Abstract
Vitamin D deficiency is associated with increased susceptibility to inflammatory arthritis. Sensory and sympathetic synovial nerves are critical to the development of inflammatory arthritis and spontaneously degenerate in the early phases of disease. These nerves contain vitamin D receptors and vitamin D influences nerve growth and neurotrophin expression. We therefore examined the density of synovial nerves and neurotrophin-containing cells in vitamin D deficient rats. Seven week old Sprague Dawley rats were fed either control or vitamin D deficient diets for four weeks. Knee synovium sections extending from patella to meniscus were immunostained for total nerves, myelinated and unmyelinated nerves, sympathetic nerves, peptidergic and non-peptidergic sensory nerves, and neurotrophins and immune cell markers. In control rats, intimal innervation by unmyelinated sensory fibers was denser than subintimal innervation. In contrast, sympathetic innervation was confined to the subintima. Many sensory axons contained markers for both peptidergic and non-peptidergic nerves. NGF was primarily expressed by intimal CD163-negative type B synoviocytes, while neurturin, a ligand selective for non-peptidergic sensory neurons, was expressed by synovial mast cells. In vitamin D deficient rats, there were significant reductions in sensory nerves in the intima and sympathetic nerves in the subintima. While there was no significant change in NGF-immunoreactivity, the number of neurturin-expressing mast cells was significantly reduced in the intima, suggesting that intimal reductions in sensory nerves may be related to reductions in neurturin. Vitamin D deficiency therefore may increase susceptibility to inflammatory arthritis by depleting sensory and sympathetic synovial nerves as a result of reduced synovial neurotrophin content.
Keywords: vitamin D, synovium, sensory, sympathetic, arthritis, nerves
Rheumatoid arthritis (RA) is a debilitating inflammatory disease affecting 1.3 million Americans (Helmick et al., 2008). While genetic risk factors for RA have been identified, environmental factors are also implicated (Arend and Firestein, 2012). For example, vitamin D deficiency has been strongly associated with RA development in humans and inflammatory arthritis in rodent models. An inverse relationship between vitamin D intake and RA risk has been established (Song et al., 2012). In addition, patients with active RA had significantly lower serum vitamin D levels than patients with silent RA (Moghimi et al., 2012). Vitamin D levels in RA patients were also inversely correlated with disease activity scores, pain, and disability (Kroger et al., 1993, Cutolo et al., 2006, Patel et al., 2007, Haque and Bartlett, 2010, Rossini et al., 2010, Turhanoglu et al., 2011, Attar, 2012), and vitamin D deficient RA patients had an increased number of tender joints (Kerr et al., 2011). A positive association has been observed between vitamin D deficiency and undifferentiated inflammatory arthritis (Heidari et al., 2012), a condition which frequently progresses to active RA, which suggests that vitamin D deficiency may play a role in initiation or early stage of the disease. In rodents, vitamin D deficiency or vitamin D receptor deletion increased disease severity and delayed resolution of inflammatory arthritis (Zwerina et al., 2011, Moghaddami et al., 2012). Conversely, supplementation with the active hormone, 1,25-dihydroxyvitamin D3 or a vitamin D analog prevented development or halted progression of collagen-induced arthritis, and reduced the severity of Lyme-induced arthritis (Cantorna et al., 1998, Larsson et al., 1998). Collectively, evidence supports the conclusion that vitamin D deficiency increases the susceptibility to and severity of inflammatory arthritis.
The exact mechanisms of how vitamin D deficiency contributes to RA severity remain poorly understood. It has been speculated that normal vitamin D levels may be protective by attenuating inflammatory cell activation (Wen and Baker, 2011). Alternatively, vitamin D may influence joint innervation, as abundant evidence has shown that joint innervation plays an important role in RA development. In humans, hemiplegia protects against RA development in the paralyzed limbs (Jacqueline, 1953, Thompson and Bywaters, 1962), and unilateral sciatic nerve transection delayed onset and severity of adjuvant arthritis in denervated limbs of rats (Courtright and Kuzell, 1965). Sensory C fibers appear to be particularly important, as systemic reductions in peptidergic sensory axons induced by capsaicin administration in rats diminished the severity of adjuvant-arthritis (Colpaert et al., 1983, Levine et al., 1986). Sympathetic nerves are also important as guanethidine-induced sympathectomy, catecholamine depletion by reserpine, and administration of β-blockers propranalol, butoxamine or ICI 118,5510 all decreased severity and increased latency to onset of adjuvant arthritis (Levine et al., 1986, Levine et al., 1988). Interestingly, both sympathetic and sensory synovial axons have been reported to undergo partial spontaneous degeneration in RA patients and in the early phases of many rodent inflammatory arthritis models (Konttinen et al., 1990, Mapp et al., 1990, Mapp et al., 1994, Imai et al., 1997, Miller et al., 2000, Hukkanen et al., 2002, Weidler et al., 2005, Dirmeier et al., 2008) and synovial axon loss has been suggested to be an early indicator of RA (Levine et al., 1984, Takeba et al., 1999). Therefore, while the relationship between synovial sensory and sympathetic innervation and RA remains to be fully elucidated, these nerves appear to be integrally involved in the development and progression of RA.
We and others have shown previously that peripheral innervation can be regulated by vitamin D levels. Both sensory and sympathetic neurons possess vitamin D receptors, and therefore have the potential to respond directly to vitamin D (Tague et al., 2011, Tague and Smith, 2011). In vitro, the extent of neurite outgrowth from cultured primary sensory or central neurons was influenced by the addition of varying levels of 1,25-dihydroxyvitamin D3 (Brown et al., 2003, Tague et al., 2011). Likewise, vitamin D deficiency, in vivo, promoted a marked increase in deep tissue sensitivity, and altered the density of skeletal muscle (but not cutaneous) nociceptor innervation (Tague et al., 2011). Vitamin D can also regulate neurotrophin expression in target cells (Kalueff et al., 2004), suggesting that it may also indirectly influence peripheral innervation. Accordingly, this study was conducted to establish whether vitamin D deficiency influences synovial innervation, which might influence the onset and modulation of RA.
2 Experimental Procedures
2.1 Animals and diets
Animal protocols and procedures were in accordance with NIH guidelines for the care and use of laboratory animals and approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. As described previously (Tague et al., 2011), weaned female Sprague Dawley rats (Harlan Laboratories Inc, Madison, WI, USA) were fed ad libitum normal chow until the introduction of experimental diet as outlined below. At 31 days of age, rats were ovariectomized via bilateral hindflank incisions and administered ketoprofen 5mg/kg s.c. (Ketofen; Fort Dodge Animal Health, Fort Dodge, IA, USA) as a postoperative analgesic. Ovariectomized rats were used to eliminate estrous cycle-driven variations in neuronal VDR expression (Tague and Smith, 2011) and because this model may incorporate risk factors associated with human populations with musculoskeletal pain, RA, and vitamin D deficiency (post-menopausal/estrogen-suppressed females) (Gaugris et al., 2005, Alexander et al., 2007, Khan et al., 2010, Myasoedova et al., 2010). At 48 days of age, rats were randomly assigned to treatment groups and fed one of two diets; Control (n=5): 2.2 IU/g vitamin D (cholecalciferol), 0.47% Ca, 0.3% P (TD.07370; Harlan TekladMadison, WI, USA), or VD- (n=4): vitamin D-depleted, 2.5% Ca, 1.5% P (TD.07541; Harlan Teklad,). The VD- diet was based on previous studies that reported that increasing dietary calcium from 0.47% to 2.5% normalized serum clacium in prolonged vitamin D deficiency (1.5% P is needed as a counterbalance) (Weishaar and Simpson, 1987) and rats fed this diet exhibited rapid increases in musculoskeletal sensitivity (Tague et al., 2011). After 4 weeks on the diet, serum 25(OH)D concentrations in VD- rats were reduced below 10nmol/L (compared 54-82nmol/L in controls) and we found no differences in serum calcium or phosphorous (Tague et al., 2011). At which time subjects were deeply anaesthetized and perfused with 50ml of cold 0.9% saline containing 10units/ml heparin (APP Pharmaceuticals, Lake Zurich, Il, USA) at a rate of 40ml/min, followed by 150-200ml of 4% formaldehyde, prepared in PBS from paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA).
2.2 Tissue processing
The bones from the left hind leg were cut out at mid-thigh and ankle leaving the knee joint intact. Knee samples were post-fixed in Zamboni’s fixative overnight at 4°C, washed in PBS changed daily for three days at 4°C, decalcified in PBS containing 10% EDTA (Sigma) (pH 7.4) for two weeks at 4°C, and cryoprotected overnight at 4°C in 30% sucrose. The knee was cut in half along the transverse plane, embedded in tissue freezing media (Electron Microscopy Sciences, Hatfield, PA, USA) frozen on dry ice, and cryosectioned at 20μm. Each slide contained two sections approximately 400μm apart. Slides from each animal were stained with hematoxylin and eosin or Giemsa to examine overt morphological changes.
2.3 Immunostaining
2.3.1 General
Each staining was completed and analyzed in a batch with a single slide from each animal containing the same section numbers. Thawed sections were pre-incubated in 1.5% donkey serum (Jackson ImmunoResearch, West Grove, PA, USA), 0.5% gelatin (Sigma-Aldrich), and 0.5% Triton X-100 (Sigma-Aldrich) prepared in Superblock (Thermo Scientific, Waltham, MA, USA) for 1hr, incubated overnight with primary antibodies, followed by a 2 hour incubation with secondary antibodies, all at room temperature. All antibodies were diluted in incubation solution (50% pre-incubation solution, 50% Superblock). Slides were washed in PBS containing 0.25% Triton-X 100 before and after the secondary antibody application. Coverslips were mounted on the slides using a solution of 50% Glycerol in PBS, and sealed with fingernail polish. Staining selectivity of all primary antibodies (with the exception of anti-neurturin, which was characterized here) has been confirmed previously as indicated, and controls in which primary antibodies were omitted were incorporated into all staining series performed in the present study.
Secondary antibodies were diluted as follows: 1:1,500 donkey anti-chicken DyLight 488 (Jackson ImmunoResearch), 1:750 Donkey anti-sheep DyLight 649 (Invitrogen, Life Technologies, Grand Island, NY), 1:1,000 donkey anti-rabbit or anti-goat Alexa 647 (Invitrogen), or 1:1000 donkey anti-rabbit 488 (Jackson). Nuclei were stained with 400 nM 4,6-diamidino-2-phenylindole (DAPI, Invitrogen) for 10 min before adding secondary antibodies.
