Summary
The vertebrate immune system is highly dependent on cell death for efficient responsiveness to microbial pathogens and oncogenically transformed cells. Cell death pathways are vital to the function of many immune cell types during innate, humoral and cellular immune responses. In addition, cell death regulation is imperative for proper adaptive immune self-tolerance and homeostasis. While apoptosis has been found to be involved in several of these roles in immunity, recent data demonstrate that alternative cell death pathways are required. Here, we describe the involvement of a programmed form of cellular necrosis called “necroptosis” in immunity. We consider the signaling pathways that promote necroptosis downstream of death receptors, type I transmembrane proteins of the tumor necrosis factor (TNF) receptor family. The involvement of necroptotic signaling through a “RIPoptosome” assembled in response to innate immune stimuli or genotoxic stress is described. We also characterize the induction of necroptosis following antigenic stimulation in T cells lacking caspase-8 or FADD function. While necroptotic signaling remains poorly understood, it is clear that this pathway is an essential component to effective vertebrate immunity.
Introduction
Necrosis was initially defined as an accidental, uncontrolled type of cell death that is typically induced by energetic starvation or plasma membrane disruption [1]. Recent studies have revolutionized the definition of necrosis, and it is now known that certain forms are highly regulated processes activated by certain pathological or physiological stimuli [2]. Necroptosis is a form of programmed necrosis that occurs when caspases are inhibited, or otherwise fail to become activated [3]. Apoptosis is a caspase-dependent mode of cell death, leading to the orderly degradation of cellular components into “apoptotic bodies” that are engulfed by surrounding cells via phagocytosis. With the efficient and rapid removal of dead cells, apoptosis has long been considered the immunologically quiescent form of cell death, whereas necrosis (and likely necroptosis due to its similarity to necrosis) is thought to provoke the immune system [4]. Given this paradigm, apoptosis is to be considered the preferred mode of cell death during the development of the immune system, particularly in regulating adaptive immune tolerance and the homeostasis of mature lymphocytes in the peripheral immune system. In contrast, necroptosis may be considered a “fail-safe” mechanism to prevent unrestrained growth of cells, particularly following infection by viruses that attempt to prevent apoptosis [5]. A key control point in the choice between necroptosis vs. apoptosis is mediated through the RIP kinase family [6]. The Serine-threonine RIP kinases, RIPK1 and RIPK3, are critical mediators of necroptosis [7], while RIPK1 is also a key regulator of apoptosis [8]. The involvement of RIP kinases in the control apoptotic vs. necroptotic cell death following death receptor stimulation is currently the most well-characterized. However, as described below, other roles for RIP kinase family members in death receptor independent forms of necroptosis are also under intense scrutiny.
Death-receptor signaling triggers cell death
TNFR1 stimulation can lead to diverse responses: anti-apoptosis, apoptosis, or necroptosis. The TNF signaling pathway is currently the most widely studied necroptotic signaling pathway. TNF binding to TNFR1 at the plasma membrane leads to the recruitment of TNFR1-associated death domain (TRADD), RIP1, cellular inhibitor of apoptosis protein 1 (cIAP1), cIAP2, TNF-receptor-associated factor 2 (TRAF2), and TRAF5 (Figure 1). This assembly is called complex I and it is situated on the plasma membrane [9]. cIAP1 and cIAP2 polyubiquitinate RIP1 and induce NF-κB activation [10, 11] while preventing apoptosis and necroptosis. Transforming growth factor-β-activated kinase 1 (TAK1)-binding proteins TAB1 and TAB2 mediate the interaction between ubiquitinated RIP1 and TAK1. TAK1 in turn activates the inhibitor of NF-κB kinase (IKK) complex, and the IKK complex phosphorylates IκB. The phosphorylated IκB is polyubiquitinated and undergoes proteasomal degradation, which enables NF-κB translocation to the nucleus. The activation of the canonical NF-κB pathway results in the transcription of pro-survival and pro-inflammatory genes [13]. In the absence of cIAPs, the canonical NF-κB pathway is suppressed [11, 14, 15]. RIP1 is not polyubiquitinated, and complex I leads to the upregulation of NF-κB-inducing kinase (NIK) and the activation of the non-canonical NF-κB pathway [16–18]. Thus, the main function of TNF-induced complex I is likely to promote anti-apoptosis pathways.
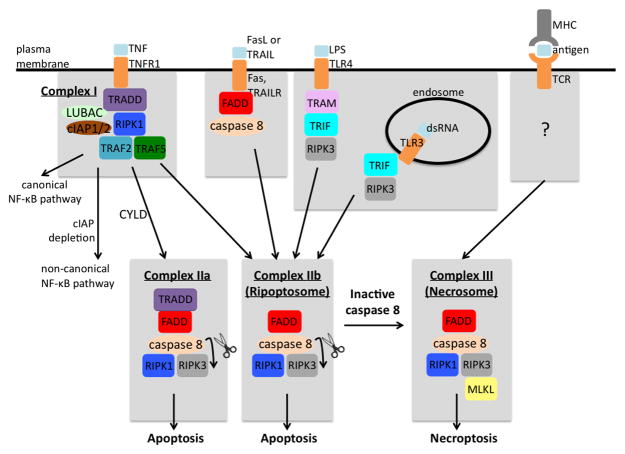
Figure 1. Molecular pathways of cell death.
