate and CD8+ T-cell-depleted mice had a 25% survival rate. Mice injected with an isotype control all survived challenge. Mice in the isotype control group recovered most of their initial weights by day 16 post-challenge. These results suggest that CD8+ or CD4+ T-cell depletion impairs the cross-protection induced by vaccination with FluMist.
Figure 6.
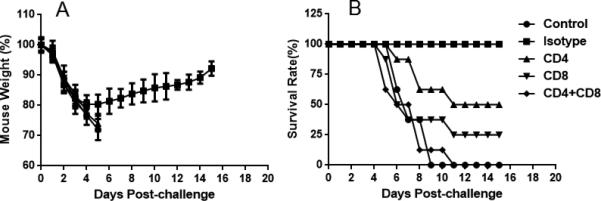
Contribution of T-cells in protective immunity determined by T-cell depletion experiments in vivo. Cross-strain protective immunity induced by prime– boost vaccination with FluMist was evaluated in mice depleted of T-cells (depletion of CD4+, CD8+, or both CD4+ and CD8+ T-cells). (A) Weight change in mice depleted of T-cells after PR8 influenza virus challenge. (B) Mouse survival rates after PR8 influenza virus challenge. (n = 8 in each group.)
FluMist vaccination induces protection against challenge with heterologous influenza viruses FM/47 (H1N1) and HK/68 (H3N2)
To further confirm that vaccination with FluMist can provide heterologous protection against various influenza viruses, we vaccinated mice with FluMist three times and challenged with FM/47(H1N1) or HK/68(H3N2) influenza virus at 14 days after the last vaccination. At 4 days after challenge, mice were euthanized and lungs were collected for harvest of lung alveolar fluid. The viruses in lung alveolar fluid were tested by plaque assay on monolayer MDCK cells. FluMist also provided protection against FM/47 and HK/68 influenza viruses, as shown in Figure 7.
Figure 7.
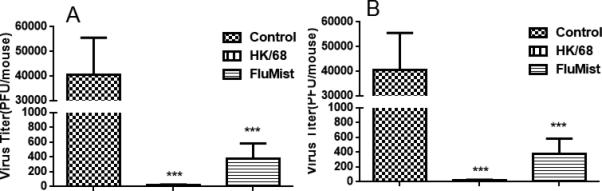
Intranasal vaccination with FluMist induced cross-strain protection. Mice in the negative control group were treated with 50 μl PBS. Mice in the positive control groups were vaccinated with 10 PFU FM/47 (H1N1) or HK/68 (H3N2) influenza viruses in 50 μl PBS on day 0. FluMist groups were vaccinated with three doses of 10 μl FluMist in 40 μl PBS on days 0, 15, and 29. On day 42, mice were challenged with 105 PFU FM/47 or HK/68 influenza virus in 50 μl PBS. (A) Viral titers in lungs of mice challenged with FM/47 at 4 days post-challenge. (B) Viral titers in lungs of mice challenged with HK/68 at 4 days post-challenge. (***p < 0.001 compared with the negative control group; n = 8 in each group.)
Discussion
Current vaccine strategies against influenza focus on generating robust antibody responses. Since the outbreak of 2009 pandemic influenza, considerable interest has been revived on the development of broadly protective influenza vaccines that would ideally afford broad protection against various subtypes of influenza A virus. The traditional strategies for creating a universal influenza vaccine focus on the conserved epitopes of influenza protein, in particular the HA2 and M2 protein regions.21–24 However, several studies showed that infection with seasonal influenza virus can provide cross-protection against 2009 pandemic influenza virus, H5N1 influenza virus, and even against the 1918 pandemic influenza virus.25–27 Moreover, some results showed that infection with 2009 pandemic influenza virus or vaccination with seasonal TIV could induce cross-reactive immunity against 1918 H1N1 influenza virus.28–30 Furthermore, previous infections with influenza virus may serve to prime future influenza virus vaccinations.15,16 These studies indicate that there are antigenic similarities among these viruses.
