Abstract
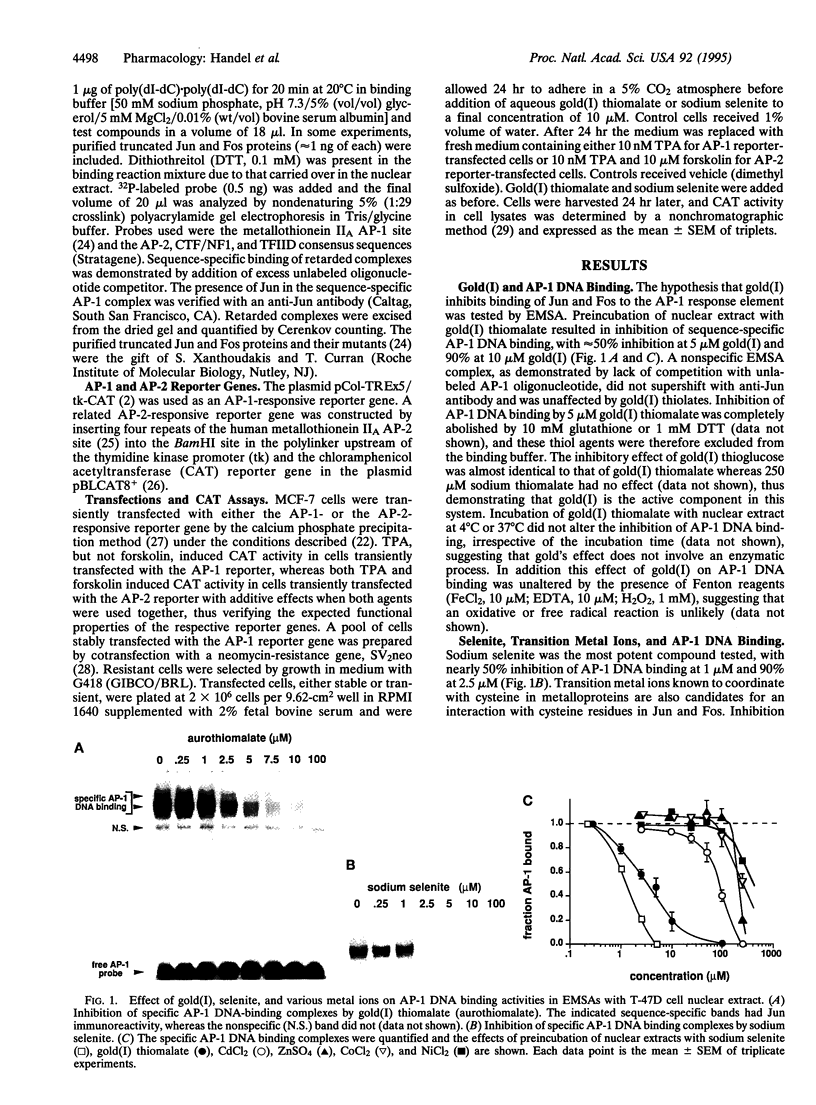
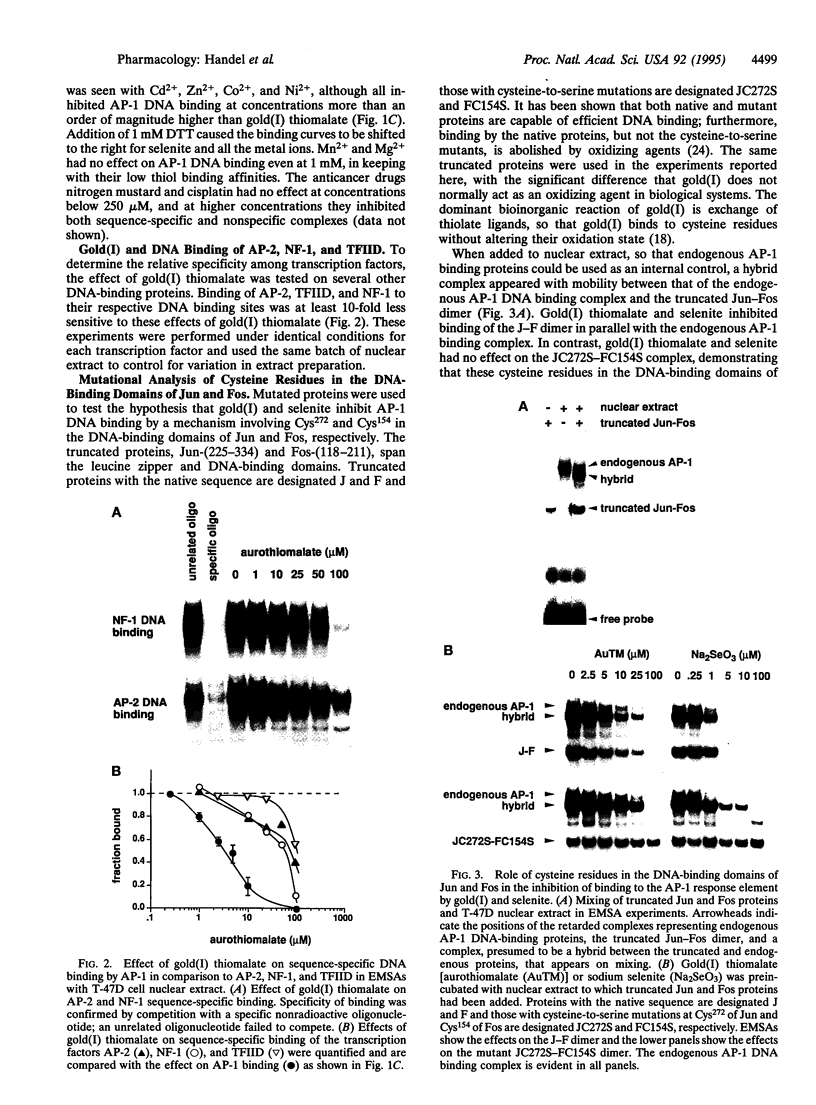
Gold(I) salts and selenite, which have diverse therapeutic and biological effects, are noted for their reactivity with thiols. Since the binding of Jun-Jun and Jun-Fos dimers to the AP-1 DNA binding site is regulated in vitro by a redox process involving conserved cysteine residues, we hypothesized that some of the biological actions of gold and selenium are mediated via these residues. In electrophoretic mobility-shift analyses, AP-1 DNA binding was inhibited by gold(I) thiolates and selenite, with 50% inhibition occurring at approximately 5 microM and 1 microM, respectively. Thiomalic acid had no effect in the absence of gold(I), and other metal ions inhibited at higher concentrations, in a rank order correlating with their thiol binding affinities. Cysteine-to-serine mutants demonstrated that these effects of gold(I) and selenite require Cys272 and Cys154 in the DNA-binding domains of Jun and Fos, respectively. Gold(I) thiolates and selenite did not inhibit nonspecific protein binding to the AP-1 site and were at least an order of magnitude less potent as inhibitors of sequence-specific binding to the AP-2, TFIID, or NF1 sites compared with the AP-1 site. In addition, 10 microM gold(I) or 10 microM selenite inhibited expression of an AP-1-dependent reporter gene, but not an AP-2-dependent reporter gene. These data suggest a mechanism regulating transcription factor activity by inorganic ions which may contribute to the known antiarthritic action of gold and cancer chemoprevention by selenium.