The activation of death receptors (TNFR1, Fas, and TRAIL), TLR3, TLR4, or the TCR can result in apoptosis or necroptosis. TNFR1 ligation to TNF leads to the assembly of complex I that can activate the canonical NF-kB pathway or the non-canonical NF-kB pathway (under cIAP depleting conditions). If CYLD deubquitinates RIPK1, then complex IIa forms and is known as a TRADD-dependent complex. Complex IIb, a TRADD-independent complex, is known as the ripoptosome. Caspase 8 cleavage of RIPK1 and RIPK3 in complexes IIa and IIb results in apoptosis. Inactive caspase 8 turns complex IIb into a RIPK1/RIPK3 necrosome. After MLKL is phosphorylated by RIPK3, necroptosis ensues. TLR3 and TLR4 activation results in the recruitment of TRIF, which recruits RIPK3 and leads to ripoptosome or necrosome formation. Currently, the necroptotic signaling mechanism that stems from TCR stimulation remains unknown.
Cylindromatosis (CYLD) is a RIP1 Lys63 deubiquitinating enzyme that prevents the pro-survival effect of RIP1 [19]. CYLD destabilizes complex I and allows RIP1 to dissociate from the plasma membrane. Subsequently, a TRADD-dependent complex (complex IIa) assembles in the cytoplasm: RIP1 associates with TRADD, FADD and pro-caspase 8 [20, 21]. Pro-caspase 8 dimerizes [22] and terminates the necroptotic signal by cleaving RIP1 and RIP3 [23–25]. It has been suggested that the caspase 8-c-FLIPL heterodimer cleaves RIP3 [26] and CYLD to promote apoptosis and prevent necroptosis. Caspase 8 activation leads to the activation of caspase 3 and caspase 7 to execute apoptosis. If cIAPs are depleted and RIP1 is not ubiquitylated, then a TRADD-independent complex forms. The assembly of RIP1, RIP3, FADD, and caspase 8 is called the RIP1-dependent complex IIb, or the ripoptosome [28]. Within this complex, caspase 8 cleaves RIP1 and RIP3, resulting in apoptosis and the prevention of further necroptotic signaling [29].
The inhibition of caspase 8 by caspase inhibitors or virally encoded proteins, such as cytokine response modifier protein A (CrmA), causes RIP1 and RIP3 to associate within necrosomes (complex III) [30]. RIP1 and RIP3 associate with one another through the RIP homotypic interaction motif (RHIM), leading to activation of downstream necroptotic signaling [31, 32]. This occurs when mixed lineage kinase domain-like protein (MLKL) is phosphorylated by RIP3 and is recruited to the necrosome [33]. The intracellular location of the necrosome is not well understood, although it has been reported that RIPK3 does not co-localize with mitochondria, golgi, endoplasmic reticulum, peroxisomes, LAMP1-associated endosomes, nor RhoB-associated endosomes [34]. However, a recent study suggests that MLKL is required for the translocation of RIPK3-containing necrosomes to membrane-associated mitochondria (MAM) upon TNF-induced necroptosis [35].
RIP1 [36–38] and RIP3 [32, 34, 37, 38] are key mediators of necroptosis induced by the DR ligands TNF, Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL). FasL binds to Fas, and in the absence of caspase 8 [39], this leads to the recruitment of FADD, RIP1 [36, 40], and RIP3 [34, 41], resulting in necroptosis. TRAIL binding to TRAIL-Rs can also result in RIP1/RIP3 necrosome formation [38]. Thus, while much remains to be determined, it is clear that ligation of death receptors of the TNF-R family can provoke apoptosis, necroptosis, or non cell-death associated events. The relative contribution of apoptosis vs. necrosis under physiological and pathological conditions in vivo remains to be fully elaborated.
Toll-like receptor signaling triggers cell death
The innate immune system is armed with pathogen recognition receptors that detect molecular components from a variety of pathogens including bacteria, viruses, yeast, and fungi. Although Toll like receptors (TLRs) bind to foreign pathogen associated molecular patterns (PAMPs), they also recognize endogenous danger associated molecular patterns (DAMPs) such as self nucleic acids released from dying cells [42]. TLR3 and TLR4 recognize double-stranded RNA [43] and lipopolysaccharide (LPS) [44], respectively. These receptors control cytokine induction, dendritic cell maturation, and antigen presentation to T cells [45]. TLR3 and TLR4 signal through the adaptor protein TRIF, but TLR4 signals through TRIF in cooperation with a TIR adaptor protein called TRAM. TLR4 is also able to signal through MyD88 [46]. RIP1 is also a critical mediator of the activation of the NF-κB pathway through TLR3 and TLR4 [47, 48]. As described below, TLR3 and TLR4 signaling pathways also regulate necrosome formation and cell death.
