Abstract
Previous work has shown that exposure to bisphenol A (BPA) during early development can alter sexual differentiation of the brain in rodents, although few studies have examined effects on areas of the brain associated with cognition. The current study examined if developmental BPA exposure alters the total number of neurons and glia in the medial prefrontal cortex (mPFC) in adulthood. Pregnant Long-Evans rats were orally exposed to 0, 4, 40, or 400 μg/kg BPA in corn oil throughout pregnancy. From postnatal days 1-9, pups were given daily oral doses of oil or BPA, at doses corresponding to those given during gestation. Brains were examined in adulthood, and the volume of layers 2/3 and layers 5/6 of the mPFC were parcellated. The density of neurons and glia in these layers was quantified stereologically with the optical disector, and density was multiplied by volume for each animal. Males exposed to 400 μg/kg BPA were found to have increased numbers of neurons and glia in layers 5/6. Although there were no significant effects of BPA in layers 2/3, the pattern of increased neuron number in males exposed to 400 μg/kg BPA was similar to that seen in layers 5/6. No effects of BPA were seen in females or in males exposed to the other doses of BPA. This study indicates that males are more susceptible to the long-lasting effects of BPA on anatomy of the mPFC, an area implicated in neurological disorders.
Keywords: BPA, prefrontal cortex, neuron number, autism, glia number
1. Bisphenol A (BPA) is an endocrine disruptor found in a large variety of consumer products, including polycarbonate plastics, resins used to line metal food and drink cans, thermal paper, and certain sealants used in dentistry (Chapin et al., 2008; Richter et al., 2007). BPA crosses the blood-brain barrier in adult rats (Sun et al., 2002) and can bind to or act through thyroid, estrogen, and androgen receptors (Kuiper et al., 1998; Moriyama et al., 2002; Zoeller et al., 2005; Sohoni and Sumpter, 1998; Lee et al., 2003). Neural development is an especially vulnerable time for exposure to endocrine disruptors. Neural proliferation and pruning (apoptosis) occur throughout development with region-specific timing (Williams and Herrup, 1988; Kole et al., 2013). Apoptosis during development, in particular, is often influenced by hormones (Simerly, 2002; Bernal, 2005), where an endocrine disruptor, such as BPA, could act. Consequently, it is imperative to determine whether endocrine disruptors, such as BPA, alter neuroanatomical development.
Developmental exposure to BPA alters neuron density or number in subcortical areas, often with a different pattern in males and females (reviewed in Golub et al., 2010; Wolstenholme et al., 2011; Masuo and Ishido, 2012). Many of these studies have concentrated on changes occurring in the hypothalamus in regions that have large sex differences, such as the AVPV (anteroventral periventricular nucleus) and SDN (sexually dimorphic nucleus). For example, at a wide range of doses, developmental BPA exposure decreased tyrosine hydroxylase-positive neuron number in the AVPV in females, while males were either not altered or decreased in this measure in an erratic dose-dependent manner (Rubin et al., 2006; McCaffrey et al., 2013). Additionally, the number of oxytocin-immunoreactive neurons in the AVPV was larger in adult female rats after daily subcutaneous injections of BPA from PND 0-3 (Adewale et al., 2011). After oral exposure to BPA during early development, the volume of the SDN was increased in male rats at PND 21 (He et al., 2012). This contrasts with a decrease in neuron number in the SDN, as measured with Calbindin D28, in adult male rats, while females are unaffected after oral BPA exposure to the dams during early development (McCaffrey et al., 2013).
Only a few studies have concentrated on BPA-induced neural changes outside of the hypothalamus. Oral BPA administration during development (gestation and lactation) resulted in more neurons in males and fewer in females in the locus coeruleus, which reversed the sex difference found in controls (Kubo et al., 2001). Additionally, oral BPA exposure during development increased the density of corticotropin-releasing hormone immunoreactive neurons in the bed nucleus of the stria terminalis in males and decreased them in females (Funabashi et al., 2004). Lastly, oral BPA given during gestation and lactation decreased the density of NeuN-positive neurons in the hippocampus at 20 days of age in males and females (Kunz et al., 2011). However, a decrease in density can be due to an increase in the size of the dendritic tree or other surrounding neuropil as readily as a change in the number of neurons (Boyce et al., 2010). Thus, the potential influence of developmental exposure to BPA on the number of neurons in neural areas directly involved in cognition remains to be investigated.
The rat prefrontal cortex (PFC) is a brain area important for many executive functions including working memory, attention, set-shifting, reversal learning and social learning (Chudasama, 2011; Uylings et al., 2003). In humans, dysfunction of this brain area has been associated with numerous neurological disorders including autism, drug addiction, schizophrenia, and disorders of attention, mood, and anxiety (Hoftman and Lewis, 2011; Price and Drevets, 2012; Teffer and Semendeferi, 2012; Van den Oever et al., 2010). There have not been any published studies of the effects of developmental exposure to BPA on cognitive tasks which rely heavily in the mPFC (e.g. delayed alternation), but studies testing complex mazes (the radial arm maze or Barnes maze) that have some reliance on the mPFC have failed to find significant effects of developmental BPA exposure (Ferguson et al., 2012; Ryan and Vandenberg, 2006; Sadowski et al., 2014). However, there is substantial evidence that anxiety behaviors, which are sensitive to disruptions of the PFC, are altered by developmental exposure to BPA (Patisaul and Bateman, 2008; Cox et al., 2010; Ryan and Vandenbergh, 2006; Adriani et al., 2003; Gioiosa et al., 2007; Rubin et al., 2006; Fujimoto et al., 2006; Kubo et al., 2001; 2003). A recent report has also found an association between neuroimmune activation in the PFC and anxiety-related behavioral changes (Luo et al., in press). Therefore, neuroanatomical changes to the PFC may result in alterations in behavior and could increase the susceptibility to a wide range of developmental and neurological disorders.