2.3.2 Nerves
Innervation density of the knee synovium was analyzed using seven nerve markers: protein gene product 9.5 (PGP5.5), peripherin, neurofilament H (NFH), tyrosine hydroxylase (TH), calcitonin gene related peptide (CGRP), substance P (SP) and GDNF Family Receptor alpha 2 (GFRα2). Total innervation was assessed by immunostaining for the pan-neuronal marker PGP9.5, a ubiquitin carboxyl-terminal hydrolase. Rabbit anti-PGP9.5 antibody was diluted 1:1,200 (AbD Serotec, Raleigh, NC (Chakrabarty et al., 2011)). Peripherin is an intermediate filament selectively expressed by small diameter nerves such as sensory and sympathetic C fibers, while NFH is selectively expressed in myelinated axons of large and medium diameter neurons (Goldstein et al., 1991, Fornaro et al., 2008). Chicken anti-peripherin antibody was used at a dilution of 1:500 (EMD Millipore, Billerica, MA, USA (Tague and Smith, 2011)) and mouse anti-NFH antibody was used at 1:400 (Sigma, (Chao et al., 2008)). TH is an enzyme present in peripheral sympathetic axons (Black et al., 1971). Rabbit anti-TH was diluted 1:200 (EMD Millipore, (Shi et al., 2008)). CGRP and SP are neuropeptides found in peptidergic Aδ and C fiber sensory nerves (Lawson, 1992). Sheep anti-CGRP (Enzo Life Sciences, Farmingdale, NY, USA [(Tague et al., 2011)]) and rabbit anti-SP (SP, DiaSorin, Stillwater, MN, USA (Kim et al., 2008)) were diluted 1:200, and rabbit anti-CGRP was diluted 1:2000 (Sigma). GFRα2 is a neurotrophin receptor, which is selectively-expressed by non-peptidergic sensory C fibers (Lindfors et al., 2006). Goat anti-GFRα2 was diluted 1:800 (R&D Systems, Minneapolis, MN, USA, (Forrest and Keast, 2008)).
2.3.3 Vasculature
Mouse anti-alpha smooth muscle actin (αSMA) antibody was used to identify vascular smooth muscle and diluted 1:400 (Sigma,(Goldshmit et al., 2006)). The endothelial marker CD34 was used to confirm that αSMA staining accurately reflected vascular structures (data not shown).
2.3.4 Neurotrophins
To detect NGF, rabbit anti-NGF was diluted 1:100 (Santa-Cruz Biotechnology, Dallas, TX, USA (Wernli et al., 2009)). The neurotrophin neurturin is the preferred GFRα2 ligand (Jing et al., 1997). For immuno-localization of neurturin, two different antibodies were used and both showed similar results; goat (Santa Cruz Biotechnology) or rabbit (EMD Millipore) anti-neurturin antibodies were diluted 1:50. Preabsorption of the goat antibody with a molar excess of blocking peptide prior to dot blot staining of recombinant neurturin protein (abcam, Cambridge, MA, USA) and immunofluorescently-labeled synovial sections (data not shown) confirmed the specificity of neurturin staining.
2.3.5 Immune cells
The intima is composed primarily of two cell types, the macrophage-like type A synoviocyte and the fibroblast-like type B synoviocyte. Macrophages/Type A synoviocytes express vitamin D receptors (Tetlow et al., 1999) and can influence the severity of rheumatoid arthritis (Kinne et al., 2000). We examined the density of Type A synoviocytes expressing the scavenger receptor and macrophage marker, CD163 (Fonseca et al., 2002). Mouse anti-CD163 was used at a dilution of 1:500 (AbD Serotec (Damm et al., 2011)). Mast cells are also present within the synovium where they are intimately associated with nerves, and their density has been correlated with synovial innervation density (Levine et al., 1990a, Hukkanen et al., 1991). Mouse anti-mast cell tryptase (MCT) diluted 1:250 (Abcam (Walls et al., 1990)) was used as a mast cell marker.
2.4 Quantitation
2.4.1 General
All quantitation was performed on de-identified, randomly sorted samples. Images were acquired on a Nikon 80i upright microscope (Nikon Instruments Inc, Melville, NY, USA), brightness and contrast was adjusted, and images were analyzed using Metamorph software (Molecular Devices, Sunnydale, CA, USA). For analysis of the subintimal area, images were acquired of the entire width of the synovium covering an area approximately 1mm proximal from the meniscus. For analysis of the intimal area, multiple images were acquired of the intima spanning from the meniscus to the patella (appox. 2-4mm) at a magnification of 200X. A white line overlay represents the intimal-subintimal junction in images presented here.
2.4.2 Morphology and Cell counts
Intimal thickness was calculated by dividing the intimal area by the length of the intima-cavity interface. Intimal cellularity was determined by counting the number of DAPI stained nuclei and dividing by the intimal area. To determine cell counts, cells with DAPI-stained nuclei that stained with CD163, MCT, or neurturin were counted and divided by the total tissue area. Because NGF staining was not confined to a distinct cell type, a threshold was set 10 fold over background. The thresholded area was divided by the total area and multiplied by 100 to determine the percent area expressing NGF.
2.4.3 Nerves
Nerve fibers included in analysis had signal intensities that were at least four fold above background tissue fluorescence. Others have shown that RA leads to the denervation of the intima, with minimal changes to the deep tissues (Mapp et al., 1990). We therefore examined innervation of the intima and subintima separately. A stereological grid (100μm2/cell) was superimposed over the images, and number of grid intersections overlying immunofluorescently-labeled axons were counted and divided by the total number of tissue intersections to provide the apparent percentage area occupied by nerves (Clarke et al., 2010). We calculated the percentage of total PGP9.5-immunoreactive (-ir) nerves containing each marker, and for each marker we co-labeled with peripherin and grid-counted nerves with and without peripherin colocalization to determine the percent co-localization for each marker. Sections were also colabeled for GFRα2 and CGRP, and the percent colocalization of CGRP and GFRα2 was determined by grid-counting.
2.5 Statistical Analysis
All values for each animal were averaged from multiple images from each section and these section averages were averaged again for each animal. The number of replicates for each analysis and the corresponding number of sections analyzed and averaged for each animal are as follows: Intimal thickness, 13 slides with a total of 26 sections; Intimal cellularity, 1 slide with 2 sections; PGP9.5, 2 slides with a total of 4 sections; NFH, 1 slide with 2 sections; peripherin, 6 slides with a total of 12 sections; CGRP, 3 slides with a total of 6 sections; Substance P, 1 slide with 2 sections; GFRα2, 3 slides with a total of 6 sections; αSMA, 1 slide with 2 sections; NGF, 1 slide with 2 sections; Neurturin, 2 slides with a total of 4 sections; MCT, 2 slides with a total of 4 sections; CD163, 2 slides with a total of 4 sections. These averages were expressed as mean±s.e.m with an n of 4-5 animals for each treatment group. Statistical significance was accepted at p<0.05 for all tests. When comparing effects between synovial location and dietary treatments, a two-way ANOVA was used. When data failed either the Shapiro-Wilk normality (p<0.05) or Levene Median equal variance (p<0.05) tests, ANOVA’s were conducted on ranks. Post-hoc multiple comparisons were made using the Student-Newman-Keuls method.
2.6 Confocal microscopy
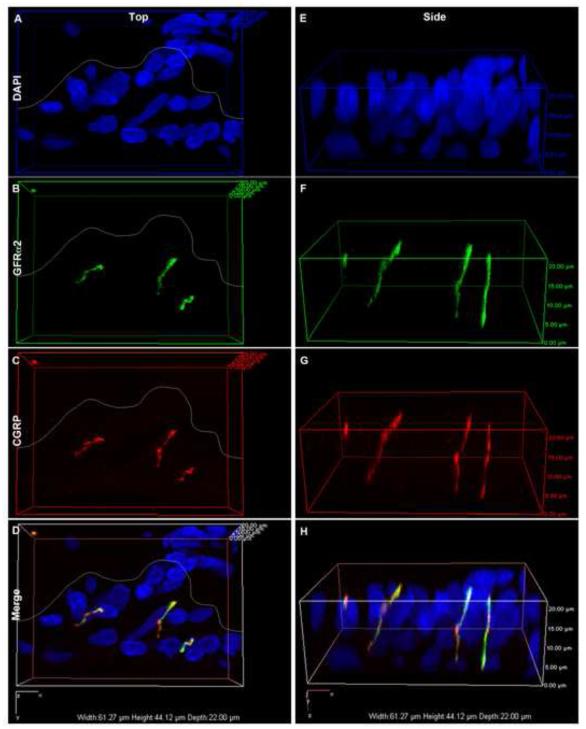
To determine whether GFRα2 and CGRP were localized within the same fibers, we employed the use of confocal microscopy. 20μm thick sections were imaged with a 60x oil objective on a Nikon A1R inverted confocal microscope using Diode 444 and 639 lasers and NIS-Elements AR software (Nikon Instruments Inc). A set of Z-stack images was acquired with a step size of 0.5μm and an optical zoom of 1.2. A volume view was rendered and rotated to examine whether the markers were found within the same axons. Shown are a set of fibers within the intima with a top and a side volume view.
3.0 Results
3.1 General morphological findings
After four weeks of on vitamin deficient diet the rats displayed no changes in the thickness of the synovium intima, nor were there changes in intima cellularity (Table 1). H&E stained sections showed no overt signs of synovial inflammation during this 4 week study in control or vitamin D deficient rats (data not shown).
Table 1.
| Intima thickness (μm) |
Intima cellularity (#/mm2) |
αSMA-ir subintima/intima (% area) |
|
|---|---|---|---|
| Control | 30.9±1.7 | 392±37 | 7.5±0.5/8.8±2.9 |
| VD- | 27.1±1.9 | 364±28 | 6.6±0.9/6.1±0.9 |
3.2 Synovial Innervation
3.2.1 PGP9.5
In control rats, PGP9.5-ir nerves were found in both the subintima and intima (Fig. 1&2A). Subintimal nerves were frequently associated with blood vessels (Fig. 1). Within the intima there were axons that traveled freely as well as nerves that followed fine microvasculature (Fig. 1). In control rats, there was a tendency toward higher mean intimal innervation density compared to subintimal innervation density (Fig. 2A&C).