Stimulation of TLR3 or TLR4 triggers the recruitment of TRIF, whose RHIM motif subsequently interacts with the RHIM motif of RIPK3 [49, 50]. The deactivation of caspases is necessary for TNFα-induced RIP1/RIP3 necrosome formation [32, 34]. Interestingly, the deactivation of caspases was not essential for the recruitment of RIP3 to TRIF in TLR3 and TLR4-mediated necroptosis in bone-marrow derived macrophages (BMDM)s [49]. It is important to note another study that showed caspase 8 inhibition in BMDMs was required for TLR4-induced necroptosis in BMDMs [51]. In addition, although TNFα is produced as a result of TLR3 or TLR4 signaling, autocrine TNFα contributes minimally to the necroptosis induced through TLR3 or TLR4 signaling [49].
Physiological importance of necroptosis to infection and tissue damage
Viruses and bacteria that can inhibit caspases could pose a threat if these organisms cannot be eliminated. Therefore, if caspase 8 is non-functional, then necroptosis may be an important way to eliminate damaged cells. Necroptosis is characterized by cellular swelling and eventual burst that releases endogenous adjuvants to surrounding tissue. This elicits an inflammatory response and the recruitment of cells to clear away damaged cells or cell debris. It has been shown that RIP3 expression is upregulated in the thymus and spleen following tissue injury [34]. Murine cytomegalovirus M45-encoded viral inhibitor of RIP activation (vIRA) is an inhibitor of RIPK3-dependent necroptosis, and it prevents the host from eliminating the virus [52]. The ability of viruses to suppress the host’s cell death pathways is an important immune evasion strategy for viruses. This provides significant implications for the role of necroptosis in the antiviral immune response, and provides at least one explanation for the need for necroptosis in normal physiology.
Necrosis-inducing complex
As described, cell death regulation in the immune system is crucial for functional immune responses and maintaining immune homeostasis. Although apoptosis has been long observed to regulate the immune system, recent findings have uncovered an alternate death pathway, termed necroptosis or programmed necrosis. Necrosis is a type of cell death characterized by rupture of the plasma membrane, and organelle swelling leading to eventual lysis of the cell [53, 54]. This death is generally regarded as unregulated. Necroptosis can be induced through ligation of tumor necrosis factor (TNF) members (through TNFR1, TNFR2, TRAILR1, and TRAILR2), Fas ligand, toll-like receptors, lipopolysaccharides (LPS), and genotoxic stress [55–59]
As described earlier, ligation of TNFα activates TNFR1 and in turn induces the recruitment of RIP1 kinase via adaptor protein TRADD [60, 61], to the membrane. RIP1 is then recruited to form what is commonly referred to as “complex I” [62, 63] containing ubiquitinated RIP1 as a scaffolding protein, NF-kB essential modulator (NEMO), and IkB kinase (IKK) complex resulting in NF-kB activation [64]. Binding of death receptors also recruits RIP1 to the cytosol for assembly of the death inducing signaling complex (DISC) or complex II [65], which is comprised of adaptor protein FADD, caspase 8, and cFLIP. In the absence of active caspase 8, cytosolic RIP1 can interact with receptor-interacting protein 3 (RIP3) kinase to generate the RIP1/RIP3-containing complex IIb, known as the “necrosome” [66]. Generation of the necrosome leads to the induction of necroptosis.
RIP1 has been demonstrated to be involved in both apoptosis and necroptosis, while RIP3 appears to participate solely in necroptosis. RIP3, which is activated following auto- and cross- phosphorylation by RIP1 has been shown to be essential for promoting necroptosis, whereas RIP1 appears to be dispensable [67–69]. RIP3 phosphorylates and activates downstream targets in the necroptotic pathway, and is considered to be the convergence point for the execution of necroptosis. RIP1 kinase activity is required for necrosome formation since necrostatin-1 [3, 70], which allosterically blocks the kinase activity of RIP1, abolishes the assembly of the RIP1-RIP3 complex. RIP1 and RIP3 have been shown to assemble only in the absence of functional caspase-8, indicating that this enzyme acts as a necrosome inhibitor. Caspase-8 has also been shown to cleave both RIP1 and RIP3 in activated T cells [71], thus acting as a negative regulator of this pathway through this mechanism.
