Abstract
Neuropathic pain affects millions of people worldwide causing substantial disability and greatly impairing quality of life. Commonly used analgesics or anti-hyperalgesic compounds are generally characterized by limited therapeutic outcomes. Thus, there is a compelling need for novel therapeutic strategies able to prevent nervous tissue alterations responsible for chronic pain. The α9α10 nAChR antagonist α-conotoxin RgIA (RgIA), a peptide isolated from the venom of a carnivorous cone snail, induces relief in both acute and chronic pain models. To evaluate potential disease-modifying effects of RgIA, the compound was given to rats following chronic constriction injury (CCI) of the sciatic nerve. Two or 10 nmol RgIA injected intramuscularly once a day for 14 days reduced the painful response to suprathreshold stimulation, increased pain threshold to non-noxious stimuli, and normalized alterations in hind limb weight bearing. Histological analysis of the sciatic nerve revealed that RgIA prevented CCI-induced decreases of axonal compactness and diameter, loss of myelin sheath and decreases in the fiber number. Moreover, RgIA significantly reduced edema and inflammatory infiltrate, including a decrease of CD86+ macrophages. In L4–L5 dors the inflammatory infiltrate consistent with a disease-modifying effect. In the dorsal horn of the spinal cord, RgIA prevented CCI-induced activation of microglia and astrocytes. These data suggest that RgIA-like compounds may represent a novel class of therapeutics for neuropathic pain that protects peripheral nervous tissues as well as prevents central maladaptive plasticity by inhibiting glial cell activation.
Keywords: alpha-conotoxin RgIA, neuropathic pain, neuroprotection, chronic constriction injury
Introduction
Damage to the somatosensory nervous system may result in the induction of neuropathic pain [8]. Metabolic or infective diseases, antiviral or anticancer chemotherapies, multiple sclerosis, and trauma each may give rise to nervous system alterations that induce painful sensory neuropathies [4]. A pathological plasticity of nervous system circuitries causes the development of a chronic pain syndrome and results in reduced efficacy of classical analgesic drugs prescribed to treat neuropathic pain [53]. The widely-used opioids, for example, are less effective in neuropathic pain since the mechanisms that contribute to chronic neuropathic pain may simultaneously contribute to diminishing the antinociceptive properties of opioids [12]. Most of the clinically available neuropathic pain treatments were originally developed for other therapeutic indications such as epilepsy or depression, or for local anesthesia. Recent additions to the pharmacopeia include ziconotide (an N-type calcium channel antagonist obtained from a cone snail) and tapentadol (an opiod agonist and norepinephrine reuptake inhibitor) [2]. These medications provide partial symptomatic relief in subsets of patients, but none has neuroprotective effects. The lack of disease modifying effects necessitates chronic treatment of patients. Compounds able to intervene in the tissue derangement or maladaptive changes of the nervous system that lead to neuropathic pain would represent optimal drugs for therapy; development of drugs that target mechanisms that lead to neurorestorative effects would represent an innovative strategy for preventing chronic pain.
Nicotinic acetylcholine receptors (nAChRs) have been suggested as potentially important signaling molecules in pain and neuroprotection [7,5,14,18,50]. nAChRs are pentameric ligand-gated ion channels composed of combinations of α and/or β subunits (α1–α10; β1–β4) [1]. Among nAChR subtypes, the α homopentamer has been implicated in neuroprotective processes [31]. In a trauma-induced model of neuropathic pain, selective activation of α7 nAChRs decreased pain perception, preserved peripheral nerve architecture and reduced degeneration and inflammation [44,37]. Furthermore, α4β2 agonists showed antinociceptive properties in different animal models of acute and chronic neuropathic pain [3]. Unfortunately, adverse effects and the lack of neuroprotective effects indicate limited potential use of these agents [45]. Recently, the α9α10 nAChR subtype has been implicated in pain because α9α10 antagonists act as pain relievers in trauma- [52] and chemotherapy-induced [54] neuropathies. Moreover, α9α10 antagonists reduced the immune response to nerve trauma [52], suggesting the possibility that such compounds might influence the pathophysiological process in the transition from acute to chronic pain. We therefore sought to test whether administration of RgIA, a peptide that selectively blocks α9α10 nAChRs [25], could prevent the nervous system alterations that underlie neuropathic pain. The effects of repeated RgIA treatments were evaluated in the peripheral and central nervous system of rats subjected to chronic constriction injury (CCI).
