Abstract
Background
To quantify and characterize duplicated tests done during the staging of localized colon cancer in Medicare population.
Methods
We used the SEER-Medicare linked database to select patients diagnosed with localized colon cancer the years 1996–2009. We considered a patient as adequately staged after having received a colonoscopy, an abdominal CT scan, and a pelvic CT scan. Abdominal and pelvic CT scans performed between complete staging and first cancer-directed treatment, if not ordered due to an acute condition, were considered duplicates. We characterized the institutions providing the tests and evaluated the association with survival using a weighted pooled logistic regression adjusted by baseline and time-varying confounders.
Results
Of 36,291 patients with a complete staging, 2,680 (7.4%) had at least one duplicated test. Patients receiving a duplicate had a higher comorbidity score, were more symptomatic, and had more visits to the emergency department and clinical evaluations. They also were treated with surgery less frequently and had worse survival (HR 1.22, 95% CI 1.16-1.28). The type of institution involved in the staging (non-profit/government centers, proprietary centers, free-standing facilities) was not associated with receiving duplicated tests.
Conclusion
We found a low frequency of duplicated abdominal or pelvic CT scans in the staging of colon cancer in Medicare population.
Keywords: colon cancer, CAT scan, SEER-Medicare, staging, prognosis
Introduction
Every year more than 100,000 cases of colon cancer are diagnosed in the United States1. Appropriate staging of these tumors is necessary for informed therapeutic decisions. Clinical staging is intended to detect metastatic disease that rules out the ability to perform curative intent surgical resection of tumor. Clinical guidelines for the diagnosis and staging of colon cancer recommend the use of colonoscopy, abdominal and pelvic CT scans2–7, and pathologic examination of the surgical specimen for localized tumors.
Health care costs in the United States are projected to account for 20% of the gross domestic product in 20208. A key measure to cut down costs is the avoidance of services that do not benefit patients9, 10. The “Choosing Wisely” campaign explicitly points at the elimination of duplicated tests as a benefit of promoting conversations between physicians and patients 11. Thus, avoidance of unnecessary tests for the diagnosis and staging of colon cancer might be a potential target for cost-containment measures.
Medicare patients receive coverage for all tests required for diagnosis and staging of colon cancer. While imposing no restrictions on the number of test covered, Medicare encourages patients to avoid unnecessary duplication of tests12. The proportion of duplicated tests is, however, unknown. If diagnostic workup includes duplicative workup, there is a potential strategy for improving care quality while also controlling health care costs. Here we quantify and characterize the frequency of duplicated tests performed in the fee for service Medicare population during the clinical staging of early stage colon cancer.
Methods
Study population
The study cohort was identified from the SEER-Medicare data, which is a linkage of patient demographic and tumor-specific variables collected by 17 SEER cancer registries across 12 states with Medicare claim files from the Centers for Medicare and Medicaid Services13. SEER data are summarized in the Patient Entitlement and Diagnosis Summary File (PEDSF), which is linked with 100% of Medicare claims. For the current study we used Medicare claims from the Inpatient, Outpatient, Home Health Agency, Durable Medical Equipment (DME), Medpar and National Claims History (NCH) files. Provider characteristics are extracted from the Hospital file, which contains information on hospital characteristics for years 1996, 1998 and 2000 to 2009.
Our analysis includes patients 66 years (to allow for at least one year of claims before diagnosis) or older, with histological diagnosis of invasive colon adenocarcinoma between 1996 (when hospital information first became available) and 2009 in a SEER area. We excluded rectal cancer and rectosigmoid tumors (which may require additional staging like MRI or endoscopic ultrasound) and cancers for which the reporting source was nursing home/hospice, autopsy or death certificate. To ensure complete ascertainment of health services, patients had to be enrolled in parts A and B and not in an HMO during the six months before and after diagnosis. We excluded patients diagnosed in Louisiana in 2005 because of the disruption of data collection following hurricane Katrina.
We considered a patient as adequately staged and ready for a therapeutic decision after having received a colonoscopy, an abdominal CT scan, and a pelvic CT scan, as prescribed by the National Comprehensive Cancer Network and European Society for Medical Oncology2–7. We did not require a chest CT scan which is considered by some guidelines 6, but not others7, 14 (see Table, Supplemental Digital Content 1, for codes used to identify these tests). Tests are extracted from the claims six months before and after SEER date of diagnosis.