Wang et al. recently discovered various proteins that act downstream of the necrosome. Mixed lineage kinase domain like protein (MLKL) has been identified as a key mediator of necroptosis signaling downstream of RIP3 kinase [72]. MLKL is phosphorylated by RIP3 at the threonine 357 and serine 358 residues, and these phosphorylation events are critical for necroptosis. Wang and colleagues [72] utilized a chemical library screening approach and identified a molecule, necrosulfonamide (NSA), that can interfere with necroptosis downstream of RIP3 activity. Interaction of MLKL1 with the necrosome is necessary for propagating the necroptotic signal in Jurkats, HT-29, and Hela cell lines. Chemical inhibition of MLKL activity leads to blockade of necroptosis, and mice genetically deficient in MLKL are unable to undergo necroptosis. MLKL−/− mice did not show defects in development or during homeostasis [73, 74], but developed signs of necrosis-associated disease. Newton et al. engineered mice expressing catalytically inactive RIP3 D161N or RIP1 D138N [75] to determine the need for kinase activity in necroptosis. Interestingly, RIP3 D161N promoted lethal RIP1-and caspase-8-dependent apoptosis. In contrast, mice expressing RIP1 D138N were viable and, like RIP3-deficient mice, resistant to tumor necrosis factor (TNF)-induced necroptosis.
RIP3 is thought to induce a switch in cellular metabolism, leading to the increase of mitochondrial ROS production that culminates in cell death [76]. Wang et al. simultaneously identified a mitochondrial protein phosphatase, phosphoglycerate mutase 5 (PGAM5) [77], as a downstream effector that binds the necrosome on the mitochondrial membrane. PGAM5-MLKL1-RIP1-3 interaction promotes activation of Drp1, a GTPase necessary for mitochondrial fission, and inhibition of Drp1 inhibition has been demonstrated to block necrosis. While the molecular mechanism of necroptosis execution is still not completely clear, current findings implicate MLKL as an important substrate of RIP3 in targeting downstream targets on cellular organelles such as mitochondria and/or lysosomes.
Necroptosis in T lymphocytes
Necroptosis regulation plays an essential role in the immune system. While the role of apoptosis has been well defined in maintaining central tolerance through the generation of self-tolerant lymphocytes and the clearance of autoreactive lymphocytes, the role of necroptosis has been recently implicated in the regulation of T cell proliferation and survival. Various studies have established that caspase-8, the key molecule mediating apoptosis in response to activation of death receptors, also has important nonapoptotic functions [78] in regulating lymphocyte survival. Several reports indicate that necroptosis regulates antigen-induced proliferation of T cells required for peripheral T cell homeostasis and T cell survival in response to activation stimuli. Mice lacking caspase-8 or expressing a dominantly interfering form of FADD unable to recruit caspase-8 (FADD DED deficient, FADDdd) [79] in T cells displayed impaired T cell homeostasis and diminished peripheral T cell numbers. Impaired expansion of T cells after TCR activation was observed in T cells deficient in caspase-8 [80, 81] or FADD [82] as well as in FADDdd-expressing T cells [83, 84]. Defective accumulation of activated T cells in the absence of caspase 8 activity is due to the induction of necroptosis. Blockade of RIP1 through chemical inhibition with RIP1 inhibitor, necrostatin-1 (Nec-1) [85] restores the expansion defect in caspase 8-deficient and FADDdd-expressing T cells [78, 79], as does genetic knockdown of RIP1 [86] in FADD deficient mice. Similarly, it has been demonstrated that the loss of RIP3 is able to rescue the defective T cell proliferation of caspase 8−/− or FADDdd mice [71, 87, 88], indicating that necroptotic signaling in T cells is regulated by caspase-8. Under physiological conditions, caspase 8 promotes survival of activated T cells by suppressing the necroptotic pathway. The interplay of these apoptotic and necroptotic pathways is critical in immune tolerance, as mice doubly deficient in both caspase 8-RIP1 and FADDdd-RIP3 develop lymphoproliferative disease and accumulated autoreactive lymphocytes.
Mice lacking caspase-8 or expressing FADDdd in T cells are also unable to mount a proper immune response when infected with murine hepatitis virus (MHV), due to defective expansion of effector cells as well as diminished cytotoxic activity [71, 89]. Necroptosis has been implicated in the elimination of excessive T cells during the contraction phase of a viral infection. Removal of activated T lymphocytes that had undergone clonal expansion in response to stimulation (infection) is essential for maintenance of T cell homeostasis and preventing an excessive immune response. However, additional deletion of RIP3 in both cases restored the proper immune responses. Under physiological conditions, the DISC proteins actively inhibit necroptosis as caspase-8 or FADD germline deficiency leads to the embryonic death of mice [1].
Work by Upton et al. have reported a function for necroptosis in the context of viral infections [90]. During these circumstances, the necroptotic pathway can be induced to clear virally infected cells. Upton et al. [91], suggests that RIP3 but not RIP1 is required for necrosis induced during viral infections. RIP3−/− mice succumb to vaccinia virus expressing caspase 8 inhibitor, and viral replication is enhanced within infected RIP3 deficient cells. Similarly, when mice were infected with cytomegalovirus [91], a virus that expresses inhibitors of caspase8 and inhibitor of RIP3, m45/vIRA, RIP3−/− mice were more susceptible to the M45/vIRA deficient virus. Under these settings, necroptosis functions as a back-up mechanism against viruses encoding apoptotic inhibitors.