Sex differences in brain development and the involvement of gonadal hormones in mediating these differences provide a foundation for the hypothesis that early exposure to endocrine disruptors, such as BPA, could perturb cortical neuron number in adulthood. A previous study found that adult male rats have more neurons in the mPFC compared to females (Markham et al., 2007). Endocrine disruptors could potentially alter neuron proliferation, which occurs prenatally in the rat cortex (Angevine and Sidman, 1962; Miller, 1985). However, there is no evidence that gonadal hormones influence proliferation in the cortex during early development, and studies of non-cortical brain regions, such as the hypothalamus and amygdala, have concluded that developmental estrogen does not impact this process (Jacobson and Gorski, 1981; Park et al., 1996; Wang et al., 2003). Nonetheless, a deficiency in maternal thyroid hormone disrupts fetal neurogenesis in the cortex (Mohan et al., 2012), which is a potential mechanism for how endocrine disruptors could alter adult neuron number.
Apoptosis during development could also be influenced by hormones, and disruptions could alter cell number. Apoptosis continues into the postnatal period in rats, especially during the first 10 days after birth (Ferrer et al., 1990; Nunez et al., 2001). Sex differences in the peaks of cell death in the posterior cortex can be altered by gonadal hormones (Nunez et al., 2001; 2002). The anterior cortex (i.e., the future mPFC) has not been studied in the detail that the posterior cortex has, but a study that broadly examined cell death in the rat cortex found that it was ubiquitous throughout the cortex during the first 10 days with some timing differences among cortical areas (Ferrer et al., 1990). Although Ferrer and colleagues did not explicitly examine the mPFC, it is not unreasonable to assume that apoptotic events occur during the early postnatal period. In addition, the expression of the estrogen receptor α gene has been found before PND 10 in the mPFC (Westberry and Wilson, 2012). Thus, developmental exposure to endocrine disruptors, such as BPA, could alter proliferation or neuronal pruning and ultimately the total number of neurons. Furthermore, it is likely that this could happen in a sex-dependent pattern. The current study investigates whether perinatal BPA exposure differentially alters adult neuron number in the mPFC of male and female rats.
The number of glia could also be altered in response to environmental exposure to BPA, given that sex differences in total glia number in the mPFC are seen in adulthood (Markham et al., 2007). GFAP (glial fibrillary acidic protein) expression increases in vitro after BPA application and in the hippocampus after developmental exposure to BPA (Kunz et al., 2011; Miyatake et al., 2006; Yamaguchi et al., 2006). Potential effects of BPA have not been examined for microglia or oligodendrocyte number. Therefore, potential effects of BPA on glia in the developing mPFC are merited.
In addition to mediating differences in cell number, gonadal hormones are also implicated in sex-specific development of white matter. Adult males have a larger volume of frontal white matter compared to females (Markham et al., 2007). Also, the splenium of the corpus callosum is larger in males than females, and administration of the estrogen receptor blocker, tamoxifen, or testosterone during early development to female rats increases the size of the corpus callosum in females (Bimonte et al., 2000; Fitch et al., 1990). This indicates that the cortical white matter may be affected by early exposure to BPA.
The present study quantifies the number of neurons and glia in the mPFC and the volume of the underlying white matter after prenatal and early postnatal exposure to BPA. We hypothesize that the role of gonadal influences in the developing mPFC will be disrupted by BPA and result in lasting changes in cortical structure.
2. Experimental Procedures
2.1 Breeding and Housing Procedures
Two cohorts (separated by approximately 1 year) of adult female and male Long Evans rats from Harlan (Indianapolis, IN) were used as breeders. Precautions were taken to minimize exposure to environmental sources of BPA or exogenous estrogens. Both breeders and their offspring were housed in polysulfone cages (except during mating). A total of 84 offspring born from 42 male and female breeding pairs were used. Rats were given access to reverse osmosis water in glass bottles and were fed a diet from Harlan (2020X) that contains low, but stable amounts of phytoestrogens throughout the experiment. All other materials used in this study (pipette tips, boxes that temporarily held cookies for dosing, etc.) were screened to ensure that they were not composed of materials that could contain BPA. All animal procedures were in compliance with the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign.
In order to acclimate female breeders to future dosing procedures, they were handled at least 3 times and fed ½ of a cookie (Newman's Own Arrowroot flavor, Westport, CT) that contained 100 μl of tocopherol-stripped corn oil (Sigma, St. Louis, MO). This method of oral administration has been used in other studies examining neural and behavioral effects of gestational exposure to BPA as well as other endocrine disruptors (McCaffrey et al., 2013; Poon et al., 2013; 2011). For breeding, each male was paired with one female in a wire mesh cage that was monitored daily for the presence of at least one sperm plug underneath the cage.
Three doses of BPA within the μg/kg range were used for several reasons. The current “safe” reference dose of BPA adopted by both the EPA and FDA is 50 μg/kg/day. The amount of BPA exposure in humans has been highly debated, but estimated exposure has been stated as between 34 ng/kg/day – 100 μg/kg/day (Kang et al., 2006; Lakind and Naiman, 2011; Thomson et al., 2003; Chapin et al., 2008). It is important to note that most of these estimates were based on non-pregnant adult humans, but there is evidence that elevated levels of BPA metabolites have been reported in women who are pregnant, compared with their pre-pregnancy levels (Braun et al., 2012). While most human exposure is thought to fall below 400 ug/kg, this dose as an oral bolus in rodents results in estimated blood levels equal those that are reported in humans (reviewed in Vandenberg et al., 2013). The wide range of estimated exposures in humans and evidence that BPA may exert nonmonotonic effects in rodents (see Vandenberg et al., 2009) was the basis for the use of several doses of BPA (0, 4, 40, or 400 μg/kg/day) in the current study.