In this study, we tested whether multiple-dose combinations of seasonal influenza vaccines FluZone or FluMist could provide cross-protection against heterologous influenza virus infection. Here, we used PR8 influenza virus as a heterologous influenza virus model. In initial experiments, the survival rate of mice primed and boosted with FluZone was only 12.5%. This result is in accordance with the result observed by Manicassamy et al. in a mouse model vaccinated intramuscularly with seasonal TIV and challenged with 2009 pandemic influenza virus.25 The survival rate of mice primed and boosted with FluMist was 75% (p < 0.001) and the survival rate of mice primed with FluMist and boosted with FluZone reached 50%. These results indicate that vaccination with FluMist provided cross-protection against lethal PR8 challenge to some extent, while vaccination with FluZone did not, although vaccination with seasonal FluZone induced a strong humoral immune response. Of note, the antibodies produced following varying prime–boost regimens with FluZone and FluMist vaccines did not neutralize PR8 virus as tested in the microneutralization assays.
Apart from the humoral immune response, some studies have indicated that there are other mechanisms capable of conferring complete protection against heterologous influenza virus challenge. Studies have shown that seasonal influenza infection can induce B-cell-dependent cross-protection or cross-reactive cytotoxic T-lymphocytes against 2009 pandemic H1N1 influenza virus, and that seasonal FluMist vaccination also induces cross-reactive T-cell immunity against 2009 H1N1 influenza virus.16,31–33 Results published by Bodewes et al. suggested that vaccination with seasonal influenza H3N2 virus induces T-cell-dependent heterosubtypic immunity against influenza H5N1 or 2009 pandemic influenza virus infection in ferrets.34,35 Infection with pre-1950 H1N1 influenza viruses can also produce T-cell-mediated cross-protection against the pandemic 2009 influenza viruses.36,37
Having observed such promising results using a prime–boost regimen with FluMist to protect against heterologous virus challenge independent of neutralizing antibody, we decided to vaccinate mice three times with FluMist and also evaluate T-cell responses in addition to protection. The survival of mice vaccinated three times with FluMist improved to 100% after challenge with PR8 virus. Although there was a substantial weight decrease in these infected mice, they recovered completely by day 16 post-challenge. Protection against two other heterologous viruses was also observed. Of significance, this cross-strain protective immunity was found to be T-cell-dependent, as depletion of CD4+, CD8+, or both T-cell types greatly diminished protection. Furthermore, significant secretion of IFN-γ and IL-2 cytokines into the lung alveolar fluid of mice primed and boosted with FluMist corroborated a role for T-cells in cross-protective immunity.
Several other studies have observed that influenza-specific CD8 T-cells are stimulated in the lung of mice infected with seasonal attenuated or wild-type influenza virus. Furthermore, CD8 T-cells contribute to viral clearance in the lungs via lysis of target cells following exocytosis of granules containing perforin and granzymes.38,39 On the other hand, cross-reactive CD4 T-cells are primarily responsible for helping other immune cells through direct cell-to-cell interactions and by secreting cytokines, but are also essential for establishing long-lasting CD8 T-cell memory, antibody, and in vivo lytic activity, and contribute to viral clearance.40,41 To date, little is known about the role of CD4 T-cells as direct mediators of effector function, but in mouse models, several influenza-specific CD4 epitopes have been identified.42–46 Some studies have shown that NP- or M2-specific CD4 epitopes are crucial for the cross-protection to heterologous influenza virus infection.31,33,46,47 Other studies using animal models have suggested that CD8 T-cells are more important than CD4 T-cells for cross-protection.29,31,37,48,49 Fang et al. reported that seasonal influenza virus infection also conferred cross-reactive protection against 2009 pandemic H1N1 influenza virus through a B-cell-dependent and CD8 T-cell-independent mechanism.32 Our study showed both CD4+ and CD8+ to be important to heterologous immunity, although depletion of CD8+ was more detrimental to survival of mice primed and boosted with FluMist than those depleted of CD4+ (25% to 50% survival, respectively). Furthermore, vaccinated mice depleted of CD4+ and CD8+ T-cells succumbed to lethal viral challenge and did not live significantly longer than the unvaccinated group of mice.