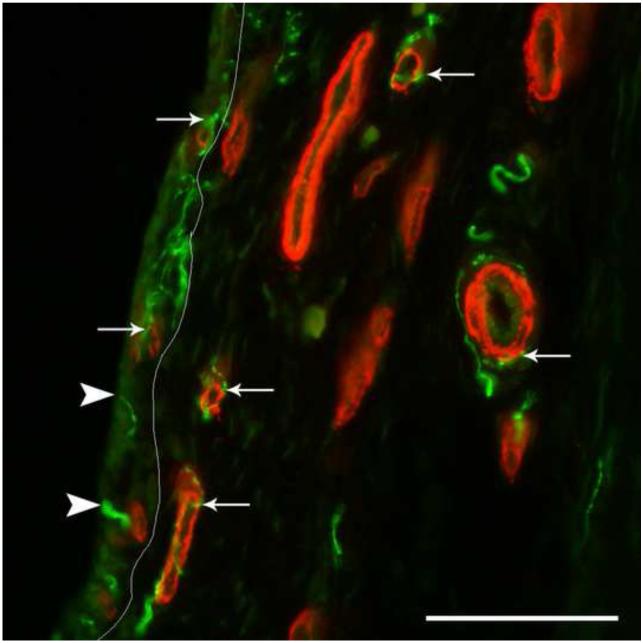
Fig. 1.
Nerves and vasculature in the rat synovium. In this immunofluorescently-labeled section of knee synovium from a control rat, the pan-neuronal marker, protein gene product 9.5 (PGP9.5, green), shows the location of nerves in relationship to the vascular marker smooth muscle actin (SMA, red). A white line superimposed on the image separates the intima (left) from the subintima (right). Larger SMA-ir vessels are found in the subintima, while the intima contains smaller microvasculature. The SMA-ir vasculature in both the subintima and intima are frequently associated with PGP9.5-ir nerves (arrows). There are also free nerve endings in the intima that appear to terminate near the intima/cavity junction (arrow heads). Scale=100μm
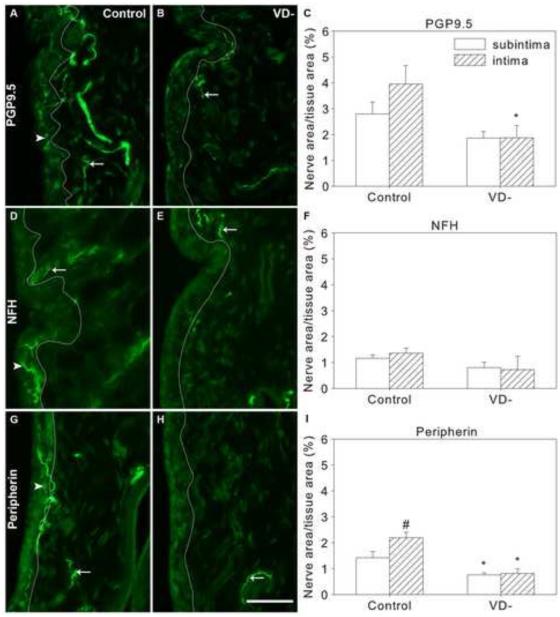
Fig. 2.
Nerves in knee synovium of control and vitamin D deficient (VD-) rats. Representative images are shown of immunofluorescently-labeled nerves in knee synovium (A,B,D,E,G,H) from control (A,D,G) and VD- (B,E,H) rats, and graphical representation of nerve density quantitation (C,F,I). Synovial sections were immunostained for PGP9.5 (A-C), Neurofilament H (NFH, D-F) and peripherin (G-I). A white line superimposed on images separates the intima (left) from the subintima (right) (A,B,D,E,G,H). Arrows point toward selected nerves in the subintima and arrowheads point toward selected intimal nerves. Data are presented as mean±s.e.m (C,F,I). # p<0.05 between intima and subintima of rats on the same diet by two-way ANOVA. * p<0.05 between control and VD- rats within the intima or subintima by two-way ANOVA. Scale=50μm
VD- rats showed an overall reduction of 45% in total synovial PGP9.5-ir innervation density compared to control rats (Fig. 2B&C, p=0.014). The subintimal innervation tended to be lower in VD- rats but did not reach statistical significance; however, mean intimal PGP9.5-ir innervation was reduced by 47% in VD- rats (Fig. 2B&C, p=0.016).
3.2.2 Neurofilament H
In the synovium of control rats, there were no significant differences in subintimal and intimal NFH-ir nerve fiber density (Fig. 2D&F). The NFH-ir nerves appeared to represent approximately 40% of the total (PGP9.5-ir) synovial innervation; 45±9% of subintimal nerves (Table 2) and 37±5% of intimal nerves (Table 3).
Table 2.
Nerve marker colocalization in the subintima
| PGP9.5 (%) | NFH (%) | Peripherin (%) | TH (%) | CGRP (%) | SP (%) | GFRa2 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contr ol |
VD- | Contr ol |
VD - |
Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | |
|
|
||||||||||||||
| PGP9.5 | 100 | 100 | 100 | 10 0 |
100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| NFH | 45±9 | 46±1 4 |
100 | 10 0 |
- | - | - | - | - | - | - | - | - | - |
| Peripheri n |
51±4 | 42±6 | - | - | 100 | 100 | 70±7 | 80±1 2 |
87±2 | 71± 8 |
69±10 | 64± 8 |
85±4 | 89± 5 |
| TH | 21±3 | 10±2 |
- | - | 39±9 | 30±1 2 |
100 | 100 | - | - | - | - | - | - |
| CGRP | 55±5 | 45±4 | - | - | 71±4 | 62±1 1 |
- | - | 100 | 100 | - | - | 63±5 | 74± 6 |
| SP | 21±3 | 24±7 | - | - | 48±10 | 46±1 8 |
- | - | - | - | 100 | 100 | - | - |
| GFRa2 | 70±6 | 76±5 | - | - | 63±8 | 71±7 | - | - | 87±5 | 93± 1 |
- | - | 100 | 100 |
% area of nerves immunoreactive to a nerve marker (columns) that also contain a second marker (rows).
p<0.05 compared to control
Table 3.
Nerve marker colocalization in the intima
| PGP9.5 (%) | NFH (%) | Peripherin (%) | TH (%) | CGRP (%) | SP (%) | GFRa2(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contr ol |
VD- | Contr ol |
VD - |
Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | Contr ol |
VD- | |
|
|
||||||||||||||
| PGP9.5 | 100 | 100 | 100 | 10 0 |
100 | 100 | - | - | 100 | 100 | 100 | 100 | 100 | 100 |
| NFH | 37±5 | 31±1 6 |
100 | 10 0 |
- | - | - | - | - | - | - | - | - | - |
| Peripher in |
67±1 7 |
46±7 | - | 100 | 100 | - | - | 40±7# | 27±1 0# |
55±9 | 51±1 8 |
72±3 | 64±2 2 |
|
| TH | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| CGRP | 53±1 1 |
69±3 7 |
- | - | 34±9# | 37±1 5 |
- | - | 100 | 100 | - | - | 64±3 | 63±2 |
| SP | 37±7 | 48±1 6 |
- | - | 39±5 | 39±1 5 |
- | - | - | - | 100 | 100 | - | - |
| GFRa2 | 70±2 1 |
55±2 0 |
- | - | 66±8 | 89±1 0* |
- | - | 86±2 | 70±7* | - | - | 100 | 100 |
% area of nerves immunoreactive to a nerve marker (columns) that also contain a second marker (rows).
p<0.05 compared to control in same location,
p<0.05 compared to subintima (Table 2)
A vitamin D deficient diet did not lead to any significant changes in NFH-ir nerve fiber density in the knee synovium (Fig 2E&F).
3.2.3 Peripherin
In synovia of control rats, the density of peripherin-ir nerves was 1.5 fold greater in the intima than the subintima (Fig. 2G&I, p=0.012). More than half of the total synovial nerves (PGP-ir) expressed peripherin-ir; 51±4% of the subintimal nerves (Table 2) and 67±17% of the intimal nerves expressed peripherin-ir (Table 3).
Overall, there was a 57% reduction in nerve fiber density in the VD- group compared to control rats (Fig 2H&I, p<0.001). There was a 47% reduction in peripherin-ir nerve fiber density in the subintima (p=0.035) and a 63% reduction in the intima (Fig 2H&I, p<0.001).
3.2.4 Tyrosine Hydroxylase
TH-ir axons were confined to the subintima of the synovium of control rats (Fig. 3A), suggesting that these presumptive sympathetic nerves do not extend into the intima. These subintimal TH-ir nerves were primarily associated with vascular structures. In the subintima, 21±3% of the total (PGP9.5-ir) nerves expressed TH and 39±9% of peripherin-ir nerves contained TH. 70±7% of TH-ir nerves co-labeled with peripherin, suggesting that they were primarily small diameter axons, which is consistent with sympathetic nerves (Table 2).
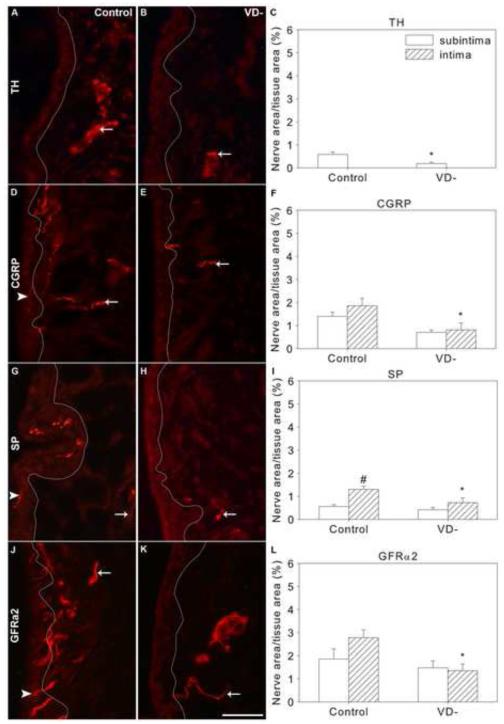
Fig. 3.