2.2 Dosing Procedure
On the first day that sperm plugs were found, females were placed in polysulfone cages, and dosing with BPA solutions began. Dosing of the pregnant rats occurred once per day starting at gestational day 0 and continued until parturition. Solutions given to pregnant females were made by suspending BPA powder (obtained from the EPA) in tocopherol-stripped corn oil at concentrations of 0, 0.01, 0.1, or 1 mg BPA/ml, which corresponded to daily doses of 0, 4, 40, or 400 μg BPA/kg of body weight/day, respectively. These solutions were mixed daily with a stir bar prior to dosing to ensure homogeneity. Females were weighed and 0.4 μl of the appropriate solution/g of body weight was pipetted on ½ of a cookie that was allowed to dry. The dams were observed until they finished their cookies, which always occurred within 5 min. The experimenter was blind to all treatments.
BPA was not administered on the day of parturition, which was designated as postnatal day (PND) 0. Doses corresponding to those given to the pregnant females were given by direct oral administration to the pups from PND 1-9 (0, 4, 40, and 400 μg BPA/kg of body weight/day). Solutions of 0, 0.002, 0.02, and 0.2 mg BPA/ml of oil were mixed daily, and a final volume of 2 μl/g of body weight was pipetted into the mouths of the pups while the dam was held temporarily in a separate cage. Oral dosing by insertion of the oil into the mouth has been used in other studies administering BPA to different ages of rodents (Adriani et al., 2003; Delcos et al., 2014; Neese et al., 2013; Wang et al., 2014) and has also been specifically used at this age in rodents for other endocrine disruptors, such as genestein (Cimafranca et al., 2010; Rodriguez-Gomez et al., 2014). This direct oral route mimics oral exposure that occurs in humans and is less stressful than gavage. Although many of the previous studies that examine the effects of early BPA exposure have relied on lactational transfer of BPA from dams to pups as a route of exposure, there is recent evidence to suggest this method results in extremely low exposures to the pups (Doerge et al., 2010).
At PND 2, litters were culled to a maximum of 10 pups, while trying to maintain a balanced sex ratio in each litter. A minimum of 7 pups per litter was required at this time for inclusion in the study. In the one litter that contained less than 7, pups were cross-fostered from litters of the same treatment group and identified by their unique color markings. These cross-fostered pups were not used for later anatomical measures. As reported in a previously published paper, there were no differences in sex ratio between the treatment groups (Sadowski et al., 2014). At PND 23, all offspring were weaned, double-housed with same-sex littermates, and handled once a week for the remainder of the experiment. Only one male and one female were used per litter for the present study.
2.3 Histology
The rats used in current study were tested in a radial arm maze task in adulthood, and the results have been reported in a separate paper (Sadowski et al., 2014). This behavioral experiment required food restriction (85-90% of free-feeding weight) that lasted approximately 5 weeks, and ad libitum food was returned to the rats after completion of the behavioral task. Rats were sacrificed around PND 140, which occurred 2-5 weeks after the rats were returned to free-feeding. This was to ensure that any potential changes to glia due to food restriction and behavioral testing would recover. On the day of sacrifice, rats were injected with 100 mg/kg sodium pentobarbital and perfused with 0.1 M phosphate buffered saline (PBS) followed by 4% parafomaldehyde in PBS. All brains were stored in the 4% paraformaldehyde solution for nine days, transferred to a 30% sucrose solution for 3 days, and then coronally sectioned on a freezing microtome. Every fourth 60 μm section was mounted on slides and stained with Methylene Blue/Azure II on the following day.
2.4 Cortical and White Matter Volume
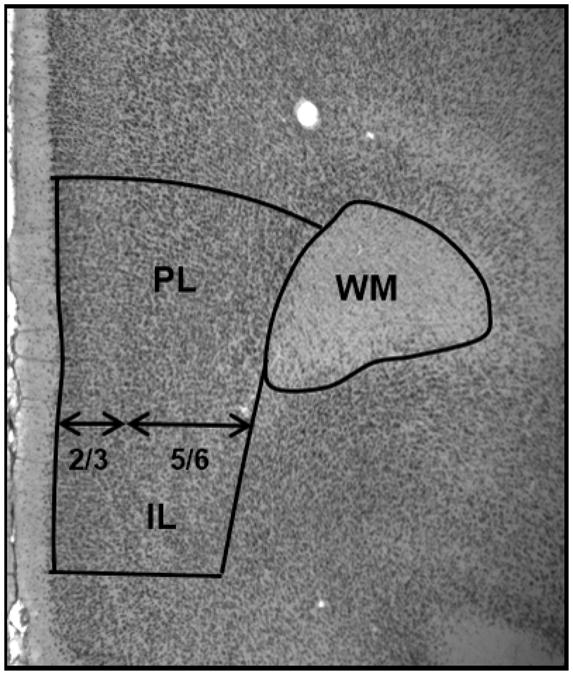
On a Zeiss microscope equipped with camera lucida, the ventral mPFC (infralimbic and prelimbic regions) was parcellated based on differences in cytoarchitecture as described previously (Krettek and Price, 1977; Markham et al., 2007; Van Eden and Uylings, 1985). The boundaries of the mPFC within a section, as shown in Figure 1, were established through cytoarchitectonic characteristics of these areas and the cortical regions bordering them. Layer 1 was not included in the analysis since it contains few neurons. The dorsal border of the mPFC is marked by an increase in the density of layer 3 cells, a broadening of layer 5, and a decrease in the thickness of layer 1 that occurs around the transition of the prelimbic region of the mPFC and dorsal anterior cingulate cortex. The infralimbic region of the mPFC is characterized by a relatively uniform lamination pattern and has a less distinct border between layers 1 and 2 compared to the prelimbic region, and ventral boundary was drawn where these characteristics end. The volumes of the prefrontal gray matter and white matter were parcellated from the most anterior section where the white matter first appeared and continued on every mounted section until the appearance of the genu of the corpus callosum, resulting in analysis of 4-6 sections for each brain. Within each parcellation, boundaries were drawn for layers 2/3 and layers 5/6 separately. Within and between cohorts, boundaries were randomly redrawn to confirm consistency within 5% of the original parcellations. Additionally, subcortical white matter underlying the mPFC was traced. All drawings were scanned into a computer, areas were measured with Image J, and post-shrinkage thickness for each layer was measured in the Stereoinvestigator program (Microbrightfield, Williston, VT) to obtain an average thickness. The volumes were calculated with the Cavalieri method as the product of the areas and the tissue thickness between the saved sections with separate calculations for the upper and lower layers of the gray matter.