It was surprising that a multiple-dose LAIV vaccination regimen was more protective than multiple doses of TIV, and induced T-cell responses. As tested in young children, two doses of seasonal LAIV is more protective against seasonal influenza than two doses of seasonal TIV, and two-dose combinations with LAIV induce influenza-specific CD4+, CD8+, and γδ T-cells.50
In conclusion, our study is noteworthy in showing that multiple doses of LAIV can provide T-cell-dependent protection against various non-vaccine strains. Moreover, as multiple doses of LAIV are approved for use in young children, it is presumed to be a safe regimen. Thus, it is possible that prime–boost vaccination with LAIV can be used in combating a potentially new influenza epidemic, or an even more dangerous pandemic.
Highlights.
Prime-boost with LAIV (FluMist) provided cross-strain protective immunity
Protective immunity with this LAIV was vaccine dose-dependent
Serum IgG responses did not contribute to virus neutralization
CD4+ and CD8+ T cell immunities were essential for cross-strain protection
Acknowledgements
This work was supported by a US Public Service research grant AI072139 (to M.Z.) from the National Institute of Allergy and Infectious Diseases and an internal fund from Texas Tech University Health Sciences Center, Paul L. Foster School of Medicine. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript. We are grateful to David Topham for providing MDCK cells, to Richard J. Webby for providing the PR8 influenza viral plasmid system and influenza FM/47 virus strain, and to the NIH-funded BEI Resources for supplying seasonal influenza vaccines and the influenza HK/68 strain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval: Animal research in this study was approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee (IACUC) under protocol number 10020.
Conflict of interest: No conflict of interest to declare.
References
- 1.Li J, Arevalo MT, Zeng M. Engineering influenza viral vectors. Bioengineered. 2013;4:9–14. doi: 10.4161/bioe.21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horimoto T, Kawaoka Y. Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol. 2005;3:591–600. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- 3.Wise HM, Foeglein A, Sun J, Dalton RM, Patel S, Howard W, et al. A complicated message: identification of a novel PB1-related protein translated from influenza A virus segment 2 mRNA. J Virol. 2009;83:8021–31. doi: 10.1128/JVI.00826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012;109:4269–74. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol. 2004;2:909–14. doi: 10.1038/nrmicro1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallat B. Flu: no sign so far that the human pandemic is spread by pigs. Nature. 2009;460:683. doi: 10.1038/460683b. [DOI] [PubMed] [Google Scholar]
- 9.Medina RA, Garcia-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol. 2011;9:590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abelin A, Colegate T, Gardner S, Hehme N, Palache A. Lessons from pandemic influenza A(H1N1): the research-based vaccine industry's perspective. Vaccine. 2011;29:1135–8. doi: 10.1016/j.vaccine.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 11.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 12.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaillant L, La Ruche G, Tarantola A, Barboza P, epidemic intelligence team at InVS Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009:14. doi: 10.2807/ese.14.33.19309-en. pii: [Au?2] [DOI] [PubMed] [Google Scholar]
- 14.Kelly H, Grant K, Williams S, Smith D. H1N1 swine origin influenza infection in the United States and Europe in 2009 may be similar to H1N1 seasonal influenza infection in two Australian states in 2007 and 2008. Influenza Other Respir Viruses. 2009;3:183–8. doi: 10.1111/j.1750-2659.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 16.Chen GL, Lau YF, Lamirande EW, McCall AW, Subbarao K. Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc Natl Acad Sci U S A. 2011;108:1140–5. doi: 10.1073/pnas.1009908108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AVMA guidelines for the euthanasia of animals: 2013 edition. AVMA; Schaumburg, IL: 2013. pp. 1–102. [Google Scholar]