Definition of duplicated test
Any abdominal CT scan or pelvic CT scan received between the date when the patient was completely staged (see above) and the date of first treatment was considered a duplicate, with the exception of scans performed because of acute conditions 15(see Table, Supplemental Digital Content 2, for the list of conditions and codes). Treatment of colon cancer was defined as colon surgery, radiotherapy, or chemotherapy (see Table, Supplemental Digital Content 1 for codes used to identify these treatments). Tests performed beyond 90 days of complete staging were not considered duplicates under the assumption that restaging might be appropriate if a patient has not been treated within 90 days.
Covariates
Demographic characteristics (age, sex, race, marital status, urbanicity), tumor features (TNM stage, grade of tumor differentiation, date of diagnosis), and census tract features (census region, percentage of black population, percentage of residents living below the poverty level, percentage of residents aged 25 or older with less than 12 years of education, percentage of residents speaking English not well/not at all at age 65+, median income) were extracted from the PEDSF file. Comorbidities were summarized using the Deyo-Charlson-Klabunde comorbidity index16, derived from the inpatient and outpatient Medicare claims for the period between 12 months to 1 month before diagnosis. To assess health services utilization, we computed a “preventive score” 17, the number of “low complexity visits” in the 24 months one year before diagnosis, and emergency room visits.
The provider performing the tests was linked with the institution information on the Hospital file. The Outsaf and Medpar files, but not the NCH file, contain a variable that allows linkage of providers with institutions without identifiers. NCH claims can correspond to either a test performed by a free-standing facility or to a professional service performed at an institutional provider (and thus also recorded in the Outsaf or Medpar files). We thus classified patients according to the type of institution involved in their staging work-up: all tests performed in institutional non-profit/government centers, at least one test in a proprietary center, and all tests in free-standing facilities or free-standing facilities plus non-profit/government centers (see table in Supplemental Digital Content 1 for the codes used to extract this information).
Mortality Analysis