Figure 1. A photograph illustrating the boundaries of parcellations for the gray matter of the mPFC and white matter. prelimbic (PL), infralimbic (IL), white matter (WM).
2.5 Neuron and Glia Number
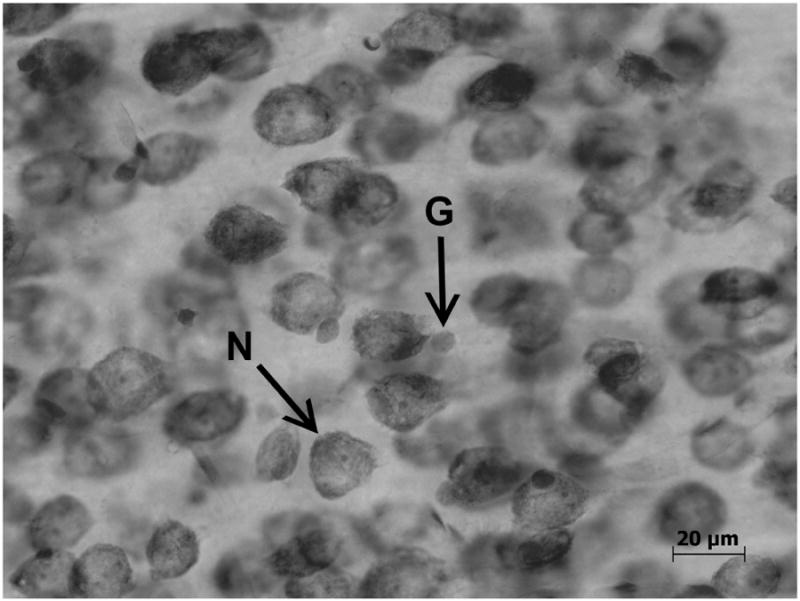
Total numbers of neurons and glia were determined as described previously (Markham et al., 2007; Koss et al., 2012) using the optical disector with the Stereoinvestigator program (Microbrightfield, Williston, VT). Neuron and glia density were quantified separately for layers 2/3 and layers 5/6 of the mPFC. The computer program randomly chose counting frames, which measured 35 μm × 35 μm (width × height), within parcellated boundaries. Guard zones were set at 1 μm at the top and bottom of each section. The investigator counted a cell only if the bottom of the cell was within the volume of the counting frame. Shown in Figure 2, neurons and glia were distinguished based on differences in morphology, size, and color (Markham et al., 2007). In the cortex, neurons are larger and are stained dark blue with a clearly defined nucleus and nucleolus. In contrast, glia are smaller, stain a turquoise blue, and have an amorphous shape. This method of distinguishing between neurons and glia in Nissl-stained sections has been used in studies from several laboratories (Benes et al., 2001; Davanlou and Smith, 2004; Day-Wilson et al., 2006; Rubinow and Juraska, 2009; Markham et al., 2007). At least 400 neurons and 140 glia were counted from layers 2/3 and from layers 5/6 for each animal. These numbers were then divided by the total volume of the counting frames to determine neuron and glia density. The densities were multiplied by the volume of the structure for each animal to determine total number of neurons and glia for individual layers.
Figure 2. A photograph showing a neuron (N) and glia (G) at high magnification. (Koss et al., 2012).
2.6 Statistical Analysis
Analyses of neuroanatomical measures were performed using ANOVAs (SAS; version 9.4; SAS Institute Inc., Cary, NC) with treatment as a factor and cohort as a covariate because of significant differences between the two cohorts in most measures (p<.05). Due to the expectation that males and females may exhibit different responses to BPA, they were analyzed separately. When significant treatment effects were found, a Dunnett's test with treatment as an independent factor and cohort as a covariate was used for posthoc tests to compare individual BPA treatment groups to the control group. This allowed for variance due to cohort to be accounted for in the post hoc comparisons. ANOVAs with sex as the independent factor and cohort as a covariate were also performed between male and female controls to examine potential sex differences. Statistical significance was set at p<.05. Sample sizes for each sex were controls = 8, 4 μg/kg = 10, 40 μg/kg = 13, 400 μg/kg = 11.
3. Results
3.1 Neuron Number
There were no significant treatment effects in neuron number in layers 2/3 or layers 5/6 in females (Figure 3A and 3B, respectively; Table 1). In contrast, there was a significant treatment effect in layers 5/6 in males (F(3,37) = 3.0; p=.04) (Figure 3B). Posthoc tests revealed more neurons in males that received 400 μg/kg BPA compared to controls (D(3,37) = 2.4; p<.05). This difference was approximately 15%. The analysis did not reveal a significant effect of treatment in layers 2/3 in males (Figure 3A), but the pattern of increased neuron number in the 400 μg/kg BPA group was similar to that seen in layers 5/6. Significant cohort effects on this measure were seen in males in layers 2/3 (F(1,37) = 13.1; p=.001) and 5/6 (F(1,37) = 10.7; p=.002) and in females in layers 2/3 (F(1,37) = 6.2; p=.02) and 5/6 (F(1,37) = 13.7; p=.001). No significant sex differences were detected when control males and females were compared.
Figure 3.
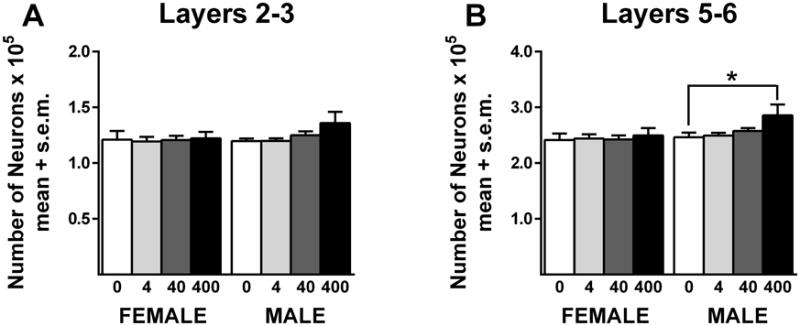
Neuron number in the mPFC. BPA did not alter neuron number in females. There was a significant difference between the controls and the 400 μg/kg BPA in males. *p<.05. Sample sizes for each sex were controls = 8, 4 μg/kg = 10, 40 μg/kg = 13, 400 μg/kg = 11.