- 18.Xu Q, Pichichero ME, Simpson LL, Elias M, Smith LA, Zeng M. An adenoviral vector-based mucosal vaccine is effective in protection against botulism. Gene Therapy. 2009;16:367–75. doi: 10.1038/gt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur R, Chen S, Arevalo MT, Xu Q, Chen Y, Zeng M. Protective immunity against tularemia provided by an adenovirus-vectored vaccine expressing Tul4 of Francisella tularensis. Clin Vaccine Immunol. 2012;19:359–64. doi: 10.1128/CVI.05384-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grund S, Adams O, Wahlisch S, Schweiger B. Comparison of hemagglutination inhibition assay, an ELISA-based microneutralization assay and colorimetric microneutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J Virol Methods. 2011;171:369–73. doi: 10.1016/j.jviromet.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Qu M, Li J, Jia L, Tan XJ, Gao ZY, Yan HQ, et al. [Etiology of hand-foot-and-mouth disease and genetic characteristics of coxsackievirus A16 in Beijing, 2009]. Bing Du Xue Bao. 2010;26:432–6. [PubMed] [Google Scholar]
- 22.Wang W, Anderson CM, De Feo CJ, Zhuang M, Yang H, Vassell R, et al. Cross-neutralizing antibodies to pandemic 2009 H1N1 and recent seasonal H1N1 influenza A strains influenced by a mutation in hemagglutinin subunit 2. PLoS Pathog. 2011;7:e1002081. doi: 10.1371/journal.ppat.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A “universal” human influenza A vaccine. Virus Res. 2004;103:173–6. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 24.De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–7. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manicassamy B, Medina RA, Hai R, Tsibane T, Stertz S, Nistal-Villan E, et al. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog. 2010;6:e1000745. doi: 10.1371/journal.ppat.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang FY, Zu RQ, Li L, Qi X, Ji H, Wu B, et al. [Serologic survey on pandemic influenza A (H1N1) virus among aged >/= 3 years population from Jiangsu Province in 2009, China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:489–93. [PubMed] [Google Scholar]
- 27.Medina RA, Rojas M, Tuin A, Huff S, Ferres M, Martinez-Valdebenito C, et al. Development and characterization of a highly specific and sensitive SYBR green reverse transcriptase PCR assay for detection of the 2009 pandemic H1N1 influenza virus on the basis of sequence signatures. J Clin Microbiol. 2011;49:335–44. doi: 10.1128/JCM.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medina RA, Manicassamy B, Stertz S, Seibert CW, Hai R, Belshe RB, et al. Pandemic 2009 H1N1 vaccine protects against 1918 Spanish influenza virus. Nat Commun. 2010;1:28. doi: 10.1038/ncomms1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gras S, Kedzierski L, Valkenburg SA, Laurie K, Liu YC, Denholm JT, et al. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci U S A. 2010;107:12599–604. doi: 10.1073/pnas.1007270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce MB, Belser JA, Gustin KM, Pappas C, Houser KV, Sun X, et al. Seasonal trivalent inactivated influenza vaccine protects against 1918 Spanish influenza virus in ferrets. J Virol. 2012 doi: 10.1128/JVI.00674-12. [Au?3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol. 2010;84:6527–35. doi: 10.1128/JVI.00519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Y, Banner D, Kelvin AA, Huang SS, Paige CJ, Corfe SA, et al. Seasonal H1N1 influenza virus infection induces cross-protective pandemic H1N1 virus immunity through a CD8-independent, B cell-dependent mechanism. J Virol. 2012;86:2229–38. doi: 10.1128/JVI.05540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T-cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol. 2011;186:987–93. doi: 10.4049/jimmunol.1002664. [DOI] [PubMed] [Google Scholar]