For each patient, follow-up started at complete staging (see above) and ended at date of death or administrative cutoff date (in PEDSF file, 12/31/2010), whichever occurred earlier. We estimated the mortality hazard ratio (HR) for “receiving at least one duplicated test”versus “not receiving any duplicated test”within 3 months of complete staging. To do so, we fit a weighted pooled logistic model that included an indicator for duplicated tests, a flexible function of time (restricted cubic splines to estimate the baseline hazard) and the baseline covariates described above. We calculated robust standard errors to compute conservative 95% confidence intervals for the effect estimate.
As in previous analyses of exposures that are not fully determined at baseline, we used data replication, censoring, and inverse probability weighting 18,19 to adjust for the time-varying covariates: visits to the emergency room, clinical evaluations, change in comorbidity index and development of large bowel obstruction. We then stabilized the weights to emulate a uniform duplicated test administration during three months20. Like previous applications of inverse probability weighting 21–23, we truncated weights at percentile 99. All analyses were conducted with SAS, version 9.3 (SAS Institute, Cary, North Carolina).
Results
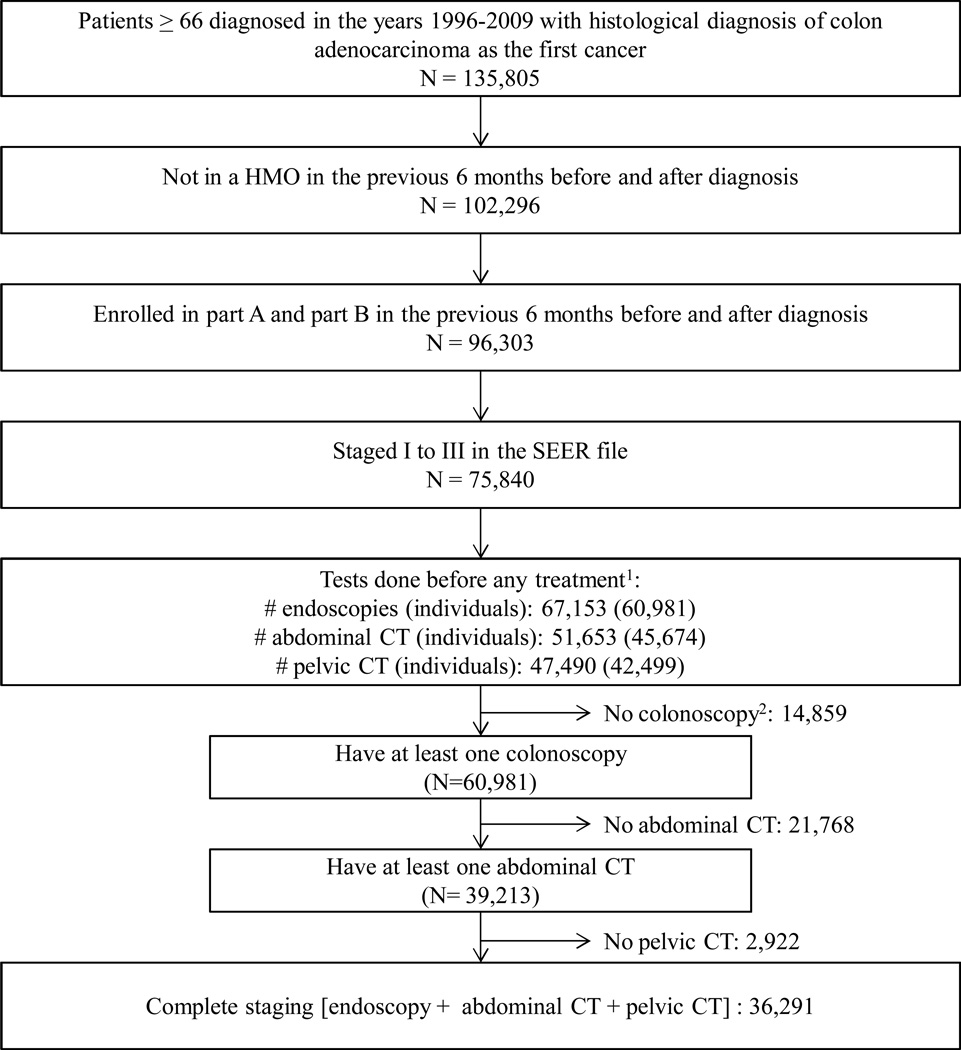
Of 75,840 eligible patients, 36,291 had complete staging: 25% stage I, 43% stage II and 33% stage III (Figure 1). We found that 2,680 (7.4%) of patients had at least one duplicated CT scan. Of the 2,680 patients with duplicated tests, 68% received one duplicated abdominal CT scan plus one duplicated pelvic CT scan, and only 8% received more than two duplicated tests (see Table, Supplemental Digital Content 3). After complete staging, a colonoscopy was repeated in 5.5% of the patients.
Figure 1. Flowchart of colon cancer patients.
1Time frame for diagnostic tests: −180 to +180 days/first treatment since diagnosis. See Methods.
24,795patients presented a diagnosis of large bowel obstruction and 1,199presented a diagnosis of large bowel perforation, both contraindications for colonoscopy.
Table 1 shows the baseline characteristics of the patients. Patients receiving a duplicated CT scan had a higher comorbidity score; lived in census areas with a higher percentage of high school drop-outs, residents below poverty line, black race/ethnicity, and lower median incomes; and were more likely to have anemia, asthenia, and gastrointestinal symptoms in the six months before diagnosis.
Table 1.
Baseline characteristics of patients with early colon cancer 66 years or older at diagnosis, enrolled in part A and B of Medicare, not in a HMO and with a complete staging(N=36,291).