Table 1. Summary of Results.
| Dose (μg/kg) | Sex | Neuron Number Layers 2/3 (× 105) | Neuron Number Layers 5/6 (× 105) | Glia Number Layers 2/3 (× 105) | Glia Number Layers 5/6 (× 105) | White Matter Volume (× 109) |
|---|---|---|---|---|---|---|
| 0 | Female | 1.21 ±0.08 | 2.41±0.12 | 0.43±0.04 | 1.32±0.11 | 1.85±0.07 |
| 4 | Female | 1.19±0.04 | 2.44±0.07 | 0.40±0.02 | 1.20±0.06 | 1.77±0.06 |
| 40 | Female | 1.21 ±0.04 | 2.43±0.07 | 0.41±0.02 | 1.25±0.07 | 1.85±0.08 |
| 400 | Female | 1.22±0.06 | 2.50±0.13 | 0.44±0.02 | 1.29±0.06 | 2.00±0.08 |
| 0 | Male | 1.20±0.02 | 2.46±0.08 | 0.38±0.04 | 1.13±0.09 | 2.15±0.11 |
| 4 | Male | 1.20±0.02 | 2.49±0.05 | 0.42±0.02 | 1.16±0.08 | 1.98±0.05 |
| 40 | Male | 1.25±0.03 | 2.58±0.05 | 0.40±0.02 | 1.23±0.05 | 2.12±0.07 |
| 400 | Male | 1.36±0.10 | 2.86±0.19* | 0.46±0.04 | 1.37±0.11* | 2.32±0.07 |
Significant difference from 0 dose male.
3.2 Glia Number
There were no significant treatment effects in glia number in layers 2/3 or layers 5/6 in females (Figure 4; Table 1). In contrast, there was significant treatment effect in layers 5/6 in males (Figure 4B) (F(3,37) = 2.9; p=.046). Post hoc tests revealed significantly more glia in males that received 400 μg/kg BPA compared to controls (D(3,37) = 2.4; p<.05). The percent increase was approximately 19%. Analysis did not reveal a significant effect of treatment in layers 2/3 in males (Figure 4A), but the pattern of increased glia number in the 400 μg/kg BPA group was similar to that seen in layers 5/6. Significant cohort effects on this measure were seen in males in layers 2/3 (F(1,37) = 23.8; p<.0001) and 5/6 (F(1,37) = 35.2; p<.0001) and in females in layers 2/3 (F(1,37) = 23.1; p<.0001) and 5/6 (F(1,37) = 19.2; p<.0001). No significant sex differences were detected when control males and females were compared.
Figure 4.
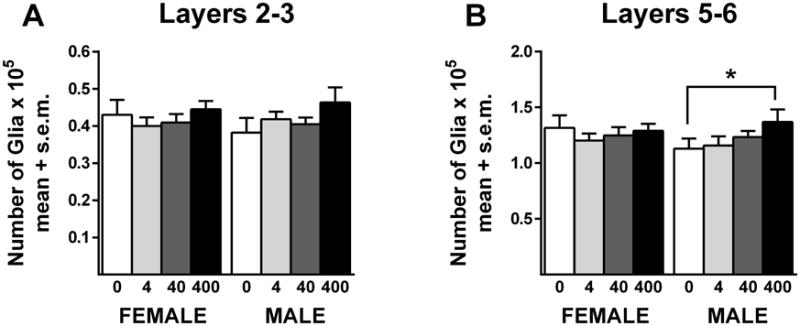
Glia number in the mPFC. BPA did not alter glia number in females. There was a significant difference between the controls and the 400 BPA group in males. *p<.05. Sample sizes for each sex were controls = 8, 4 μg/kg = 10, 40 μg/kg = 13, 400 μg/kg = 11.
3.3 White Matter Volume
There were no significant treatment effects in the white matter of females (Figure 5; Table 1). There was a significant effect of treatment in the males (F(3,37) = 3.1; p=.04), but no differences were found between controls and any of the individual treated groups. Significant cohort effects on this measure were seen in males (F(1,37) = 9.8; p=.003) and in females (F(1,37) = 5.3; p=.03). Comparisons between control males and females revealed a sex difference (F(1,13) = 7.1; p=.02) in white matter volume, as reported previously by our laboratory (Markham et al., 2007).
Figure 5.
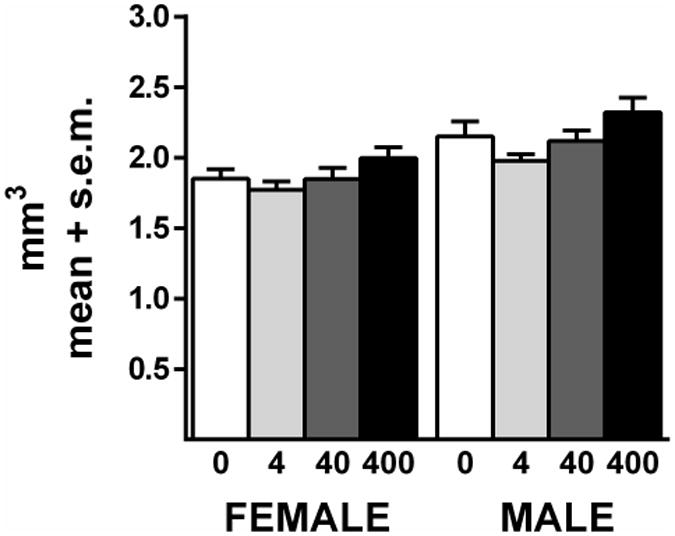
White matter volume. BPA did not alter the volume of the white matter in females. In males, there was a significant overall treatment effect (p<.05) but no significant differences between controls and individual treatment groups. Sample sizes for each sex were controls = 8, 4 μg/kg = 10, 40 μg/kg = 13, 400 μg/kg = 11.