A recent study by Bohgaki et al. [92] demonstrated that the inactivation of caspase-8 in T cells suppressed the autoimmune phenotype characteristic of Bim (Bcl-2-interacting mediator of cell death) knockout mice [93]. Bim is a proapoptotic BH3-only protein of the Bcl-2 protein family, involved in facilitating the intrinsic apoptosis pathway [94]. Bim-deficient mice develop autoimmunity due to the defective apoptotic pathway in T cells [93], and inactivation of caspase-8 in Bim−/− T cells promotes activation-induced necroptosis of autoreactive Bim−/− T cells. Thus loss of caspase-8 in T cells appears to have antagonizing effects on Bim-associated autoimmunity, suggesting a role for necroptosis in preventing overactive immune responses. These findings indicate that apoptosis and necroptosis must be tightly regulated in order to maintain immune homeostasis.
Ripoptosome and host defense
Recent work from Tenev et al. and Feoktistova et al. has revealed a cytosolic complex, termed the “Ripoptosome” [55, 56], that can induce both apoptosis and necroptosis through interactions between TLR domain-containing adaptor protein inducing interferon-B (TRIF) [95] and the RHIM of RIP1. This ~2-megadalton signaling platform assembles in response to pattern recognition receptor (PRR) activation and genotoxic stress, and is also mediated by complex IIb proteins caspase 8, FADD, cFLIP, and RIP1. The ripoptosome is a signaling platform that regulates caspase-8 dependent apoptosis or RIP1 mediated necroptosis independent of TNF, TRAIL, or CD95L, which distinguishes it from the necrosome.
The immune system defends itself against pathogens through Pattern Recognition Receptors (PRRs) [96]. Activation through TLRs by certain PAMPs and DAMPs induces RIP-mediated apoptotic or pyroptotic (inflammasome-mediated) cell death in particular cell types and conditions. It has been shown that stimulation of TLR3 by dsRNA (poly[I:C]) or TLR4 by LPS in combination with caspase inhibition leads to RIP3-mediated necroptosis in murine macrophages [97] and FADD-deficient Jurkat cells [98]. When TLR3 is activated, TRIF interacts with RIP3 leading to ROS production and necroptosis. Macrophage cell death and cytokine expression were both inhibited when LPS and zVAD-fmk or poly(I:C) and zVAD-fmk were injected into RIP3-deficient or TrifLPS2/LPS2 dominant-negative mutant mice [99], suggesting the involvement of RIP3 and TRIF in TLR-mediated necroptosis. Wang et al. showed that the TLR4 pathway is involved in High Mobility Group Box 1 protein (HMGB1) secretion from macrophages by a mechanism that depends on interleukin-1 (IL-1) receptor-associated kinase 4 (IRAK4) [100], suggesting that HMGB1 might be released during TLR4-mediated necroptosis.
Caspase 8 and RIP1 are also known to be recruited to the RNA sensor retinoic acid-inducible gene I (RIG1) complex [101], and cytosolic DNA sensor, DNA-dependent activator of interferon regulatory factors (DAI) [102], which directly engage RIPK1 and RIP3 through the RHIM to assemble the ripoptosome. Expression of DAI sensitizes cells to virus-induced necrosis and DAI knockdown or knockout cells are resistant to this death pathway. Importantly, as with RIP3−/− mice, vIRA mutant MCMV pathogenesis is restored in DAI−/− mice, consistent with a DAI-RIP3 complex being the natural target of vIRA. Thus, DAI interacts with RIP3 to mediate virus-induced necrosis analogous to the RIP1-RIP3 complex controlling death receptor-induced necroptosis. These observations emphasize the various cellular contexts where the necroptotic pathway is crucial in host defense.
Modulation of necroptosis in T cells
Current studies still aim to indentify a physiological role for necroptosis in lymphocytes. It is clear that caspase 8−/− or FADDdd T cells succumb to necroptosis when stimulated with antigens. The BCL-10-CARMA-1-MALT1 (BCM) complex, which activates NF-kB following TCR stimulation, is proposed to also dictate DISC formation [103, 104]. However, Arechiga et al. demonstrated that NF-kB signaling is intact in FADDdd and caspase-8 deficient T cells [105]. It has been suggested that perhaps there is a role for necroptosis in FasL/R regulation. However, extracellular blockade of DR ligation failed to rescue caspase 8−/− or FADDdd-expressing T cells, suggesting that the nucleation of RIP1/RIP3 necrosomes occurs in a manner independent of DR signaling. Findings from Newton et al. indicate RIP3 activity determines the switch between apoptosis and necroptosis [75]. The mechanism by which caspase 8 is activated is still unclear, and understanding the temporal kinetics of its activity would provide additional insight into the events that promote necroptosis.