| Not receiving any duplicate scan(N=33,611) |
Receiving at least one duplicate scan(N=2,680) |
|
|---|---|---|
| Sentinel symptom(%) | ||
| Anemia | 26,007(77) | 2,202(82) |
| Gastrointestinal symptoms1 | 15,194(45) | 1,540(57) |
| Large bowel obstruction | 6,993(21) | 794(30) |
| Abnormal weight loss | 5,775(17) | 629(23) |
| Asthenia | 14,015(42) | 1,391(52) |
| Comorbidity score(%) | ||
| 0 | 17,645(53) | 1,117(42) |
| 1 | 8,514(25) | 717(27) |
| 2+ | 6,121(18) | 743(28) |
| Unknown | 1,331(4) | 103(4) |
| Median age when staged(range) | 78.4(65.9–106.3) | 78.6(65.8–99.2) |
| Female(%) | 19,620(58) | 1,564(58) |
| Race(%) | ||
| Caucasian NOS | 27,947(83) | 2,171(81) |
| Caucasian, Spanish origin or surname | 1,384(4) | 117(4) |
| African American | 2,590(8) | 250(9) |
| Asian/Pacific islander | 1,526(5) | 131(5) |
| Other/Unknown/unspecified | 164(0) | 11(0) |
| Stage(%) | ||
| I | 8,623(25) | 671(25) |
| II | 14,449(43) | 1,088(41) |
| III | 10,899(32) | 921(34) |
| Grade of differentiation(%) | ||
| Well differentiated | 2,711(8) | 201(8) |
| Moderately differentiated | 22,841(68) | 1,749(65) |
| Poorly differentiated | 6,907(21) | 589(22) |
| Unknown | 1,152(3) | 141(5) |
| Median number of low complexity visits(Q1–Q3)2 | 7(2–13) | 7(3–15) |
| Median preventive score(Q1–Q3)3 | 2(1–3) | 2(1–3) |
| Urbanicity(%) | ||
| Big Metro (≥1 million population) | 19,361(58) | 1,595(60) |
| Metro (250,000 to 1 million) | 9,135(27) | 650(24) |
| Urban (20,000 to 250,000) | 1,821(5) | 127(5) |
| Less Urban (2,500 to 20,000) | 2,672(8) | 246(9) |
| Rural (rural or < 2,500 population) | 622(2) | 62(2) |
| Marital Status(%) | ||
| Single | 2,593(8) | 219(8) |
| Married | 16,168(48) | 1,176(44) |
| Separated/divorced | 2,059(6) | 173(6) |
| Widowed | 11,446(34) | 1,021(38) |
| Unknown | 1,345(4) | 101(4) |
| SEER registry census region(%) | ||
| West | 11,309(34) | 867(32) |
| Northeast | 9,897(29) | 809(30) |
| Midwest | 5,203(15) | 395(15) |
| South | 6,741(20) | 588(22) |
| Pacific | 461(1) | 21(1) |
| Year of diagnosis(%) | ||
| 1996–2000 | 5,580(17) | 318(12) |
| 2001–2005 | 15,812(47) | 1,184(44) |
| 2006–2009 | 12,219(36) | 1,178(44) |
| Census tract features4[median(Q1–Q3)] | ||
| % did not complete high school | 15.7(9.4–25.5) | 17.1(10.4–27.5) |
| % below poverty line | 7.6(4.1–14.3) | 8.2(4.4–15.9) |
| % black race/ethnicity | 2.1(0.6–7.7) | 2.4(0.7–9.5) |
| % English not well/at all at 65+ | 1.6(0–5.5) | 1.5(0–5.8) |
| Median income(USD) | 46,163(34,742–61,152) | 44,712(33,099–60,007) |
Gastrointestinal symptoms include abdominal distention, change in bowel habit, constipation, irritable bowel syndrome, diarrhea, obstruction, anemia, abnormal weight loss, asthenia.
Low complexity visits(as defined by CPT codes, see appendix Table A1) during the years -2 and -3 of diagnosis.
See methods section for details.
Census tract features are missing for 218 individuals
Patients with duplicated CT scans had more clinical evaluations and were more likely to visit the emergency department in the time span from being completely staged to first treatment (Table 2). Patients with duplicated CT scans also had a longer median time from staging to first treatment (17 days, interquartile range [IQR] from 7 to 35 versus9days, IQR from 3 to 20). First treatment received was surgery in 89% and 96% of the patients with and without duplicates, respectively. The use of chemotherapy or radiotherapy as first treatment was marginal (Table 2).
Table 2.
Cancer treatment-related interventions and outcomes by duplicates.