4. Discussion
This study provides the first evidence that early exposure to bisphenol A results in a higher number of neurons and glia in the PFC of male rats. This effect was only seen at the highest dose used, 400 μg/kg BPA. This dose is relevant to prenatal exposure that occurs in humans because it can produce serum blood levels in female adult rodents and female rhesus monkeys that are similar to those observed in female humans (Taylor et al., 2011).
The effect on neuron number in males at the 400 μg/kg BPA dose was only significant in the lower layers (5/6) of the cortex, although the direction of change in upper layers (2/3) was similar. The number of neurons is especially important because it is formed early in life and is less plastic than other morphological components. Unlike many other alterations, such as those in dendritic spines, glia number or neurotransmitter levels, there are no known pharmacological or environmental interventions (e.g. environmental enrichment) that could be given later in life to normalize neuron number in the cortex. Proliferation of cortical neurons occurs during specific periods of development in contrast to glia, for which proliferation occurs throughout life (Lee et al., 2000).
Mechanisms responsible for the higher number of neurons and glia in males in layers 5/6 at the highest BPA dose are unknown. The timing of the peak of postnatal apoptosis has been shown to differ between male and female rat pups in the developing posterior (visual) cortex. Males exhibit a higher peak in apoptosis in the early postnatal period (PND 6/7), which corresponds to the time of postnatal BPA exposure in the present study (PND 1-9), while females have higher peaks in apoptosis after PND 10 (Nuñez et al., 2001). The height of these peaks of apoptosis can be altered by early exposure to gonadal hormones. Exposure to 5α-dihydrotestosterone increased the amount of apoptosis in females to control male values at PND 6 in the posterior cortex (Nuñez et al., 2000). Interestingly, estrogen exposure in females had no effects on this measure. This study establishes that the rate of apoptosis can be altered by gonadal steroids, and it offers the possibility that gonadal hormone receptors are mediating the increase in neuron and glia number in the present study. The hormones involved and the timing in the prefrontal cortex is probably different than in the posterior cortex because differences in the density of androgen receptors have been documented between the posterior cortex and the developing anterior (cingulate/motor) cortex during early development (Nunez et al., 2003). In addition, a recent study reported that early BPA exposure altered estrogen receptor gene expression in the PFC of male and female mice in a dose and sex-specific manner (Kundakovic et al., 2013). Future studies are needed to determine the role of gonadal hormones and their receptors in mediating cell death in the developing mPFC of males and females to elucidate the role of endocrine disruptors.
The animals examined in the current experiment were also behaviorally tested in a radial arm maze as adults, and hormone levels were assessed in their littermates at weaning age (Sadowski et al., 2014). There were no significant changes in thyroxine or thyroid stimulating hormone at weaning in males, suggesting that long-term changes in thyroid hormones are not responsible for the neural changes in males reported here. Similarly, there were no significant changes in reference or working memory observed in any of the groups exposed to BPA. This suggests that the higher number of neurons and glia in the mPFC of 400 μg/kg BPA males did not impact radial arm maze behavior. Other studies examining cognitive performance in land-based tasks in rodents after developmental exposure to BPA also found no effects (Ryan and Vandenbergh, 2006; Ferguson et al., 2012). However, there is evidence that early exposure to BPA can alter social and anxiety behavior (Adriani et al., 2003; Cox et al., 2010; Dessi-Fulgheri et al., 2002; Gioiosa et al., 2007; Kubo et al., 2001; 2003; Kundakovic et al., 2013; Patisaul and Bateman, 2008; Porrini et al., 2005; Ryan and Vandenbergh, 2006; Wolstenholme et al., 2012). The PFC is implicated in modulating social and anxiety behaviors in humans and in animal models (Anderson et al., 1999; Ball et al., 2013; Fossati, 2012; Nelson and Guyer, 2011). Therefore, alterations in the number of neurons and glia in the mPFC in the current study may affect performance on tasks that include emotional and social processing while not affecting other cognitive tasks.
The increases in glia number in the PFC of males exposed to 400 μg/kg BPA could be due to changes in astrocytes, oligodendrocytes, or microglia because the Nissl stain employed in the current study does not distinguish between different types of glia. There is limited evidence that GFAP density is acutely increased after exposure to BPA (Yamaguchi et al., 2006; Miyatake et al., 2006; Kunz et al., 2011), but long-term effects are unknown. No studies to date have investigated whether BPA directly impacts oligodendrocytes, but an in vitro study did report concentration-dependent alterations in neural progenitor cells and in NG2-positive precursor cells (Okada et al., 2008). However, it is difficult to ascertain how the concentrations of BPA used in culture studies correspond to doses of BPA used in in vivo studies or to exposure that occurs in humans. Therefore, more studies with in vivo administration of BPA are needed to determine what types of glia are contributing to the increases seen in this current study.
There is a growing literature that suggests that early exposure to environmental contaminants, specifically those with endocrine-disrupting properties, may be associated with an increased risk of neurological disorders, such as autism. Examples include studies investigating early exposure to phthalates, polybrominated diphenyl ethers, and polychlorinated biphenyls (Larsson et al., 2009; Miodovnik et al., 2011; Mitchell et al., 2012; Testa et al., 2012; Woods et al., 2012). Although the magnitude seen in the present study is relatively small, there is evidence that large increases in neurons and glia are found in human males diagnosed with autism (Courchesne et al. 2011; Edmonson et al., 2014). This suggests that increases in cell number are not necessarily advantageous. It is speculative to suggest that the effects found here could be an indication of a predisposition toward autism, but it does add to the recent examination of the roles that endocrine disruptors may play in the development of neurological disorders (de Cock et al., 2012).