Sympathetic and sensory nerves in knee synovium of control and vitamin D deficient (VD-) rats. Representative images are shown of immunofluorescently-labeled nerves in knee synovium (A,B,D,E,G,H,J,K) from control (A,D,G,J) and VD- (B,E,H,K) rats, and graphical representation of nerve density quantitation (C,F,I,L). Synovial sections were immunofluorescently-labeled for tyrosine hydroxylase (TH, A-C), Calcitonin gene-related peptide (CGRP, D-F), substance P (SP,G-I), and GDNF family receptor alpha 2 (GFRα2, J-K). A white line has been superimposed on images to separate the intima (left) from the subintima (right) (A,B,D,E,G,H,J,K). Arrows point toward selected nerves in the subintima and arrowheads point toward selected intimal nerves. Data are presented as mean±s.e.m (C,F,I,L). # p<0.05 between intima and subintima of rats on the same diet by two-way ANOVA. * p<0.05 between control and VD- rats within the intima or subintima by ANOVA. Scale=50μm
As in control animals, TH-ir nerves were not observed in the intima of the synovium of VD- rats (Fig. 3B). Mean subintimal TH-ir nerve fiber density was reduced by 68% in VD- rats compared to control rats (Fig. 3B&C, p=0.018). The percent of total subintimal nerves with TH-ir dropped to 10±2%, which is a reduction of 55% compared to controls.
3.2.5 Calcitonin Gene Related Peptide
CGRP-ir nerves were found in both the subintima and the intima of control rat synovium (Fig. 3D). Roughly half of the total synovial PGP9.5-ir nerves co-stained with CGRP-ir; 55±5% of the subintimal nerves (Table 2) and 53±11% of the intimal nerves (Table 3). CGRP-ir was observed in 71±4% of subintimal peripherin-ir nerves and 34±9% of peripherin-ir intimal nerves in control rats (Table 3). Likewise, there were 53% fewer CGRP-ir nerves that also contained peripherin-ir in the intima compared to the subintima (Table 2&3, p<0.001); 87±2% and 40±7% of CGRP-ir nerves contained peripherin-ir in the subintima and intima, respectively. This suggests that while most of the subintimal CGRP-ir nerves are C fibers, more than half of the intimal nerves containing CGRP may be larger fiber types such as Aδ.
Compared to controls, total synovial CGRP-ir innervation was reduced by 53% in the VD- rats (Fig. 3E&F, p=0.004). While there was a tendency for subintimal innervation to be reduced (Fig. 3F, p=0.072), intimal innervation was significantly reduced by 56% (Fig. 3F, p=0.011).
3.2.6 Substance P
Consistent with previous findings (Hukkanen et al., 2002), synovial SP-ir innervation was less dense than CGRP-ir innervation in control rats. SP-ir was found in both the subintima and intima of control rat synovia (Fig. 3G), with intimal SP-ir innervation being 2.3 fold greater than subintimal SP-ir nerve density (Fig. 3I, p<0.001). Approximately one third of PGP-ir synovial nerves contained SP; 21±3% of subintimal nerves (Table 2) and 37±7% of intimal nerves (Table 3). 48±10% of subintimal peripherin-ir contained SP-ir (Table 2) and 39±5% of intimal peripherin-ir nerve area contained SP-ir (Table 3). In control rats, 69±10% of subintimal (Table 2) and 55±9% of intimal SP-ir nerves contained peripherin (Table 3).
Overall synovial SP-ir innervation density was reduced by 38% in the VD- rats compared to the control group (Fig. 3H&I, p=0.027). This was primarily due to changes in the intima, where SP-ir innervation was reduced by 44% in VD- rats (Fig 3I, p=0.013).
3.2.7 GDNF Family Receptor alpha 2
In control rats, GFRα2-ir nerves were found in both the subintima and intima (Fig. 3J). More than two thirds of the PGP9.5-ir nerves in the control rat synovia contained GFRα2; 70±6% of the subintimal nerves (Table 2) and 70±21% of the intimal nerves (Table 3). 63±8% and 66±8% of peripherin-ir subintimal and intimal nerves contained GFRα2-ir, respectively (Table 2&3). 85±4% of the subintimal and 72±3% of the intimal GFRα2-ir nerves also contained peripherin (Table 2). This suggests that GFRα2 nerves in the synovium were primarily small diameter C fibers.
Overall, the innervation density of GFRα2-ir nerves was reduced by 56% in VD- rats compared to controls (Fig. 3K&L, p=0.003). This was primarily driven by a 65% reduction in GFRα2-ir innervation within the intima (Fig. 3L, p=0.001). Within the intima, there was a 30% increase in the percentage of peripherin-ir nerves containing GFRα2 in VD- rats compared to controls (Table 3, p=0.048)
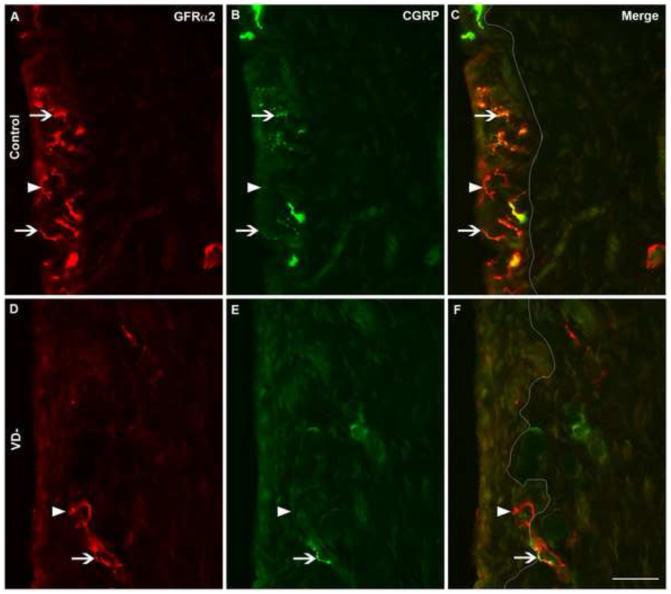
3.2.8 CGRP & GFRα2
Based on the observation that CGRP-ir and GFRα2-ir were both visualized in more than 50% of the total PGP9.5-ir population in the synovium, we stained sections with both antisera to determine the extent of CGRP-ir and GFRα2-ir overlap. Many synovial fibers appeared to contain both CGRP and GFRα2 in both control (Fig. 4A-C) and VD- (Fig. 4D-F) rats. In control rats, almost 90% of the CGRP-ir nerve fibers in the synovium co-localized with GFRα2-ir; 87±5% in the subintima and 86±5% in the intima (Tables 2&3). On the other hand, 64% of GFRα2-ir nerve fibers co-localized with CGRP-ir; 63±5% in the subintima and 64±3% in the intima.
Fig. 4.
Colocalization of CGRP and GFRα2 immunoreactivity in synovial nerves. Representative images are shown of immunofluorescently-labeled nerves in knee synovium (A-F) from control (A-C) and vitamin D deficient (VD-, D-F) rats. Synovial sections were immunofluorescently-labeled for both GDNF family receptor alpha 2 (GFRα2, A&D) and calcitonin gene-related peptide (CGRP, B&E) and shown in merged images (C&F). A white line has been superimposed on images to separate the intima (left) from the subintima (right) (C&F). Arrowheads point toward selected nerves with GFRα2-only labeling, while arrows point toward selected nerves with CGRP and GFRα2 co-labeling. Scale=50μm
To determine whether the markers were indeed found within the same axons and not just in adjacent fibers, we examined the synovium with high resolution confocal microscopy (Fig. 5). While it remains possible that some of the fibers may be tightly interwoven, it appears that CGRP and GFRα2 are co-expressed within many synovial nerves (Fig. 5).
Fig. 5.
Confocal volume view images showing CGRP and GFRα2 within the same axons. Representative images of a group of axons within the synovial intima of a control rat (A-H). Synovial sections were stained with DAPI (A&E) and immunofluorescently-labeled for both GDNF family receptor alpha 2 (GFRα2, B&F) and calcitonin gene-related peptide (CGRP, C&G). D&H show merged images. Volume view images are shown from top-down (A-D) and side (E-H) views. In A-D, a white line has been superimposed on images to separate the subintima (above) from the intima (below) (C&F). GFRα2 and CGRP appear to be located within many of the same nerve fibers.
3.3 Vascular density
Immunoreactivity for alpha smooth muscle actin (αSMA) or the endothelial marker CD34 (data not shown) showed blood vessels throughout the subintima and directly adjacent to the intima, as well as very fine vessels within the intima (Fig. 1). There were no changes in density of αSMA-ir vessels between diet groups (Table 1).
3.4 Neurotrophic factors
3.4.1 Nerve Growth Factor
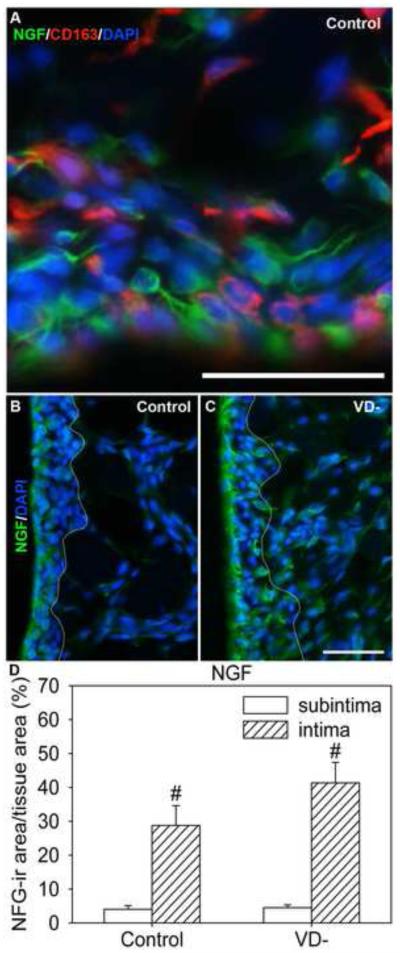
Some CD163-ir type A synoviocytes in the intima expressed NGF, but this protein was primarily expressed by the CD163-negative (type B) synoviocytes (Fig 6A). NGF was expressed by cells in both the subintima and the intima; however, the percent area of NGF-ir was over 7 fold greater in the intima relative to the subintima in control rats (Fig. 6B&D, p=0.002).
Fig. 6.