| Not receiving any duplicate scan (N=33,611) |
Receiving at least one duplicate scan (N=2,680) |
|
|---|---|---|
|
Median(Q1–Q3) time from complete staging to first duplicate, days |
N/A | 4(1–14) |
|
Median(Q1–Q3) time from complete staging to first treatment, days |
9(3–20) | 17(7–35) |
|
Patients with a clinical evaluation between complete staging and first treatment1(%) |
26,564(79) | 1,930(72) |
| Median(Q1–Q3) number of evaluations |
2(2–4) | 4(2–6) |
|
Patients visiting the emergency department between complete staging and first treatment(%) |
3,587(11) | 788(29) |
| Median(Q1–Q3) number of visits | 1(1–1) | 1(1–1) |
| First treatment(%) | ||
| Surgery | 32,190(96) | 2,383(89) |
| Chemotherapy | 78(0) | 32(1) |
| Radiotherapy | 99(0) | 21(1) |
| No cancer-specific therapy | 1,244(4) | 244(9) |
| Months of follow-up | 1,885,882 | 119,649 |
| Number of deaths(%) | 18,296(54) | 1,697(63) |
| Number of colon cancer deaths(%) | 6,596(20) | 673(25) |
| Adjusted rate ratio for all cause mortality | Reference | 1.22(1.16–1.28) |
| Adjusted rate ratio for colon cancer mortality | Reference | 1.23(1.14–1.32) |
Clinical evaluation consists on any of the following: new outpatient, established outpatient, hospital observation services, new inpatient, established inpatient, observation/inpatient care services, outpatient consultation, inpatient consultation, follow-up inpatient consultation, confirmatory consultation, nursing facility services and team conference.
Fifty percent of patients received complete staging in non-profit/government centers, 8% received at least one staging test in a proprietary center, and 42% received staging tests in free-standing facilities with or without tests in institutional non-profit/government centers. The percentage of patients receiving duplicates was 6%, 9% and 8% in these three groups, respectively.
The all-cause mortality HR for having received a duplicated CT scan was 1.22 (95% CI 1.16-1.28). The corresponding HR for colon cancer-specific mortality was 1.23 (95% CI 1.14-1.32) (see Table, Supplemental Digital Content 4, for more details on the survival analysis).
Discussion
We found that 7%of abdominal or pelvic CT scans were duplicated in the staging of localized colon cancer in Medicare patients. Compared with patients without duplicated CT scans, those with duplicates had a higher comorbidity index, were more symptomatic, visited the emergency room more often, and received surgery as first treatment less often. These findings suggest that patients receiving duplicate tests were more frail and complex, which may warrant the additional testing.
The higher mortality among patients receiving duplicate CT scans also suggests that a the duplicates may often be clinically indicated for reasons not captured in the Medicare data, such as performance status and abnormal test results. This explanation is further supported by the attenuation of the mortality HR after adjusting for baseline and time-varying confounders, together with the smaller attenuation observed for cancer-specific mortality(see Table, Supplemental Digital Content 4, for more details on the survival analysis).
The short time span from complete staging to the first treatment (median 9 days) indicates a timely administration of treatment to patients with localized colon cancer. Though patients receiving duplicates are treated a few days later on average, it is unlikely that this delay can explain the association between duplicated CT scans and mortality.
The cost of cancer care is estimated to grow from $125 billion in 2010 to $173 billion in 2020 in the US 24. Aging of the US population is argued as one of the drivers of this cost increase 25 and, in the case of colorectal cancer, the 12 months following diagnosis account for most of the expenses 24. Our analysis targeted elderly population in the initial phase of colorectal cancer diagnosis, and provided reassurance of an adequate use of Medicare resources in this population.
Our analysis has the data limitations inherent to claim-based analyses and is restricted to patients over 66 years residing in SEER states. There is a possibility of occasional coding of rectal cancer as colon cancer, or vice versa. However, the small proportion of radiotherapy as first therapy suggest this potential miscoding would have been infrequent. Some diagnostic tests may have been missed if some patients were using health care providers outside Medicare. However, when we restricted the analysis to the 27,158 individuals with an evaluation for a colon cancer-related symptom in the six months before diagnosis (i.e., those more likely to have been diagnosed and staged within Medicare), results did not change materially.
In summary, we found a 7% frequency of duplicated CT scans for disease staging, which may be partly explained by the higher complexity of these patients, and timely delivery of treatment among elderly Medicare patients with localized colon cancer.
Supplementary Material
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement # U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding sources: This work was partly funded by NIH grant P01-CA134294. XGA is a recipient of an "ASISA Fellowship".
Footnotes
Financial disclosures: there are not financial disclosures from any of the authors.
Contributor Information
Xabier García-Albéniz, Department of Epidemiology, Harvard School of Public Health. Boston, MA.
Roger W. Logan, Department of Epidemiology, Harvard School of Public Health. Boston, MA.
Deborah Schrag, Dana Farber Cancer Institute, Boston, MA.