Material and Methods
Animals
Male Sprague-Dawley rats (Harlan, Varese, Italy), weighing 200–250 g at the beginning of the experimental procedure, were used for all the experiments. Animals were housed in CeSAL (Centro Stabulazione Animali da Laboratorio, University of Florence) and used no earlier than one week after their arrival. Four rats were housed per cage (size 26 × 41 cm); animals were fed with standard laboratory diet and tap water ad libitum, and kept at 23 ± 1 °C with a 12 h light/dark cycle, light at 7 a.m. All animal manipulations were carried out according to the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive of 24 November 1986; 86/609/EEC). The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85–23, revised 1996; University of Florence assurance number: A5278-01). Formal approval to conduct the described experiments was obtained from the Animal Subjects Review Board of the University of Florence. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Induction of peripheral mononeuropathy by CCI
Neuropathy was induced according to the procedure described by [6]. Briefly, rats were anaesthetized with 2% isoflurane. Under aseptic conditions, the right (ipsilateral) common sciatic nerve was exposed at the level of the middle thigh by blunt dissection. Proximal to the trifurcation, the nerve was carefully freed from the surrounding connective tissue, and four chromic cat gut ligatures (4-0, Ethicon, Norderstedt, Germany) were tied loosely around the nerve with about a 1 mm spacing between ligatures. After hemostasis was confirmed, the incision was closed in layers. The animals were allowed to recover from surgery and then housed one per cage with free access to water and standard laboratory chow. Another group of rats were subjected to sham surgery in which the sciatic nerve was only exposed but not ligated.
Pharmacological treatments
RgIA, synthesized as previously described [24], was dissolved in sterile saline (vehicle) to obtain a concentration of either 2 nmol/100 µL or 10 nmol/100 µL. For “chronic” treatment, 100 µL of RgIA was injected intramuscularly (i.m.) into the vastus lateralis muscle of the ipsilateral (to facilitate reaching the sciatic nerve) paw starting 60 min after surgery (after awakening from anesthesia) and daily thereafter out to day 13. Behavioural tests were performed 24 hours after the last treatment (day 7 and 14). On day 7 and 14, after behavioural measurements, animals received a new i.m. administration of 2 nmol or 10 nmol RgIA and behavioural tests were performed 30 min later. This results were reported as “acute” effect. Control animals received equivalent volumes of vehicle alone. Morphological measurements were performed on day 14. Dosages were chosen on the basis of a previous study [52].
Paw-pressure test
The nociceptive threshold in the rat was determined with an analgesimeter (Ugo Basile, Varese, Italy), according to the method described by [35]. Briefly, constantly increasing pressure was applied to a small area of the dorsal surface of the hind paw using a blunt conical mechanical probe. Mechanical pressure was increased until vocalization or a withdrawal reflex occurred while rats were lightly restrained. Vocalization or withdrawal reflex thresholds were expressed in grams. Rats scoring below 40 g or over 75 g during the test before drug administration were rejected (25%). For analgesia measures, mechanical pressure application was stopped at 120 g.
Von Frey test
The animals were placed in 20 cm × 20 cm Plexiglass boxes equipped with a metallic mesh floor, 20 cm above the bench. Rats were habituated to the environment for 15 minutes before the test. An electronic Von Frey hair unit (Ugo Basile, Varese, Italy) was used: the withdrawal threshold was evaluated by applying forces ranging from 0 to 50 g with a 0.2 g accuracy. A punctate stimulus was delivered with a plastic-tipped probe to the mid-plantar area of each posterior paw from below the mesh floor, and the withdrawal threshold was automatically displayed on the screen. Paw sensitivity threshold was defined as the minimum pressure required to elicit a robust and immediate withdrawal reflex of the paw. Voluntary movements associated with locomotion were not considered as a withdrawal response. Stimuli were applied alternately to each posterior paw with an interval of 5 seconds. Measurements were repeated 5 times and the final value was the average of these 5 measurements [46].
Incapacitance test
Weight bearing changes were measured using an incapacitance apparatus (Linton Instrumentation, Norfolk, UK) to detect changes in postural equilibrium after a hind limb injury [9]. Rats were trained to stand on their hind paws in a box with an inclined plane (65° from horizontal). This box was placed above the incapacitance apparatus. This allowed us to independently measure the weight that the animal applied on each hind limb. The value reported for each animal was the mean of 5 consecutive measurements. In the absence of hind limb injury, rats applied an equal weight on both hind limbs, indicating a postural equilibrium, whereas an unequal distribution of the weight on hind limbs indicated a monolateral decreased pain threshold. Data are expressed as the difference between the weight applied to the limb contralateral to the injury and the weight applied to the ipsilateral one (Δ Weight).
Histologic and morphometric studies on nerve and dorsal root ganglia
On day 14, the ipsilateral (2 cm spanning the ligation) and contralateral sciatic nerves and the L4–L5 dorsal root ganglia (DRG) were dissected and processed for Azan-Mallory staining. Alternatively, sciatic nerves were fixed with osmium. Experimental and analytical procedures were previously described [44,16].