Nerve growth factor (NGF) in knee synovium of control and vitamin D deficient (VD-) rats. Representative images are shown of NGF-labeled cells in knee synovium (A,B,C) from control (A,B) and VD- (C) rats, and graphical representation of NGF-ir density (D). Synovial sections were immunofluorescently labeled for NGF (green, A-C), CD163-ir (red) macrophages/type A synoviocytes (A), and stained with DAPI (blue, A-C). A white line has been superimposed on images to separate the intima (left) from the subintima (right) (B&C). Data are presented as mean±s.e.m (D). # p<0.05 between intima and subintima of rats on the same diet by two-way ANOVA. * p<0.05 between control and VD- deficient rats within the intima or subintima by two-way ANOVA. Scale=50μm
As in controls, NGF expression in VD- rats was greater in the intima than the subintima (Fig. 6C&D, p<0.001). There were no significant differences in subintimal NGF expression in VD- rats compared to controls (Fig. 6D). There was a tendency toward increased NGF-ir area in the intima of VD- rats compared to controls, but it was not significant.
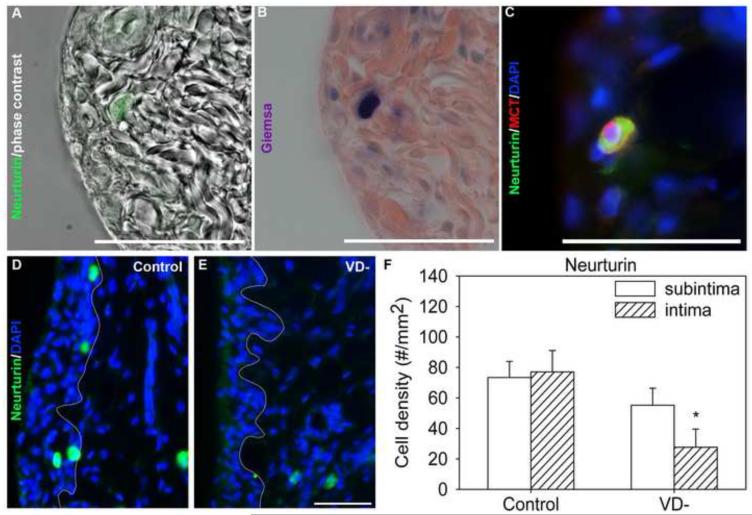
3.4.2 Neurturin
In control rats, a distinct set of synovial cells showed intense neurturin-ir (Fig 7A,C,&D). To determine the identity of these cells, we stained for neurturin together with markers for several cell types. Neurturin staining did not colocalize with the macrophage markers CD163 (Type A synoviocytes) or CD68, the T-cell marker TCR, αSMA, or the fibroblast/Type B synoviocyte marker vimentin (data not shown). Neurturin-expressing cells had a granular appearance (Fig. 7A) and stained deeply with Giemsa stain, indicative of mast cells (Fig. 7B). Mast cell tryptase immunoreactivity was observed in 100% of neurturin-ir cells, suggesting that neurturin is expressed solely by synovial mast cells (Fig. 7C). In control animals, neurturin-expressing cells were heterogeneously scattered throughout the synovium, but were distributed comparably within the intima and subintima (Fig. 7D&F).
Fig. 7.
Neurturin in knee synovium of control and vitamin D deficient (VD-) rats. An image from a synovial section from control rat was immunofluorescently-labeled for neurturin and superimposed over a phase contrast image of the same area (A) and subsequently stained with Giemsa, indicative of mast cells (B). A synovial section from a control rat was immunoflourescently-labeled for neurturin (green), mast cell tryptase (red), and DAPI (blue, C). Representative images are shown of neurturin-ir (green) cells in the in knee synovium from control (D) and vitamin D deficient (VD-, E) rats, and graphical representation neurturin-ir cell density (F). A DAPI nuclear stain (blue) shows the tissue morphology and a white line has been superimposed on images to separate the intima (left) from the subintima (right). Data are presented as mean±s.e.m (F). # p<0.05 between intima and subintima of rats on the same diet by two-way ANOVA. * p<0.05 between control and VD- rats within the intima or subintima by two-way ANOVA. Scale=50μm
Overall, the density of synovial neurturin-ir cells in VD- rats was reduced by 45% compared to controls (Fig. 7E&F, p=0.015). The overall reduction in VD- rats was largely due to a 64% reduction in intimal neurturin-ir cell density (Fig. 7F, p=0.013), with subintimal changes failing to achieve statistical significance.
3.5 Immune cells
3.5.1 CD163
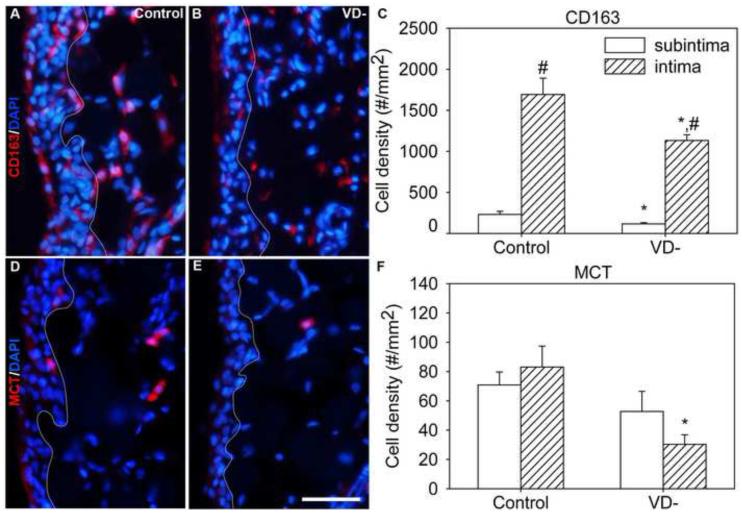
CD163-ir cells were primarily found in the intima, consistent with the localization of this marker to type A synoviocytes (Fig. 8A). Compared to the subintima, the density of CD163-ir cells was 7.3 times higher in the intima (Fig. 8C, p<0.001), where they accounted for 19±2% of all cells.
Fig. 8.
Immune cells in the synovium. Representative images are shown of immunofluorescently-labeled immune cells in knee synovium (A,B,D,E) from control (A,D) and vitamin D deficient (VD-, B,E) rats, and graphical representation of immune cell density quantitation (C,F). Synovial sections were immunofluorescently-labeled for CD163 (macrophages, (A,B) or mast cell tryptase (MCT, D,E) and stained with DAPI (A,B,D,E). A white line has been superimposed on images to separate the intima (left) from the subintima (right) (A,B,D,E). Data are presented as mean±s.e.m (C,F). # p<0.05 between intima and subintima of rats on the same diet by two-way ANOVA. * p<0.05 between control and VD- rats within the intima or subintima by two-way ANOVA. Scale=50μm
In VD- rats, there was an overall drop in average density of CD163-ir cells in the synovium (Fig. 8B&C, p=0.003). CD163-ir cells were decreased by 50% in the subintima (Fig. 8C, p=0.024) and 33% in the intima (Fig. 8C, p=0.024).
3.5.2 Mast cell tryptase
MCT-ir mast cells were found in heterogeneously distributed clusters throughout the synovium and their numbers were similar in the subintima and intima (Fig. 8D).
There was a significant reduction in MCT-ir cell density in VD- rat synovia compared to controls (Fig. 8E&F, p=0.009). This was primarily due to a 64% reduction in the density of MCT-ir cells in the intima (Fig. 8F, p=0.006).
4.0 Discussion
4.1 Normal rat knee synovium
4.1.1 Innervation
The rat knee synovium is a well innervated structure, as indicated by the abundant staining of nerve fibers with the pan-neuronal marker, PGP9.5. The synovium can be divided into two layers. The intima is comprised of a thin layer of macrophage-like type A synoviocytes and fibroblast-like type B synoviocytes that secrete synovial fluid. The subintima is primarily comprised of fatty, fibrous, and vascular tissue (Smith, 2011). In the subintima, nerves are mostly associated with vascular structures, while the intima appears to have both free nerve endings and nerves that follow fine microvascular structures.
Intimal and subintimal PGP9.5-ir axons appear to represent several heterogeneous populations. In the subintima, it appears that roughly half the axons are unmyelinated C fibers, as evidenced by their immunoreactivity to peripherin, and the other half are larger myelinated fibers that express NFH. On the other hand, axons in the intima are predominantly unmyelinated C fibers, as evidenced by the observation that roughly two-thirds contained the C fiber intermediate filament, peripherin, whereas about one-third expressed the myelinated fiber marker NFH (Goldstein et al., 1991, Fornaro et al., 2008). Sympathetic nerves, as evidenced by robust expression of the catecholamine-synthesizing enzyme, TH, were confined exclusively to the subintima. These sympathetic axons were primarily C fibers, as 70% contained peripherin. While the sympathetic nerves only accounted for about 20% of subintimal axons, the majority of remaining nerves were likely sensory nerves. Given the dense vascularity of this tissue, it seems plausible that sympathetic and some sensory fibers serve a vaso-regulatory role in the subintima; however, the sensory nerves likely also function as mechanoreceptors or ‘pain-sensing’ nociceptors.
Based on their immunostaining for several sensory nerve markers, synovial innervation in both the subintima and intima appears to be comprised predominantly of sensory axons. Previous reports have described a major population of ‘peptidergic’ sensory axons, which exhibit CGRP-ir and less frequently, SP-ir (Mapp, 1995). Our findings confirm the presence of an abundant population of fibers with CGRP-ir, and a smaller population of axons with SP-ir, which presumably overlaps with the CGRP-ir population (Hukkanen et al., 2002). Unlike the sympathetic nerves, these nerves extended into the intima. Peptidergic neurons express the NGF-responsive TrkA receptor, and their axons represent primarily Aδ and C fibers that respond to noxious stimuli including mechanical pain, heat, and changes in pH (Cavanaugh et al., 2009), and therefore presumably play a protective role in preventing joint injury. Nerve conduction from Aδ fibers is faster than C fibers, conveying acute sharp pain, while C fiber conduction is slower and may feel more spatially diffuse; however, both may contribute to persistent pain and inflammation (Le Pichon and Chesler, 2014). Interestingly, while 87% of the CGRP-ir nerves in the subintima contain the C fiber marker peripherin, only about 40% of the intimal nerves are peripherin-ir, suggesting that many intimal CGRP-ir nerves may be Aδ or larger fibers. It is therefore interesting to speculate that these faster conducting sensory fibers might play a role sharp pains that are associated with joint use. These ‘peptidergic’ nerves may also play another role in the intima, as CGRP promotes production of anti-inflammatory cytokines and inhibits the production of collagenases, while SP appears to have opposing effects (Takeba et al., 1999). Therefore, peptidergic sensory nerves may play a protective nociceptive role and also directly influence synoviocyte secretions that regulate joint health.