Other mechanisms, such as autophagy, may coordinate the sensitivity of cells to necroptosis [106]. T lymphocyte proliferation is impaired in the absence of autophagy proteins Atg5, Atg7, Atg3, or Beclin-1 [107]. Interestingly, a hyperautophagic phenotype has been observed in FADDdd- and caspase-8-deficient lymphocytes after TCR stimulation [78, 79]. Treatment with Nec-1 in these models not only prevented cell death but also reduced autophagy levels.
Another aspect of the crosstalk between necroptosis and autophagy deals with its role in removal damaged organelles [108]. It was proposed that selective autophagy of mitochondria, or mitophagy, may be used to compartmentalize mitochondria producing ROS and protect cells from excess ROS production. Interference with the mechanism responsible for the removal of damaged mitochondria could sensitize cells to necroptotic death. However, deletion of Atg7 in the genetic background of a caspase 8 deficiency exacerbated the loss of T cells [80], indicating that the defects observed in T cells of mice lacking the capacity to induce autophagy are independent of RIP1 activity. As ROS have been linked to necroptotic induction, the molecular mechanisms of the interplay between mitophagy and necroptosis are currently being investigated. As described above, Wang et al. recently discovered a connection between RIP3 kinase activity and the MLKL protein, which is recruited to mitochondrial membranes [77]. This interaction is responsible for mitochondrial fission and fragmentation and decreased mitochondrial fragmentation was observed in MLKL knockout T cells. Taken together, decreased mitophagy and increased rupture of mitochondria may enable the release of mitochondrial DNA and other related factors that serve as DAMPs and promote ROS production.
Conclusions
As described above, the choice between necroptosis vs. apoptosis is clearly important for proper immune function. The loss of caspase 8 mediated cell leads to the induction of necroptosis and hyper-autophagy instead [109]. While it has been proposed that necroptosis may simply serve as a redundant pathway to ensure the demise of cells following viral infection [90], it is also possible that it may serve other physiologic roles. Supporting this is the demonstration that the signals that promote apoptosis are counter-inhibitory toward necroptotic signaling; loss of caspase 8-dependent processing of RIP1 and CYLD leads to hypersensitivity to necroptosis [71, 110]. Further elaboration of mice bearing defects in other necroptosis regulatory molecules will likely reveal additional physiological roles for necroptotic signaling. Clearly, this counter-regulation between apoptosis and necroptosis is vital, since loss of caspase 8 or FADD in the germline results in embryonic lethality [111–113], lethality that is rescued by the loss of RIP1 mediated necroptosis [86]. Given this, it will be important to understand the signals that lead to necrosome and/or ripoptosome formation under different cell types during embryonic development as well as in the maintenance and function of mature tissues. Clearly, many discoveries lay ahead that will enhance our understanding of the physiological functions mediated by necroptotic signaling.
Acknowledgments
This work is supported by grants from the National Multiple Sclerosis Society (CA 1058-A-8), the California Institute for Regenerative Medicine (CIRM RM1-01717 and TR3-05603) and the National Institutes of Health (GM099040).
Abbreviations
- TNF
tumor necrosis factor
- RIP
receptor interacting protein
- cIAP
cellular inhibitor of apoptosis protein
- TRADD
TNF receptor associated death domain
- FADD
Fas associated death domain
- TAK1
Transforming growth factor-β-activated kinase 1
- NF-kB
nuclear factor kappa B
- IKK
inhibitor of kappa B kinase
- IKB
inhibitor of kappa B
- CYLD
Cylindromatosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh CM, Luhrs KA, Arechiga AF. The “fuzzy logic” of the death-inducing signaling complex in lymphocytes. J Clin Immunol. 2003;23(5):333–53. doi: 10.1023/a:1025313415487. [DOI] [PubMed] [Google Scholar]
- 2.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death and Differentiation. 2012;19(1):75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degterev A, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 4.Walsh CM, Bell BD. T cell intrinsic roles of autophagy in promoting adaptive immunity. Curr Opin Immunol. 2010;22(3):321–5. doi: 10.1016/j.coi.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocarski ES, et al. True grit: programmed necrosis in antiviral host defense, inflammation, and immunogenicity. J Immunol. 2014;192(5):2019–26. doi: 10.4049/jimmunol.1302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinlich R, Dillon CP, Green DR. Ripped to death. Trends Cell Biol. 2011;21(11):630–7. doi: 10.1016/j.tcb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micheau O, Tschopp J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell. 2003;114(2):181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 9.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114(2):181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471(7340):591. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand MJ, et al. cIAP1 and cIAP2 Facilitate Cancer Cell Survival by Functioning as E3 Ligases that Promote RIP1 Ubiquitination. Molecular Cell. 2008;30(6):689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell MA, Ting AT. NFκB and ubiquitination: partners in disarming RIPK1-mediated cell death. Immunologic Research. 2012;54(1–3):214–226. doi: 10.1007/s12026-012-8321-7. [DOI] [PubMed] [Google Scholar]