Full text
PDF
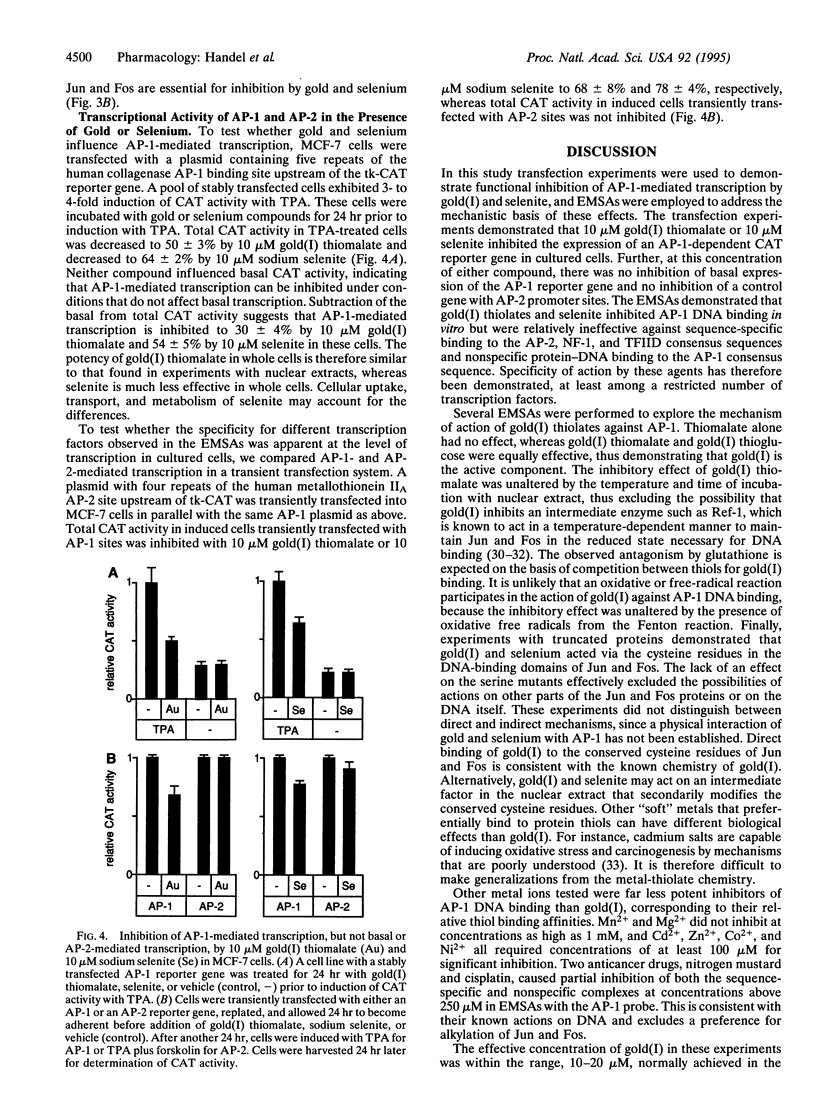

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. A ubiquitous nuclear protein stimulates the DNA-binding activity of fos and jun indirectly. Cell Growth Differ. 1990 Oct;1(10):455–462. [PubMed] [Google Scholar]
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Littman B. H. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991 Sep;34(9):1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Ganther H. E. Selenotrisulfides. Formation by the reaction of thiols with selenious acid. Biochemistry. 1968 Aug;7(8):2898–2905. doi: 10.1021/bi00848a029. [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Handel M. L., deFazio A., Watts C. K., Day R. O., Sutherland R. L. Inhibition of DNA binding and transcriptional activity of a nuclear receptor transcription factor by aurothiomalate and other metal ions. Mol Pharmacol. 1991 Nov;40(5):613–618. [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kawakami A., Eguchi K., Migita K., Nakao H., Otsubo T., Ueki Y., Shimomura C., Tezuka H., Matsunaga M., Ishimaru T. Inhibitory effects of gold sodium thiomalate on the proliferation and interferon-gamma induced HLA-DR expression in human endothelial cells. J Rheumatol. 1990 Apr;17(4):430–435. [PubMed] [Google Scholar]
- Klein-Hitpass L., Ryffel G. U., Heitlinger E., Cato A. C. A 13 bp palindrome is a functional estrogen responsive element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988 Jan 25;16(2):647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M., Musgrove E. A., Sutherland R. L. Differential effects of phorbol ester on epidermal growth factor receptors in estrogen receptor-positive and -negative breast cancer cell lines. Cancer Res. 1990 Aug 15;50(16):4849–4855. [PubMed] [Google Scholar]
- Kumar S., Rabson A. B., Gélinas C. The RxxRxRxxC motif conserved in all Rel/kappa B proteins is essential for the DNA-binding activity and redox regulation of the v-Rel oncoprotein. Mol Cell Biol. 1992 Jul;12(7):3094–3106. doi: 10.1128/mcb.12.7.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkainen R., Isomäki H., Kajander A. Effect of gold treatment on the progression of erosions in RA patients. Scand J Rheumatol. 1977;6(2):123–127. doi: 10.3109/03009747709095434. [DOI] [PubMed] [Google Scholar]
- Matthews J. R., Wakasugi N., Virelizier J. L., Yodoi J., Hay R. T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992 Aug 11;20(15):3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Ono S. J., Bazil V., Levi B. Z., Ozato K., Strominger J. L. Transcription of a subset of human class II major histocompatibility complex genes is regulated by a nucleoprotein complex that contains c-fos or an antigenically related protein. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4304–4308. doi: 10.1073/pnas.88.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M., Whelan A., Feighery C., Bresnihan B. Changes in lymphocyte infiltration of the synovial membrane and the clinical course of rheumatoid arthritis. Arthritis Rheum. 1989 Apr;32(4):361–369. doi: 10.1002/anr.1780320402. [DOI] [PubMed] [Google Scholar]