Most synovial axons in the current study did not appear to be of the classical peptidergic phenotype. While not previously described in knee synovium, nociceptors also comprise a separate, non-peptidergic population that are responsive to the GDNF family of ligands (GFL) through effects on GDNF-family receptors (GFRs) (Lindfors et al., 2006). These nociceptors are believed to be mostly responsible for detection of noxious mechanical stimuli (Cavanaugh et al., 2009), which would be extremely important in structures such as joints where mechanical stress is common and injury needs to be avoided. In addition, about 45% of non-peptidergic cutaneous nerves are capable of responding to heat (Stucky and Lewin, 1999), and GFRα2/neurturin signaling is required for heat responsiveness and inflammatory pain from the non-peptidergic nerves (Stucky et al., 2002, Lindfors et al., 2006). Here, CGRP-ir nerve density was equally distributed between the subintima and intima, whereas GFRα2-ir nerves were enriched only in the intima, where they were also significantly more abundant than peptidergic nerves (70% vs. 55% of total nerves). About two-thirds of GFRα2-ir nerves co-labeled with peripherin, which is consistent with a C fiber nociceptive phenotype. However, we found that two-thirds of the GFRα2-ir nerves in the intima also had immunoreactivity to CGRP. In fact, only 10% of the CGRP-ir nerves did not contain GFRα2-ir. The presence of non-peptidegic axons in the synovium is supported by an earlier report concerning the tempomandibular joint synovium where another non-peptidergic marker, P2X3, was observed in more than half of the nerves (Ichikawa et al., 2004). Interestingly, this report noted significant co-localization of P2X3-ir and CGRP-ir in trigeminal neurons that project to the tempomandibular joint. We suggest that the synovium may contain traditional peptidergic and non-peptidergic innervation, but also a large percentage of hybrid fibers that have properties of both peptidergic and non-peptidergic sensory nerves. The presence of GFRα2-ir in both peptidergic and non-peptidergic innervation in the intima suggests that GFRα2 signaling may have an important role in the synovium. These GFRα2-ir axons within the intima are well-positioned to respond to mechanical or inflammatory changes in the synovial fluid.
4.1.2 Neurotrophins
Neurotrophins are target-derived growth factors that have been studied for decades for their influence on the maintenance, phenotype, and excitability of sensory and sympathetic neurons (Ernsberger, 2009). NGF is a potent neurotrophin which promotes the development and postnatal maintenance of sensory and sympathetic nerves (Levi-Montalcini, 1987). We observed that NGF-ir was most prominent within the intima. While some CD163-ir and some mast cells contained NGF, it was mostly confined to CD163-negative cells, which are presumably the fibroblast-like type-B synoviocytes. Localized NGF-ir did not correlate with the innervation density of either identified NGF-responsive nerve population, as sympathetic axons were confined to the NGF-sparse subintima and peptidergic sensory nerve fiber density was equally distributed between the intima and subintima.
Neurturin is important for maintaining GFRα2 containing nerve terminals (Lindfors et al., 2006), and was confined to a distinct set of cells which, based on their affinity for Geimsa stain and expression of mast cell tryptase, were identified as mast cells. It is noteworthy that neurturin was expressed by these inflammatory cells, given that GFRα2/neurturin signaling is required for inflammatory/heat pain signaling in non-peptidergic neurons (Stucky et al., 2002, Lindfors et al., 2006). These neurturin-ir mast cells were typically found in small clusters scattered throughout the synovium, in both the subintima and intima. These mast cells, as others have described, were often closely associated with nerves in the synovium (Hukkanen et al., 1991), suggesting that they may interact. Others have reported that synapses form between mast cells and peripheral nerves and that nerve stimulation can activate mast cells, which can reciprocally influence neuronal activity (Suzuki et al., 2004, Ito et al., 2008).
4.2 Vitamin D deficient synovium
4.2.1 Innervation
Previous studies have shown that vitamin D regulates neurite outgrowth from both hippocampal and sensory neurons (Brown et al., 2003, Tague et al., 2011). In dorsal root ganglion neuron cultures, low physiological levels of 1,25-dihydroxyvitamin D3 resulted in increased sprouting by unmyelinated (but not myelinated) neurons relative to euphysiological concentrations (Tague et al., 2011). However the in vivo effects of vitamin D deficiency on the sensory nerves were not uniform. It resulted in nociceptor hyperinnervation of skeletal muscle, with corresponding increases in muscle mechanical sensitivity, without changes in cutaneous sensitivity or innervation density (Tague et al., 2011). In addition, vitamin D had no effect on sympathetic axon growth in culture or in muscle (Tague et al., 2011). In contrast, the present study revealed a distinct effect of vitamin D deficiency on both sensory and sympathetic knee joint innervation, leading to a 45% drop in total synovial innervation. While many of these nerves followed the vasculature, the reductions in synovial innervation were not due to decreased vascularization, because no changes in vascular density were found.
Within the subintima, vitamin D deficiency did not result in a significant decrease in total PGP9.5-ir innervation and did not alter innervation density of myelinated NFH-ir axons. It did, however, result in a significant reduction in unmyelinated peripherin-ir axons in the subintima. This appeared to be primarily due to a reduction in sympathetic TH-ir fibers and not decreased sensory SP-, CGRP-, or GFRα2-ir innervation. This suggests that vitamin D deficiency selectively reduces sympathetic innervation of the subintima. Given the established vasoconstrictive role of these nerves in regulating blood flow (McDougall et al., 1997), vitamin D deficiency is likely to have impact on perfusion of synovial tissues.
Within the intima, the vitamin D deficient diet reduced PGP9.5-ir total nerves by more than half. While there was no significant change in NFH-ir innervation, intimal peripherin-ir nerves were markedly reduced and attributable to a decrease in both CGRP-/SP-ir and GFRα2-ir axon densities. Thus, vitamin D deficiency led to reductions in small fiber sensory nerves, which has implications for detection of noxious stimuli, inflammation, and wound repair (Widgerow and Kalaria, 2012). The factors responsible for the wide repertoire of vitamin D’s effects in different tissues remain to be fully elucidated. However, in the synovium intima, evidence from this study suggests that alterations in content of neurotrophin-expressing cells may be, in part, responsible.
4.2.2 Neurotrophins and immune cells
Sympathetic and sensory peptidergic neurons are both responsive to NGF (Levi-Montalcini, 1987). Vitamin D has been shown to increase NGF expression (Wion et al., 1991, Brown et al., 2003) and vitamin D deficiency is associated with reductions in NGF expression in the developing brain (Eyles et al., 2003, Feron et al., 2005). However, our findings do not support the idea that there was a substantial decrease in synovial NGF expression. In fact, the area occupied by NGF-ir cells was unchanged, if not slightly elevated, in both intima and subintima in VD- rats. The slight increase in NGF-ir area may have been due to the decreased number of CD163-ir type A synoviocytes, compared to the NGF-ir CD163-negative type B synoviocytes. This decrease in CD163-ir macrophage-like cells was consistent with previous studies where vitamin D deficiency reduced proliferation and differentiation of bone marrow-derived macrophages and decreased macrophage migration (Bar-Shavit et al., 1981, Abu-Amer and Bar-Shavit, 1993), although we cannot exclude the possibility that the reduction in CD163-ir may reflect reduced expression of this scavenger receptor. Nonetheless, these findings may suggest that rather than NGF, other proteins such as semaphorins, IgLON cell adhesion molecules, and brain derived neurotrophic factor, known to be repulsive to peptidergic sensory and sympathetic axons (Miller et al., 2004, Krizsan-Agbas et al., 2008, Richeri et al., 2011), may play a role in reduced synovial innervation in vitamin D deficiency.
Non-peptidergic GFRα2-ir sensory neurons are responsive to the GFL member, neurturin (Jing et al., 1997). Consistent with the reduction in these GFRα2-ir nerves in the intima, we also observed a reduction in the number of neurturin-ir cells within the intima. This appeared to be due to an overall diminution in the number of infiltrating mast cells, which appear to be the sole source of neurturin within the synovium. However, it is also possible that the vitamin D deficient diet may have resulted in mast cell degranulation, which reduced the number of resident neurturin-ir mast cells. While we cannot rule out the possibility that the reduction in mast cells occurs secondary to the loss of innervation, as has been previously suggested (Levine et al., 1990a), the most straightforward explanation is that non-peptidergic axons were reduced in VD- rats in response to a decrease in neurturin-synthesizing mast cells.
4.3 A comparison of Vitamin D deficiency and Rheumatoid arthritis
A number of studies have suggested that vitamin D deficiency increases the susceptibility and severity of rheumatoid arthritis (Kroger et al., 1993, Cantorna et al., 1998, Larsson et al., 1998, Cutolo et al., 2006, Patel et al., 2007, Haque and Bartlett, 2010, Rossini et al., 2010, Kerr et al., 2011, Turhanoglu et al., 2011, Zwerina et al., 2011, Attar, 2012, Moghaddami et al., 2012, Moghimi et al., 2012, Song et al., 2012). At least in the short time frame of our study, vitamin D deficiency did not appear to cause overt rheumatoid arthritis; however we can make some significant comparisons.
4.3.1 Innervation and Rheumatoid arthritis
Similar to our findings in vitamin D deficiency, reductions in sensory and sympathetic innervation are well documented in RA. In humans and animals, the sensory and sympathetic nerves in the synovium degenerate during inflammatory arthritis (Konttinen et al., 1990, Mapp et al., 1990, Mapp et al., 1994, Imai et al., 1997, Miller et al., 2000, Hukkanen et al., 2002, Weidler et al., 2005, Dirmeier et al., 2008). In some animal models, reinnervation may occur during the later stages of disease (Imai et al., 1997); however, it is unclear whether reinnervation occurs in humans. Also similar to our findings in vitamin D deficient rats, the loss of synovial innervation was not associated with reductions in NGF, as NGF is reported to be elevated in RA (Seidel et al., 2010). In addition, localized synovial reductions in mast cell density are associated with reductions in innervation density in RA patients (Hukkanen et al., 1991), as they were in VD- rats.