- 13.Van Antwerp D, et al. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 14.Varfolomeev E, et al. c-IAP1 and c-IAP2 Are Critical Mediators of Tumor Necrosis Factor α (TNFα)-induced NF-κB Activation. Journal of Biological Chemistry. 2008;283(36):24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahoney DJ, et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proceedings of the National Academy of Sciences. 2008;105(33):11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varfolomeev E, et al. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-κB Activation, and TNFα-Dependent Apoptosis. Cell. 2007;131(4):669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131(4):682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Zarnegar BJ, et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nature Immunology. 2008;9(12):1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moquin DM, McQuade T, Chan FKM. CYLD Deubiquitinates RIP1 in the TNFα-Induced Necrosome to Facilitate Kinase Activation and Programmed Necrosis. PLoS ONE. 2013;8(10):e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitomi J, et al. Identification of a Molecular Signaling Network that Regulates a Cellular Necrotic Cell Death Pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Du F, Wang X. TNF-α Induces Two Distinct Caspase-8 Activation Pathways. Cell. 2008;133(4):693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Chang DW, et al. Interdimer processing mechanism of procaspase-8 activation. The EMBO journal. 2003;22(16):4132–4142. doi: 10.1093/emboj/cdg414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, et al. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & development. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rébé C, et al. Caspase-8 prevents sustained activation of NF-kappaB in monocytes undergoing macrophagic differentiation. Blood. 2007;109(4):1442–1450. doi: 10.1182/blood-2006-03-011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cellular Signalling. 2007;19(10):2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Oberst A, et al. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nature Cell Biology. 2011;13(12):1437. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand MJ, Vandenabeele P. The Ripoptosome: death decision in the cytosol. Mol Cell. 2011;43(3):323–5. doi: 10.1016/j.molcel.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Lu JV, Walsh CM. Programmed necrosis and autophagy in immune function. Immunol Rev. 2012;249(1):205–17. doi: 10.1111/j.1600-065X.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, et al. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277(11):9505–11. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 32.Cho Y, et al. Phosphorylation-Driven Assembly of the RIP1-RIP3 Complex Regulates Programmed Necrosis and Virus-Induced Inflammation. Cell. 2009;137(6):1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Z, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nature Cell Biology. 2013;16(1):55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, et al. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-α. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288(23):16247–61. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holler N, et al. Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nature Immunology. 2000;1(6):489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D-W, et al. Science. American Association for the Advancement of Science; 2009. RIP3, an Energy Metabolism Regulator That Switches TNF-Induced Cell Death from Apoptosis to Necrosis; pp. 332–336. [DOI] [PubMed] [Google Scholar]
- 38.Jouan-Lanhouet S, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death & Differentiation. 2012;19(12):2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura H, et al. Necrotic death pathway in Fas receptor signaling. The Journal of cell biology. 2000;151(6):1247–1256. doi: 10.1083/jcb.151.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanger B. RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81(4):513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 41.Li JX, et al. The B-RafV600E inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death and Disease. 2014;5(6):e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nature Medicine. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulou L, et al. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 44.Lien E, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. The Journal of clinical investigation. 2000;105(4):497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunology. 2004;5(10):987. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 46.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 47.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3--induced NF-kappaB activation. Nature immunology. 2004;5(5):503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 48.Cusson-Hermance N, et al. Rip1 mediates the Trif-dependent toll-like receptor 3-and 4-induced NF-kappaB activation but does not contribute to interferon regulatory factor 3 activation. Journal of Biological Chemistry. 2005;280(44):36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 49.He S, et al. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3–mediated pathway. Proceedings of the National Academy of Sciences. 2011;108(50):20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaiser WJ, Offermann MK. Apoptosis Induced by the Toll-Like Receptor Adaptor TRIF Is Dependent on Its Receptor Interacting Protein Homotypic Interaction Motif. The Journal of Immunology. 2005;174(8):4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y, et al. NF-κB Protects Macrophages from Lipopolysaccharide-induced Cell Death. Journal of Biological Chemistry. 2005;280(51):41827–41834. doi: 10.1074/jbc.M510849200. [DOI] [PubMed] [Google Scholar]
- 52.Upton JW, Kaiser WJ, Mocarski ES. Virus Inhibition of RIP3-Dependent Necrosis. Cell Host & Microbe. 2010;7(4):302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24(23):2592–602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shulga N, Pastorino JG. GRIM-19-mediated translocation of STAT3 to mitochondria is necessary for TNF-induced necroptosis. J Cell Sci. 2012;125(Pt 12):2995–3003. doi: 10.1242/jcs.103093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Tenev T, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43(3):432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Feoktistova M, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43(3):449–63. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donepudi M, et al. Insights into the regulatory mechanism for caspase-8 activation. Mol Cell. 2003;11(2):543–9. doi: 10.1016/s1097-2765(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 58.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19(1):75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vercammen D, et al. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9(11):801–8. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 60.Ermolaeva MA, et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9(9):1037–46. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 61.Harper N, et al. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J Biol Chem. 2003;278(28):25534–41. doi: 10.1074/jbc.M303399200. [DOI] [PubMed] [Google Scholar]