- 34.Bodewes R, Kreijtz JH, Geelhoed-Mieras MM, van Amerongen G, Verburgh RJ, van Trierum SE, et al. Vaccination against seasonal influenza A/H3N2 virus reduces the induction of heterosubtypic immunity against influenza A/H5N1 virus infection in ferrets. J Virol. 2011;85:2695–702. doi: 10.1128/JVI.02371-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hillaire ML, van Trierum SE, Kreijtz JH, Bodewes R, Geelhoed-Mieras MM, Nieuwkoop NJ, et al. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J Gen Virol. 2011;92:2339–49. doi: 10.1099/vir.0.033076-0. [DOI] [PubMed] [Google Scholar]
- 36.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T-cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. 2011;85:448–55. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skountzou I, Koutsonanos DG, Kim JH, Powers R, Satyabhama L, Masseoud F, et al. Immunity to pre-1950 H1N1 influenza viruses confers cross-protection against the pandemic swine-origin 2009 A (H1N1) influenza virus. J Immunol. 2010;185:1642–9. doi: 10.4049/jimmunol.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topham DJ, Tripp RA, Doherty PC. CD8+ T-cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–200. [PubMed] [Google Scholar]
- 39.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown DM, Dilzer AM, Meents DL, Swain SL. CD4 T-cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J Immunol. 2006;177:2888–98. doi: 10.4049/jimmunol.177.5.2888. [DOI] [PubMed] [Google Scholar]
- 41.Topham DJ, Tripp RA, Sarawar SR, Sangster MY, Doherty PC. Immune CD4+ T-cells promote the clearance of influenza virus from major histocompatibility complex class II −/− respiratory epithelium. J Virol. 1996;70:1288–91. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowe SR, Miller SC, Brown DM, Adams PS, Dutton RW, Harmsen AG, et al. Uneven distribution of MHC class II epitopes within the influenza virus. Vaccine. 2006;24:457–67. doi: 10.1016/j.vaccine.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 43.Babon JA, Cruz J, Ennis FA, Yin L, Terajima M. A human CD4+ T cell epitope in the influenza hemagglutinin is cross-reactive to influenza A virus subtypes and to influenza B virus. J Virol. 2012;86:9233–43. doi: 10.1128/JVI.06325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duvvuri VR, Marchand-Austin A, Eshaghi A, Patel SN, Low DE, Gubbay JB. Potential T-cell epitopes within swine-origin triple reassortant influenza A (H3N2) variant virus which emerged in 2011: an immunoinformatics study. Vaccine. 2012;30:6054–63. doi: 10.1016/j.vaccine.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 45.McKinstry KK, Strutt TM, Kuang Y, Brown DM, Sell S, Dutton RW, Swain SL. Memory CD4+ T-cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122:2847–56. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T-cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–80. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt T, Dirks J, Enders M, Gartner BC, Uhlmann-Schiffler H, Sester U, Sester M. CD4+ T-cell immunity after pandemic influenza vaccination cross-reacts with seasonal antigens and functionally differs from active influenza infection. Eur J Immunol. 2012;42:1755–66. doi: 10.1002/eji.201242393. [DOI] [PubMed] [Google Scholar]
- 48.Weinfurter JT, Brunner K, Capuano SV, 3rd, Li C, Broman KW, Kawaoka Y, Friedrich TC. Cross-reactive T-cells are involved in rapid clearance of 2009 pandemic H1N1 influenza virus in nonhuman primates. PLoS pathogens. 2011;7:e1002381. doi: 10.1371/journal.ppat.1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge X, Tan V, Bollyky PL, Standifer NE, James EA, Kwok WW. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J Virol. 2010;84:3312–9. doi: 10.1128/JVI.02226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis. 2011;204:845–53. doi: 10.1093/infdis/jir436. [DOI] [PMC free article] [PubMed] [Google Scholar]