Despite the fact that sensory and sympathetic nerves degenerate, these nerves play a vital role in the development of inflammatory arthritis. Hemiplegia protected against arthritis in the paralyzed limbs (Jacqueline, 1953, Thompson and Bywaters, 1962). Similarly, while unilateral nerve transection caused a transient swelling of the affected limb (Levine et al., 1990b), it also protected against the induction of inflammatory arthritis (Courtright and Kuzell, 1965). Similarly systemic depletions of sensory C fibers or sympathetic nerves significantly diminished the severity of adjuvant-arthritis in rats (Colpaert et al., 1983, Levine et al., 1986, Levine et al., 1988). However, unlike systemic removal of sensory and sympathetic nerves, intra-articular injection of capsaicin, which leads to localized denervation of both sensory and sympathetic nerves, caused severe inflammation and joint destruction (Mapp et al., 1996). Similarly, localized ablation of nerves with a selective immunotoxin led to joint destruction of the injected joint (Salo et al., 1997). Interestingly, unlike peripheral nerve transection, dorsal rhizotomy, which would injure the neuron, but maintain the connection of the soma to the peripheral nerve ending, exacerbated inflammatory arthritis (Levine et al., 1986). Therefore, while the presence of sympathetic and sensory nerves is required for RA development, it is likely that a localized and active degeneration of these nerves may be responsible for development of inflammatory arthritis. This could be due to an active release of neurotransmitters, neuropeptides, and other pro-inflammatory factors from degenerating nerves and/or a hyperexcitability created by the degeneration process. Since vitamin D deficiency leads to localized depletion of sensory and sympathetic nerves in the synovium presumably through nerve degeneration, this may contribute to hypovitaminosis D-induced increases in susceptibility to and severity of inflammatory arthritis.
5.0 Conclusion
Four weeks of vitamin D deficient diet was sufficient to lead to sensory and sympathetic denervation of the synovium. Given that vitamin D deficiency has been shown to increase susceptibility to RA and RA is associated with sensory and sympathetic denervation, we believe that hypovitaminosis-D induced synovial degeneration may be an important finding. While vitamin D deficiency may not cause RA, it may create a neuro-inflammatory process that promotes the development of the disorder.
Highlights.
Inflammatory arthritis is linked to vitamin D deficiency and synovial denervation.
Intimal synovial nerves are sensory and most contain both CGRP & GFRα2.
Vitamin D deficiency reduces intimal density of neurturin(GFRα2 ligand)-ir cells.
Vitamin D deficiency reduces the intimal density of sensory nerves.
Vitamin D deficiency reduces subintimal density of sympathetic (TH-ir) nerves.
Acknowledgements
We would like to thank the Kansas Intellectual and Developmental Disabilities Research Center imaging and histology cores for the use of their facilities and equipment. We would also like to thank Dr. Dora Agbas, Dr. Julie Christianson, and Dr. Aritra Bhattacherjee for thoughtful proof-reading and suggestions to the manuscript. Funding for this work was provided by NIH NIA F31AG032943, NCCAM R21AT006629, and NICHD RO1HD049615, with core support from NICHD P30HD002528.
Glossary
- αSMA
alpha smooth muscle actin
- CGRP
calcitonin gene related peptide
- CD163
cluster of differentiation 163
- DAPI
40,6-diamidino-2-phenylindole
- GFRα2
glial derived neurotrophic factor family receptor alpha 2
- MCT
mast cell tryptase
- NGF
nerve growth factor
- PGP9.5
protein gene product 9.5
- RA
rheumatoid arthritis
- SP
substance P
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol. 1993;151:356–368. doi: 10.1006/cimm.1993.1245. [DOI] [PubMed] [Google Scholar]
- Alexander JL, Dennerstein L, Woods NF, Halbreich U, Kotz K, Richardson G, Graziottin A, Sherman JJ. Arthralgias, bodily aches and pains and somatic complaints in midlife women: etiology, pathophysiology and differential diagnosis. Expert Rev Neurother. 2007;7:S15–26. doi: 10.1586/14737175.7.11s.S15. [DOI] [PubMed] [Google Scholar]
- Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8:573–586. doi: 10.1038/nrrheum.2012.134. [DOI] [PubMed] [Google Scholar]
- Attar SM. Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia. Saudi Med J. 2012;33:520–525. [PubMed] [Google Scholar]
- Bar-Shavit Z, Noff D, Edelstein S, Meyer M, Shibolet S, Goldman R. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33:673–676. doi: 10.1007/BF02409507. [DOI] [PubMed] [Google Scholar]
- Black IB, Hendry I, Iversen LL. Differences in the regulation of tyrosine hydroxylase and dopa decarboxylase in sympathetic ganglia and adrenals. Nat New Biol. 1971;231:27–29. [PubMed] [Google Scholar]
- Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A, McCarson KE, Smith PG. Hypersensitivity and hyperinnervation of the rat hind paw following carrageenan-induced inflammation. Neurosci Lett. 2011;495:67–71. doi: 10.1016/j.neulet.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Clarke GL, Bhattacherjee A, Tague SE, Hasan W, Smith PG. Beta-adrenoceptor blockers increase cardiac sympathetic innervation by inhibiting autoreceptor suppression of axon growth. J Neurosci. 2010;30:12446–12454. doi: 10.1523/JNEUROSCI.1667-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC, Donnerer J, Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983;32:1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Courtright LJ, Kuzell WC. Sparing effect of neurological deficit and trauma on the course of adjuvant arthritis in the rat. Ann Rheum Dis. 1965;24:360–368. doi: 10.1136/ard.24.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, Sulli A, Paolino S, Seriolo B. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol. 2006;24:702–704. [PubMed] [Google Scholar]
- Damm J, Luheshi GN, Gerstberger R, Roth J, Rummel C. Spatiotemporal nuclear factor interleukin-6 expression in the rat brain during lipopolysaccharide-induced fever is linked to sustained hypothalamic inflammatory target gene induction. The Journal of comparative neurology. 2011;519:480–505. doi: 10.1002/cne.22529. [DOI] [PubMed] [Google Scholar]
- Dirmeier M, Capellino S, Schubert T, Angele P, Anders S, Straub RH. Lower density of synovial nerve fibres positive for calcitonin gene-related peptide relative to substance P in rheumatoid arthritis but not in osteoarthritis. Rheumatology (Oxford) 2008;47:36–40. doi: 10.1093/rheumatology/kem301. [DOI] [PubMed] [Google Scholar]
- Ernsberger U. Role of neurotrophin signalling in the differentiation of neurons from dorsal root ganglia and sympathetic ganglia. Cell and tissue research. 2009;336:349–384. doi: 10.1007/s00441-009-0784-z. [DOI] [PubMed] [Google Scholar]
- Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Feron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65:141–148. doi: 10.1016/j.brainresbull.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Fonseca JE, Edwards JC, Blades S, Goulding NJ. Macrophage subpopulations in rheumatoid synovium: reduced CD163 expression in CD4+ T lymphocyte-rich microenvironments. Arthritis and rheumatism. 2002;46:1210–1216. doi: 10.1002/art.10207. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Lee JM, Raimondo S, Nicolino S, Geuna S, Giacobini-Robecchi M. Neuronal intermediate filament expression in rat dorsal root ganglia sensory neurons: an in vivo and in vitro study. Neuroscience. 2008;153:1153–1163. doi: 10.1016/j.neuroscience.2008.02.080. [DOI] [PubMed] [Google Scholar]
- Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. The Journal of comparative neurology. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: a systematic review. QJM. 2005;98:667–676. doi: 10.1093/qjmed/hci096. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Galea MP, Bartlett PF, Turnley AM. EphA4 regulates central nervous system vascular formation. The Journal of comparative neurology. 2006;497:864–875. doi: 10.1002/cne.21029. [DOI] [PubMed] [Google Scholar]
- Goldstein ME, House SB, Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. J Neurosci Res. 1991;30:92–104. doi: 10.1002/jnr.490300111. [DOI] [PubMed] [Google Scholar]
- Haque UJ, Bartlett SJ. Relationships among vitamin D, disease activity, pain and disability in rheumatoid arthritis. Clin Exp Rheumatol. 2010;28:745–747. [PubMed] [Google Scholar]
- Heidari B, Hajian-Tilaki K, Heidari P. The status of serum vitamin D in patients with rheumatoid arthritis and undifferentiated inflammatory arthritis compared with controls. Rheumatol Int. 2012;32:991–995. doi: 10.1007/s00296-010-1736-3. [DOI] [PubMed] [Google Scholar]
- Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- Hukkanen M, Gronblad M, Rees R, Kottinen YT, Gibson SJ, Hietanen J, Polak JM, Brewerton DA. Regional distribution of mast cells and peptide containing nerves in normal and adjuvant arthritic rat synovium. J Rheumatol. 1991;18:177–183. [PubMed] [Google Scholar]
- Hukkanen M, Platts LA, Corbett SA, Santavirta S, Polak JM, Konttinen YT. Reciprocal age-related changes in GAP-43/B-50, substance P and calcitonin gene-related peptide (CGRP) expression in rat primary sensory neurones and their terminals in the dorsal horn of the spinal cord and subintima of the knee synovium. Neurosci Res. 2002;42:251–260. doi: 10.1016/s0168-0102(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Fukunaga T, Jin HW, Fujita M, Takano-Yamamoto T, Sugimoto T. VR1-, VRL-1- and P2X3 receptor-immunoreactive innervation of the rat temporomandibular joint. Brain Res. 2004;1008:131–136. doi: 10.1016/j.brainres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Imai S, Tokunaga Y, Konttinen YT, Maeda T, Hukuda S, Santavirta S. Ultrastructure of the synovial sensory peptidergic fibers is distinctively altered in different phases of adjuvant induced arthritis in rats: ultramorphological characterization combined with morphometric and immunohistochemical study for substance P, calcitonin gene related peptide, and protein gene product 9.5. J Rheumatol. 1997;24:2177–2187. [PubMed] [Google Scholar]
- Ito A, Hagiyama M, Oonuma J. Nerve-mast cell and smooth muscle-mast cell interaction mediated by cell adhesion molecule-1, CADM1. J Smooth Muscle Res. 2008;44:83–93. doi: 10.1540/jsmr.44.83. [DOI] [PubMed] [Google Scholar]