There were main effects of cohort for both the number of glia and neurons. Although we tried to keep all variables the same (sex, strain, testing facility, season, etc. housing conditions), a few of these are not under our control (animal colony conditions and animal caretakers) (Chesler et al, 2002; Sorge et al., 2014). Similar effects of cohort have been reported in other neurotoxicology studies (e.g., Neese et al., 2013) and in some of the variables reported from these animals in Sadowski et al. (2014), most notably in timing of puberty. The cohort effect was consistent across all groups which resulted in the large statistical significance. However, it did not interact with the BPA treatment. The fact that there was a treatment effect in spite of the variability introduced by cohort gives us confidence in our results.
No significant sex differences were seen in either neuron or glia number in the mPFC when male and female controls were compared, despite our previously reported sex differences or indications for sex differences when assessing these measures in adulthood (Markham et al., 2007; Koss et al., 2012). This discrepancy may be due to handling of both the dam during gestation and pups during early postnatal development that was necessary for the administration of the BPA dose. There is evidence for different neural effects between the sexes after early postnatal handling, such as in shock-induced release of adrenocorticotropic hormone (Erskine et al., 1975), area of the corpus callosum (Berrebi et al., 1988), and turnover of serotonin in PFC (Duchesne et al., 2009). Also, it is unknown how administration of corn oil vehicle during postnatal development impacts sex differences, or neural development in general, given that it is not a natural substance for young lactating rat pups.
In conclusion, exposure to 400 μg/kg BPA during development resulted in more neurons and glia in the mPFC of male, but not female, rats. The reason for this particular susceptibility in males is unknown, but the binding of BPA to gonadal steroid receptors and differential timing in naturally-occurring cell death between the sexes are possibilities. Future studies are needed to assess the mechanism for these changes and the specific type of cells that are involved.
Highlights.
Pregnant females received 0, 4, 40, or 400 μg/kg/day BPA throughout gestation.
Male and female offspring were orally given the same dose from postnatal days 1-9.
In adulthood, neuron and glia number in the medial prefrontal cortex were quantified using stereological techniques
Males exposed to 400 μg/kg/day BPA during development had more neurons and glia in layers 5/6 compared to male controls.
Females were unaffected.
Acknowledgments
We thank Nioka Lowry and Wendy Koss for their assistance. We would also like to thank the Microscopy Suite at the Beckman Institute for support and use of their facilities and Ghazal Naseri Kouzehgarani for help with the statistics. This work was supported by NIEHS P20 ES 018163, EPA RD 83459301 Project 4, NIEHS T32 ES007326.
Abbreviations
- BPA
bisphenol A
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- AVPV
anteroventral periventricular nucleus
- PND
postnatal day
- GFAP
glial fibrillary acidic protein
- SDN
sexually dimorphic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32:38–49. doi: 10.1016/j.neuro.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriani W, Seta DD, Dessi-Fulgheri F, Farabollini F, Laviola G. Altered profiles of spontaneous novelty seeking, impulsive behavior, and response to D-amphetamine in rats perinatally exposed to bisphenol A. Environ Health Perspect. 2003;111:395–401. doi: 10.1289/ehp.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL. Autoradiographi study of histogenesis in the cerebral cortex of the mouse. Anat Rec. 1962;142:210. [Google Scholar]
- Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med. 2013;43:1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- Berrebi AS, Fitch RH, Ralphe DL, Denenberg JO, Friedrich VL, Jr, Denenberg VH. Corpus callosum: region-specific effects of sex, early experience and age. Brain Res. 1988;438:216–224. doi: 10.1016/0006-8993(88)91340-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Fitch RH, Denenberg VH. Neonatal estrogen blockade prevents normal callosal responsiveness to estradiol in adulthood. Brain Res Dev Brain Res. 2000;122:149–155. doi: 10.1016/s0165-3806(00)00067-5. [DOI] [PubMed] [Google Scholar]
- Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJ. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 2010;38:1011–1025. doi: 10.1177/0192623310385140. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Cimafranca MA, Davila J, Ekman GC, Andrews RN, Neese SL, Peretz J, Woodling KA, Helferich WG, Sarkar J, Flaws JA, Schantz SL, Doerge DR, Cooke PS. Acute and chronic effects of oral genistein administration in neonatal mice. Biol Reprod. 2010;83:114–121. doi: 10.1095/biolreprod.109.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanlou M, Smith DF. Unbiased stereological estimation of different cell types in rat cerebral cortex. Image Anal and Stereol. 2004;23:1–11. [Google Scholar]
- Day-Wilson KM, Jones DN, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- de Cock M, Maas YG, van de Bor M. Does perinatal exposure to endo crine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr. 2012;101:811–818. doi: 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, Gamboa da Costa G, Woodling KA, Bryant MS, Chidambaram M, Trbojevich R, Juliar BE, Felton RP, Thorn BT. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–197. doi: 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol Lett. 2010;199:372–376. doi: 10.1016/j.toxlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Edmonson C, Ziats MN, Rennert OM. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism. 2014;5:3-2392–5-3. doi: 10.1186/2040-2392-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS, Stern JM, Levine S. Effects of prepubertal handling on shock-induced fighting and ACTH in male and female rats. Physiol Behav. 1975;14:413–420. doi: 10.1016/0031-9384(75)90005-0. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Berrebi AS, Cowell PE, Schrott LM, Denenberg VH. Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res. 1990;515:111–116. doi: 10.1016/0006-8993(90)90584-x. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Law CD, Abshire JS. Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicol Teratol. 2012;34:598–606. doi: 10.1016/j.ntt.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol. 2012;22(Suppl 3):S487–91. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats. Brain Res. 2006;1068:49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, Kimura F. Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology. 2004;29:475–485. doi: 10.1016/s0306-4530(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Golub MS, Wu KL, Kaufman FL, Li LH, Moran-Messen F, Zeise L, Alexeeff GV, Donald JM. Bisphenol A: Developmental toxicity from early prenatal exposure. Birth Defects Res B Dev Reprod Toxicol. 2010;89:441–466. doi: 10.1002/bdrb.20275. [DOI] [PubMed] [Google Scholar]