- Sanders K. M., Carlson P. L., Littman B. H. Effects of gold sodium thiomalate on interferon stimulation of C2 synthesis and HLA-DR expression by human monocytes. Arthritis Rheum. 1987 Sep;30(9):1032–1039. doi: 10.1002/art.1780300910. [DOI] [PubMed] [Google Scholar]
- Shamberger R. J. Relationship of selenium to cancer. I. Inhibitory effect of selenium on carcinogenesis. J Natl Cancer Inst. 1970 Apr;44(4):931–936. [PubMed] [Google Scholar]
- Sigler J. W., Bluhm G. B., Duncan H., Sharp J. T., Ensign D. C., McCrum W. R. Gold salts in the treatment of rheumatoid arthritis. A double-blind study. Ann Intern Med. 1974 Jan;80(1):21–26. doi: 10.7326/0003-4819-80-1-21. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Snow E. T. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53(1):31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Snyder G. H., Cennerazzo M. J., Karalis A. J., Field D. Electrostatic influence of local cysteine environments on disulfide exchange kinetics. Biochemistry. 1981 Nov 10;20(23):6509–6519. doi: 10.1021/bi00526a001. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spalding D. M., Darby W. L., 3rd, Heck L. W. Alterations in macrophage collagenase secretion induced by gold sodium thiomalate. Arthritis Rheum. 1986 Jan;29(1):75–81. doi: 10.1002/art.1780290110. [DOI] [PubMed] [Google Scholar]
- Van Riel P. L., Larsen A., Van de Putte L. B., Gribnau F. W. Effects of aurothioglucose and auranofin on radiographic progression in rheumatoid arthritis. Clin Rheumatol. 1986 Sep;5(3):359–364. doi: 10.1007/BF02054254. [DOI] [PubMed] [Google Scholar]
- Vernon-Roberts B., Doré J. L., Jessop J. D., Henderson W. J. Selective concentration and localization of gold in macrophages of synovial and other tissues during and after chrysotherapy in rheumatoid patients. Ann Rheum Dis. 1976 Dec;35(6):477–486. doi: 10.1136/ard.35.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters M. T., Smith J. L., Moore K., Evans P. R., Cawley M. I. An investigation of the action of disease modifying antirheumatic drugs on the rheumatoid synovial membrane: reduction in T lymphocyte subpopulations and HLA-DP and DQ antigen expression after gold or penicillamine therapy. Ann Rheum Dis. 1987 Jan;46(1):7–16. doi: 10.1136/ard.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992 Feb;11(2):653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]