- 62.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. Embo J. 1996;15(22):6189–96. [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu H, et al. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4(4):387–96. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 64.Ea CK, et al. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–57. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 65.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14(22):5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138(2):229–32. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 67.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137(6):1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Zhang DW, et al. RIP3, an Energy Metabolism Regulator that Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009;325(5938):332–6. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 70.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu JV, et al. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108(37):15312–7. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L, et al. Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell. 2012;148(1–2):213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 73.Murphy JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39(3):443–53. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Wu J, et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23(8):994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343(6177):1357–60. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 76.Schulze-Osthoff K, et al. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267(8):5317–23. [PubMed] [Google Scholar]
- 77.Wang Z, et al. The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways. Cell. 2012;148(1–2):228–43. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 78.Ch’en IL, et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105(45):17463–8. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bell BD, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105(43):16677–82. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ch’en IL, et al. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208(4):633–41. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salmena L, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17(7):883–95. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osborn SL, et al. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A. 2010;107(29):13034–9. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newton K, et al. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17(3):706–18. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walsh C, et al. A role for FADD in T cell activation and development. Immunity. 1998;8(4):439–49. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 85.Degterev A, Maki JL, Yuan J. Activity and specificity of necrostatin-1, small-molecule inhibitor of RIP1 kinase. Cell Death Differ. 2013;20(2):366. doi: 10.1038/cdd.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471(7338):373–6. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–72. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beisner DR, et al. The requirements for fas-associated death domain signaling in mature T cell activation and survival. J Immunol. 2003;171(1):247–56. doi: 10.4049/jimmunol.171.1.247. [DOI] [PubMed] [Google Scholar]
- 90.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol. 2012;12(2):79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7(4):302–13. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bohgaki T, et al. Caspase-8 inactivation in T cells increases necroptosis and suppresses autoimmunity in Bim−/− mice. J Cell Biol. 2011;195(2):277–91. doi: 10.1083/jcb.201103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 94.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5(3):189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 95.Weber A, et al. Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ. 2010;17(6):942–51. doi: 10.1038/cdd.2009.190. [DOI] [PubMed] [Google Scholar]
- 96.Martinon F, et al. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29(3):213–29. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- 97.Ma Y, et al. NF-kappaB protects macrophages from lipopolysaccharide-induced cell death: the role of caspase 8 and receptor-interacting protein. J Biol Chem. 2005;280(51):41827–34. doi: 10.1074/jbc.M510849200. [DOI] [PubMed] [Google Scholar]
- 98.Kalai M, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 2002;9(9):981–94. doi: 10.1038/sj.cdd.4401051. [DOI] [PubMed] [Google Scholar]
- 99.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 100.Wang F, et al. Fas (CD95) induces rapid, TLR4/IRAK4-dependent release of pro-inflammatory HMGB1 from macrophages. J Inflamm (Lond) 2010;7:30. doi: 10.1186/1476-9255-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajput A, et al. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34(3):340–51. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 102.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 Complexes with RIP3 to Mediate Virus-Induced Programmed Necrosis that Is Targeted by Murine Cytomegalovirus vIRA. Cell Host Microbe. 2012;11(3):290–7. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawadler H, et al. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol Cell. 2008;31(3):415–21. doi: 10.1016/j.molcel.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Misra RS, et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. J Biol Chem. 2007;282(27):19365–74. doi: 10.1074/jbc.M610610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arechiga AF, et al. A Fas-Associated Death Domain Protein/Caspase-8-Signaling Axis Promotes S-Phase Entry and Maintains S6 Kinase Activity in T Cells Responding to IL-2. J Immunol. 2007;179(8):5291–300. doi: 10.4049/jimmunol.179.8.5291. [DOI] [PubMed] [Google Scholar]
- 106.Bray K, et al. Autophagy suppresses RIP kinase-dependent necrosis enabling survival to mTOR inhibition. PLoS One. 2012;7(7):e41831. doi: 10.1371/journal.pone.0041831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He MX, et al. Macroautophagy in T lymphocyte development and function. Front Immunol. 2012;3:22. doi: 10.3389/fimmu.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bell BD, Walsh CM. Coordinate regulation of autophagy and apoptosis in T cells by death effectors: FADD or foundation. Autophagy. 2009;5(2):238–40. doi: 10.4161/auto.5.2.7512. [DOI] [PubMed] [Google Scholar]
- 110.O’Donnell MA, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13(12):1437–42. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9(2):267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 112.Zhang J, et al. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392(6673):296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 113.Yeh WC, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279(5358):1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]