Immunohistochemical assessment of glial cells in L4–L5 DRG and spinal cord
Paraffin sections of sciatic nerves (14-days post surgery) were analyzed to detect CD86-positive cells, as previously described [15]. L4–L5 DRGs and the L4/L5 segments of the spinal cord, exposed from the lumbovertebral column via laminectomy and identified by tracing the dorsal roots from their respective DRG, were analyzed to assess glial cells. Eight-µm cryostat sections of formalin-fixed DRG were incubated with antibody against glutamine synthetase (Millipore, Milan, Italy) at a dilution of 1:100 overnight at 4°C, followed by a rinse in phosphate-buffered saline with Tween-2 PBST), and reaction with secondary antibody conjugated to Alexa Fluor 594 (1:1000, Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Twenty-µm cryostat sections of formalin-fixed spinal cord were incubated with primary antibody directed against Iba1 (rabbit, 1:1000; Wako Chemicals, Richmond, VA, USA) for microglia staining and against glial fibrillary acidic protein GFAP; mouse, 1:5000; Chemicon, Billerica, MA, USA) for astrocytes staining. After rinsing in PBST, sections were incubated in donkey anti-rabbit IgG secondary antibody labeled with Alexa Fluor 488 (1:1000, Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. For all immunohistochemical studies, negative control sections (no exposure to the primary antisera) were processed concurrently with the other sections. Analytical procedures were previously described [15,16], briefly, DRG serial sections were captured under a fluorescent microscope and the signal intensity was analyzed by “ImageJ” analysis software (NIH, Bethesda, MD, USA). Microglia and astrocyte morphology was assessed by inspection of at least three fields (40× 0.75NA objective) in the dorsal horn and cerebral areas per section. Quantitative analysis of GFAP and Iba1-positive cells was performed by collecting at least three independent fields through a 20× 0.5NA objective. GFAP-positive cells were counted using the “cell counter” plugin of ImageJ, while Iba1-positive cells were quantified by means of the automatic thresholding and segmentation features of ImageJ. The GFAP signal in immunostained sections was quantified using FIJI software (distributed by ImageJ, NIH, Bethesda, MD, USA) by automatic thresholding images with the aid of the "Moments" algorithm.
Statistical analysis
Behavioral measurements were performed on 12 rats for each treatment carried out in 2 different experimental sets. For behavioral experiments as well as for immunoblot quantitation, standard ANOVA followed by Fisher‘s protected least significant difference procedure were used. Repeated measures ANOVA followed by Fisher‘s protected least significant difference procedure were used for behavioral experiments when two different time points were compared for the same group. Histological, morphometric and immunohistochemical analyses were performed on 6 rats per group, evaluating 6 sections each of sciatic nerve, L4–L5 DRG, and spinal cord for each animal. DRG values are reported as means of L4 and L5. One-way repeated measure ANOVA followed by the Mann–Whitney test was used. All behavioral assessments were made by researchers blinded to rat treatment. Slides from control and experimental groups were labeled with numbers so that the person performing the image analysis was blinded as to the experimental group. In addition, all images were captured and analyzed by an investigator other than the one who performed measurements to avoid possible bias. Data were analyzed using the “Origin 7.5” software. Differences were considered significant at a P<0.05.