- Jacqueline F. [A case of evolutive polyarthritis with localisation controlateral to a hemiplegia] Rev Rhum Mal Osteoartic. 1953;20:323–324. [PubMed] [Google Scholar]
- Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney J, Schultz H, Zhou R, Fox GM. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J Biol Chem. 1997;272:33111–33117. doi: 10.1074/jbc.272.52.33111. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of neuroprotective action of vitamin D(3) Biochemistry (Mosc) 2004;69:738–741. doi: 10.1023/b:biry.0000040196.65686.2f. [DOI] [PubMed] [Google Scholar]
- Kerr GS, Sabahi I, Richards JS, Caplan L, Cannon GW, Reimold A, Thiele GM, Johnson D, Mikuls TR. Prevalence of vitamin D insufficiency/deficiency in rheumatoid arthritis and associations with disease severity and activity. J Rheumatol. 2011;38:53–59. doi: 10.3899/jrheum.100516. [DOI] [PubMed] [Google Scholar]
- Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O'Dea AP, Klemp JR, Fabian CJ. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. The Journal of comparative neurology. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. Macrophages in rheumatoid arthritis. Arthritis Res. 2000;2:189–202. doi: 10.1186/ar86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Rees R, Hukkanen M, Gronblad M, Tolvanen E, Gibson SJ, Polak JM, Brewerton DA. Nerves in inflammatory synovium: immunohistochemical observations on the adjuvant arthritis rat model. J Rheumatol. 1990;17:1586–1591. [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Smith PG. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res. 2008;86:3086–3095. doi: 10.1002/jnr.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger H, Penttila IM, Alhava EM. Low serum vitamin D metabolites in women with rheumatoid arthritis. Scand J Rheumatol. 1993;22:172–177. doi: 10.3109/03009749309099266. [DOI] [PubMed] [Google Scholar]
- Larsson P, Mattsson L, Klareskog L, Johnsson C. A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol. 1998;114:277–283. doi: 10.1046/j.1365-2249.1998.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurons. In: Scott SA, editor. Sensory neurons: diversity, development, and plasticity. Oxford Press; New York: 1992. pp. 27–59. [Google Scholar]
- Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Frontiers in neuroanatomy. 2014;8:21. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Levine JD, Clark R, Devor M, Helms C, Moskowitz MA, Basbaum AI. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984;226:547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Levine JD, Coderre TJ, Covinsky K, Basbaum AI. Neural influences on synovial mast cell density in rat. J Neurosci Res. 1990a;26:301–307. doi: 10.1002/jnr.490260306. [DOI] [PubMed] [Google Scholar]
- Levine JD, Coderre TJ, Helms C, Basbaum AI. Beta 2-adrenergic mechanisms in experimental arthritis. Proc Natl Acad Sci U S A. 1988;85:4553–4556. doi: 10.1073/pnas.85.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Coderre TJ, White DM, Finkbeiner WE, Basbaum AI. Denervation-induced inflammation in the rat. Neurosci Lett. 1990b;119:37–40. doi: 10.1016/0304-3940(90)90749-y. [DOI] [PubMed] [Google Scholar]
- Levine JD, Dardick SJ, Roizen MF, Helms C, Basbaum AI. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986;6:3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci. 2006;26:1953–1960. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI. Innervation of the synovium. Annals of the rheumatic diseases. 1995;54:398–403. doi: 10.1136/ard.54.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI, Kerslake S, Brain SD, Blake DR, Cambridge H. The effect of intra-articular capsaicin on nerve fibres within the synovium of the rat knee joint. J Chem Neuroanat. 1996;10:11–18. doi: 10.1016/0891-0618(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Kidd BL, Gibson SJ, Terry JM, Revell PA, Ibrahim NB, Blake DR, Polak JM. Substance P-, calcitonin gene-related peptide- and C-flanking peptide of neuropeptide Y-immunoreactive fibres are present in normal synovium but depleted in patients with rheumatoid arthritis. Neuroscience. 1990;37:143–153. doi: 10.1016/0306-4522(90)90199-e. [DOI] [PubMed] [Google Scholar]
- Mapp PI, Walsh DA, Garrett NE, Kidd BL, Cruwys SC, Polak JM, Blake DR. Effect of three animal models of inflammation on nerve fibres in the synovium. Ann Rheum Dis. 1994;53:240–246. doi: 10.1136/ard.53.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall JJ, Ferrell WR, Bray RC. Spatial variation in sympathetic influences on the vasculature of the synovium and medial collateral ligament of the rabbit knee joint. J Physiol. 1997;503:435–443. doi: 10.1111/j.1469-7793.1997.435bh.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Justen HP, Scholmerich J, Straub RH. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 2000;14:2097–2107. doi: 10.1096/fj.99-1082com. [DOI] [PubMed] [Google Scholar]
- Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Scholmerich J, Straub RH. Increased prevalence of semaphorin 3C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–1163. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- Moghaddami M, Mayrhofer G, Anderson PH, Morris HA, Van Der Hoek M, Cleland LG. Efficacy and mechanisms of action of vitamin D in experimental polyarthritis. Immunol Cell Biol. 2012;90:168–177. doi: 10.1038/icb.2011.22. [DOI] [PubMed] [Google Scholar]
- Moghimi J, Sadeghi A, Malek M, Ghorbani R. Relationship between disease activity and serum levels of vitamin D and parathyroid hormone in rheumatoid arthritis. Endocr Regul. 2012;46:61–66. doi: 10.4149/endo_2012_02_61. [DOI] [PubMed] [Google Scholar]
- Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 2010;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56:2143–2149. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- Richeri A, Chalar C, Martinez G, Greif G, Bianchimano P, Brauer MM. Estrogen up-regulation of semaphorin 3F correlates with sympathetic denervation of the rat uterus. Auton Neurosci. 2011;164:43–50. doi: 10.1016/j.autneu.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, Bernini L, Cacace E, Sinigaglia L, Di Munno O, Adami S. Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther. 2010;12:R216. doi: 10.1186/ar3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PT, Theriault E, Wiley RG. Selective ablation of rat knee joint innervation with injected immunotoxin: a potential new model for the study of neuropathic arthritis. J Orthop Res. 1997;15:622–628. doi: 10.1002/jor.1100150420. [DOI] [PubMed] [Google Scholar]
- Seidel MF, Herguijuela M, Forkert R, Otten U. Nerve growth factor in rheumatic diseases. Semin Arthritis Rheum. 2010;40:109–126. doi: 10.1016/j.semarthrit.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Shi H, Cui H, Alam G, Gunning WT, Nestor A, Giovannucci D, Zhang M, Ding HF. Nestin expression defines both glial and neuronal progenitors in postnatal sympathetic ganglia. J Comp Neurol. 2008;508:867–878. doi: 10.1002/cne.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100–106. doi: 10.2174/1874312901105010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GG, Bae SC, Lee YH. Association between vitamin D intake and the risk of rheumatoid arthritis: a meta-analysis. Clin Rheumatol. 2012 doi: 10.1007/s10067-012-2080-7. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Rossi J, Airaksinen MS, Lewin GR. GFR alpha2/neurturin signalling regulates noxious heat transduction in isolectin B4-binding mouse sensory neurons. J Physiol. 2002;545:43–50. doi: 10.1113/jphysiol.2002.027656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Suzuki R, Furuno T, Teshima R, Nakanishi M. N-cadherin plays a role in the synapse-like structures between mast cells and neurites. Biol Pharm Bull. 2004;27:1891–1894. doi: 10.1248/bpb.27.1891. [DOI] [PubMed] [Google Scholar]
- Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci. 2011;31:13728–13738. doi: 10.1523/JNEUROSCI.3637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat. 2011;41:1–12. doi: 10.1016/j.jchemneu.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeba Y, Suzuki N, Kaneko A, Asai T, Sakane T. Evidence for neural regulation of inflammatory synovial cell functions by secreting calcitonin gene-related peptide and vasoactive intestinal peptide in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2418–2429. doi: 10.1002/1529-0131(199911)42:11<2418::AID-ANR21>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Smith SJ, Mawer EB, Woolley DE. Vitamin D receptors in the rheumatoid lesion: expression by chondrocytes, macrophages, and synoviocytes. Ann Rheum Dis. 1999;58:118–121. doi: 10.1136/ard.58.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M, Bywaters EG. Unilateral rheumatoid arthritis following hemiplegia. Ann Rheum Dis. 1962;21:370–377. doi: 10.1136/ard.21.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turhanoglu AD, Guler H, Yonden Z, Aslan F, Mansuroglu A, Ozer C. The relationship between vitamin D and disease activity and functional health status in rheumatoid arthritis. Rheumatol Int. 2011;31:911–914. doi: 10.1007/s00296-010-1393-6. [DOI] [PubMed] [Google Scholar]
- Walls AF, Bennett AR, McBride HM, Glennie MJ, Holgate ST, Church MK. Production and characterization of monoclonal antibodies specific for human mast cell tryptase. Clin Exp Allergy. 1990;20:581–589. doi: 10.1111/j.1365-2222.1990.tb03153.x. [DOI] [PubMed] [Google Scholar]
- Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Scholmerich J, Straub RH. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis. 2005;64:13–20. doi: 10.1136/ard.2003.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Baker JF. Vitamin D, immunoregulation, and rheumatoid arthritis. J Clin Rheumatol. 2011;17:102–107. doi: 10.1097/RHU.0b013e31820edd18. [DOI] [PubMed] [Google Scholar]
- Wernli G, Hasan W, Bhattacherjee A, van Rooijen N, Smith PG. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res Cardiol. 2009;104:681–693. doi: 10.1007/s00395-009-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widgerow AD, Kalaria S. Pain mediators and wound healing--establishing the connection. Burns. 2012;38:951–959. doi: 10.1016/j.burns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res. 1991;28:110–114. doi: 10.1002/jnr.490280111. [DOI] [PubMed] [Google Scholar]
- Zwerina K, Baum W, Axmann R, Heiland GR, Distler JH, Smolen J, Hayer S, Zwerina J, Schett G. Vitamin D receptor regulates TNF-mediated arthritis. Ann Rheum Dis. 2011;70:1122–1129. doi: 10.1136/ard.2010.142331. [DOI] [PubMed] [Google Scholar]