- He Z, Paule MG, Ferguson SA. Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol Teratol. 2012;34:331–337. doi: 10.1016/j.ntt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kole AJ, Annis RP, Deshmukh M. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689. doi: 10.1038/cddis.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Ogata R, Omura M, Hori T, Aou S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci Lett. 2001;304:73–76. doi: 10.1016/s0304-3940(01)01760-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz N, Camm EJ, Somm E, Lodygensky G, Darbre S, Aubert ML, Huppi PS, Sizonenko SV, Gruetter R. Developmental and metabolic brain alterations in rats exposed to bisphenol A during gestation and lactation. Int J Dev Neurosci. 2011;29:37–43. doi: 10.1016/j.ijdevneu.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005-2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Luo G, Wang S, Li Z, Wei R, Zhang L, Liu H, Wang C, Niu R, Wang J. Maternal Bisphenol A Diet Induces Anxiety-like Behavior in Female Juvenile with Neuroimmune Activation. Toxicol Sci. doi: 10.1093/toxsci/kfu085. In press. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ishido M. Neurotoxicity of endocrine disruptors: possible involvement in brain development and neurodegeneration. J Toxicol Environ Health B Crit Rev. 2011;14:346–369. doi: 10.1080/10937404.2011.578557. [DOI] [PubMed] [Google Scholar]
- McCaffrey KA, Jones B, Mabrey N, Weiss B, Swan SH, Patisaul HB. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36:55–62. doi: 10.1016/j.neuro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Dev Brain Res. 1985;23:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. Levels of select PCB and PBDE congeners in human postmortem brainreveal possible environmental involvement in 15q11-q13 duplication autismspectrum disorder. Environ Mol Mutagen. 2012;53:589–598. doi: 10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake M, Miyagawa K, Mizuo K, Narita M, Suzuki T. Dynamic changes in dopaminergic neurotransmission induced by a low concentration of bisphenol-A in neurones and astrocytes. J Neuroendocrinol. 2006;18:434–444. doi: 10.1111/j.1365-2826.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A, Godbole MM. Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol. 2012;237:477–488. doi: 10.1016/j.expneurol.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Neese SL, Bandara SB, Schantz SL. Working memory in bisphenol-A treated middle-aged ovariectomized rats. Neurotoxicol Teratol. 2013;35:46–53. doi: 10.1016/j.ntt.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Dev Cogn Neurosci. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Huppenbauer CB, McAbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Brain Res Dev Brain Res. 2000;125:83–88. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, Furukawa Y. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008;29:163–170. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- Park JJ, Baum MJ, Paredes RG, Tobet SA. Neurogenesis and cell migration into the sexually dimorphic preoptic area/anterior hypothalamus of the fetal ferret. J Neurobiol. 1996;30:315–328. doi: 10.1002/(SICI)1097-4695(199607)30:3<315::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav. 2008;53:580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Poon E, Monaikul S, Kostyniak PJ, Chi LH, Schantz SL, Sable HJ. Developmental exposure to polychlorinated biphenyls reduces amphetamine behavioral sensitization in Long-Evans rats. Neurotoxicol Teratol. 2013;38:6–12. doi: 10.1016/j.ntt.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon E, Powers BE, McAlonan RM, Ferguson DC, Schantz SL. Effects of developmental exposure to polychlorinated biphenyls and/or polybrominated diphenyl ethers on cochlear function. Toxicol Sci. 2011;124:161–168. doi: 10.1093/toxsci/kfr214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrini S, Belloni V, Della Seta D, Farabollini F, Giannelli G, Dessi-Fulgheri F. Early exposure to a low dose of bisphenol A affects socio-sexual behavior of juvenile female rats. Brain Res Bull. 2005;65:261–266. doi: 10.1016/j.brainresbull.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gomez A, Filice F, Gotti S, Panzica G. Perinatal exposure to genistein affects the normal development of anxiety and aggressive behaviors and nitric oxide system in CD1 male mice. Physiol Behav. 2014;133C:107–114. doi: 10.1016/j.physbeh.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sadowski RN, Park P, Neese SL, Ferguson DC, Schantz SL, Juraska JM. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicol Teratol. 2014;42C:17–24. doi: 10.1016/j.ntt.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Sun Y, Nakashima MN, Takahashi M, Kuroda N, Nakashima K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2002;16:319–326. doi: 10.1002/bmc.161. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, Toutain PL, Laffont CM, VandeVoort CA. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res. 2012;195:191–218. doi: 10.1016/B978-0-444-53860-4.00009-X. [DOI] [PubMed] [Google Scholar]
- Testa C, Nuti F, Hayek J, De Felice C, Chelli M, Rovero P, Latini G, Papini AM. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4:223–229. doi: 10.1042/AN20120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson BM, Cressey PJ, Shaw IC. Dietary exposure to xenoestrogens in New Zealand. J Environ Monit. 2003;5:229–235. doi: 10.1039/b211323f. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health. 2013;28:37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci U S A. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Niu R, Zhu Y, Han H, Luo G, Zhou B, Wang J. Changes in memory and synaptic plasticity induced in male rats after maternal exposure to bisphenol A. Toxicology. 2014;322C:51–60. doi: 10.1016/j.tox.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Wilson ME. Regulation of estrogen receptor alpha gene expression in the mouse prefrontal cortex during early postnatal development. Neurogenetics. 2012;13:159–167. doi: 10.1007/s10048-012-0323-z. [DOI] [PubMed] [Google Scholar]
- Williams RW, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet. 2012;21:2399–2411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Zhu J, Yu T, Sasaki K, Umetsu H, Kidachi Y, Ryoyama K. Low-level bisphenol A increases production of glial fibrillary acidic protein in differentiating astrocyte progenitor cells through excessive STAT3 and Smad1 activation. Toxicology. 2006;226:131–142. doi: 10.1016/j.tox.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]