Miguel A Hernán, Departments of Epidemiology and Biostatistics, Harvard School of Public Health, Boston, MA; Harvard-MIT Division of Health Sciences and Technology, Boston, MA.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Engstrom PF, Benson AB, Cohen A, Doroshow J, Kiel K, Niederhuber J, et al. NCCN Colorectal Cancer Practice Guidelines. The National Comprehensive Cancer Network. Oncology (Williston Park) 1996 Nov;10(11 Suppl):140–175. [PubMed] [Google Scholar]
- 3.Benson AB, Choti MA, Cohen AM, Doroshow JH, Fuchs C, Kiel K, et al. NCCN Practice Guidelines for Colorectal Cancer. Oncology (Williston Park) 2000 Nov;14(11A):203–212. [PubMed] [Google Scholar]
- 4.ESMO Minimum Clinical Recommendations for diagnosis, adjuvant treatment and follow-up of colon cancer. Ann Oncol. 2001 Aug;12(8):1053–1054. doi: 10.1023/a:1017492920758. [DOI] [PubMed] [Google Scholar]
- 5.Engstrom PF, Benson AB, Saltz L. Colon cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2003 Jan;1(1):40–53. doi: 10.6004/jnccn.2003.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. Colon Cancer (Version 1.2012) [Internet] Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 7.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24(Suppl 6):vi64–vi72. doi: 10.1093/annonc/mdt354. (April 2002) [DOI] [PubMed] [Google Scholar]
- 8.Shatto J, Clemens M. Projected Medicare Expenditures Under an Illustrative Scenario With Alternative Payment Updates to Medicare Providers. [[Accessed March 1, 2014]];Whasington, DC Centers Medicare Medicaid Serv Off Actuar [Internet] 2011 Available from: https://www.cms.gov/ReportsTrustFunds/Downloads/2011TRAlternativeScenario.pdf.
- 9.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012 Apr 11;307(14):1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan R, Peppercorn J, Sikora K, Zalcberg J, Meropol NJ, Amir E, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011 Sep;12(10):933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 11.Choosing Wisely. [cited 2014 Apr 10];n initiative of the ABIM Foundation [Internet] Available from: http://www.choosingwisely.org/about-us/
- 12.What Part B covers. [cited 2014 Mar 1];Getting a second opinion before surgery [Internet] Available from: http://www.medicare.gov/what-medicare-covers/part-b/second-opinions-before-surgery.html.
- 13.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl) doi: 10.1097/01.MLR.0000020942.47004.03. IV–3–18. [DOI] [PubMed] [Google Scholar]
- 14.Kim HY, Lee SJ, Lee G, Song L, Kim S-A, Kim JY, et al. Should Preoperative Chest CT Be Recommended to All Colon Cancer Patients? Ann Surg. 2013 Feb 19; doi: 10.1097/SLA.0b013e3182865080. [DOI] [PubMed] [Google Scholar]
- 15.ACR-SPR PRACTICE GUIDELINE FOR THE PERFORMANCE OF COMPUTED TOMOGRAPHY (CT) OF THE ABDOMEN AND COMPUTED TOMOGRAPHY (CT) OF THE PELVIS [Internet] Available from: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/CT_Abdomen_Pelvis.pdf.
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000 Dec;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Ackermann RT, Williams B, Nguyen HQ, Berke EM, Maciejewski ML, LoGerfo JP. Healthcare cost differences with participation in a community-based group physical activity benefit for medicare managed care health plan members. J Am Geriatr Soc. 2008 Aug;56(8):1459–1465. doi: 10.1111/j.1532-5415.2008.01804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006 Mar;98(3):237–242. doi: 10.1111/j.1742-7843.2006.pto_329.x. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 20.Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernán MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat. 2010 Jan;6(2) doi: 10.2202/1557-4679.1212. Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray M, Logan R, Sterne JAC, Hernández-Díaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010 Jan 2;24(1):123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008 Sep 15;168(6):656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cain LE, Logan R, Robins JM, Sterne JAC, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011 Apr 19;154(8):509–515. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010 – 2020. J Natl Cancer Inst. 2011 Jan 19;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih Y-CT, Ganz PA, Aberle D, Abernethy A, Bekelman J, Brawley O, et al. Delivering high-quality and affordable care throughout the cancer care continuum. J Clin Oncol. 2013 Nov 10;31(32):4151–4157. doi: 10.1200/JCO.2013.51.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.