Results
Effects of RgIA on development of neuropathic pain after CCI
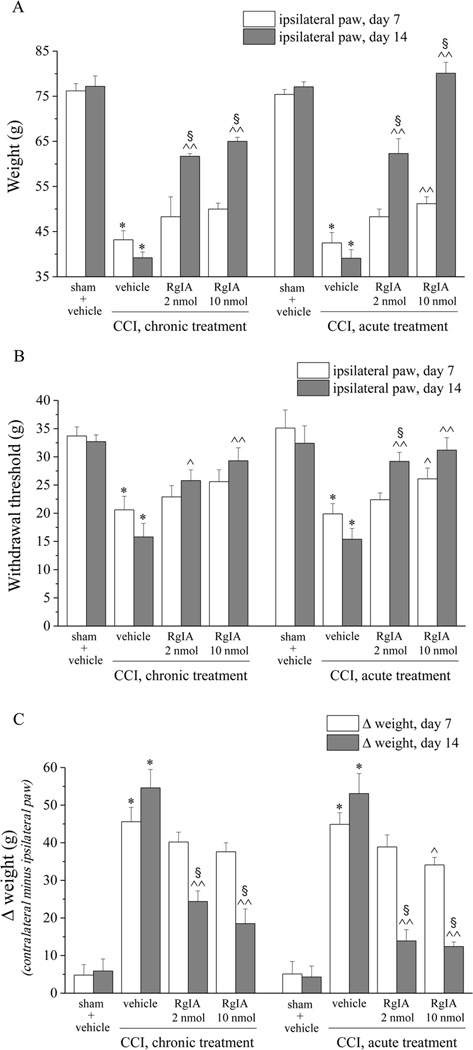
The CCI model of mononeuropathy elicits a pain syndrome, characterized by increased response to suprathreshold stimulation, that begins about 3 days after nerve injury and reaches a plateau that lasts between days 7 to 30 [44]. Seven days after right (ipsilateral) sciatic nerve ligation, rats tolerated a paw pressure of 43.2 ± 2.0 g (Figure 1A; paw pressure test). The left, contralateral (non-operated) side had a threshold of 78.1 ± 4.1 g (not illustrated), which was not significantly different than that of the ipsilateral side in sham-operated animals (76.2 ± 1.6 g) (Figure 1A). RgIA (2 nmol or 10 nmol) was administered daily in the ipsilateral vastus lateralis muscle, and seven days of RgIA injections did not reduce pain as measured 24h after the last RgIA injection; however, new administration of 10 nmol RgIA, to chronically-treated animals, 30-min before testing significantly reduced pain hypersensitivity (Figure 1A, acute). In contrast, 14 days of treatment with RgIA prevented hypersensitivity and the weight tolerated by the ipsilateral paw increased to 61.7 ± 0.6 g and 65.0 ± 0.9 g in 2 nmol- and 10 nmol-treated group, respectively, compared to 39.2 ± 1.3 g in the CCI + vehicle group (Figure 1A). In addition, when tested 30 min after a new injection of 10 nmol RgIA, the weight tolerated increased to 80.1 ± 2.4 g (Figure 1A, acute). Thus, RgIA provided anti-nociception 30 min after its injection on both days 7 and 14, indicating no tolerance to the acute effects. In addition, however, repeated administration of RgIA reduced the development of pain present 24 hours after the last injection. This longer-lasting effect was not significant after 7 days of treatment but was clearly evident by day 14, consistent with a progressive disease-modifying effect.
Figure 1.
RgIA inhibits pain behaviors induced by CCI. Peripheral neuropathy was induced by chronic constriction injury (CCI) of the right sciatic nerve (ipsilateral), and RgIA (2 and 10 nmol) was injected daily by i.m. into the ipsilateral paw starting on the day of surgery. A) Sensitivity to a noxious mechanical stimulus as measured by the paw-pressure test. B) Pain threshold to a non-noxious mechanical stimulus as measured by the Von Frey test. C) Pain assessed by hind limb weight bearing alterations using an incapacitance test that measured postural unbalance related to pain; data are expressed as the difference between the weight applied on the limb contralateral to the injury and the weight applied on the ipsilateral limb. All behavioral tests were performed 7 and 14 days after operation: either 24 hours after the last treatment (chronic) or 30 minutes after a new i.m. administration (acute). Control animals were subjected to sham surgery and treated with vehicle. Each value represents the mean ± SEM of 12 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle; §;P<0.05 versus the same group tested on day 7.
Pain threshold was also evaluated by the Von Frey test, which employs a mechanical stimulus that does not normally provoke pain (Figure 1B). The progressive decrease of paw withdrawal threshold (CCI + vehicle, 20.6 ± 2.4 g on day 7 and 15.8 ± 2.4 g on day 14, with respect to sham + vehicle, 33.7 ± 1.6 g) was significantly reversed on day 7 by 10 nmol RgIA (Figure 1B, acute), and also significantly prevented on day 14 by both dosages (Figure 1B, chronic). A subsequent administration did not significantly increase paw withdrawal threshold (Figure 1B, acute).
The hind limb weight-bearing alterations induced by CCI are shown in Figure 1C. The difference between the weight applied on the limb contralateral to the injured limb and the weight applied on the injured limb (Δ Weight) was measured by an incapacitance test (see Methods). On day 14, postural imbalance was relieved by 62% and 74% by repeated administration of 2 and 10 nmol RgIA, respectively (Figure 1C, chronic). Thirty min after the new injection on day 14 relief increased to 80% (Figure 1C, acute).
Thus, by all three tests (paw pressure, Von Frey, and incapacitance) 14 days of treatment with 10 nmol RgIA relieved pain. In paw pressure and Von Frey tests, RgIA was had no effect on pain threshold of the contralateral paw indicating that RgIA did not produce a general analgesic effect (Supplemental information, Figure S1A and Figure S1B). Moreover, 2 and 10 mol RgIA acutely administered did not alter pain threshold of sham rats as measured by the paw pressure test (Supplemental information, Table S1).
Effect of RgIA on morphological and morphometric derangement of the sciatic nerve
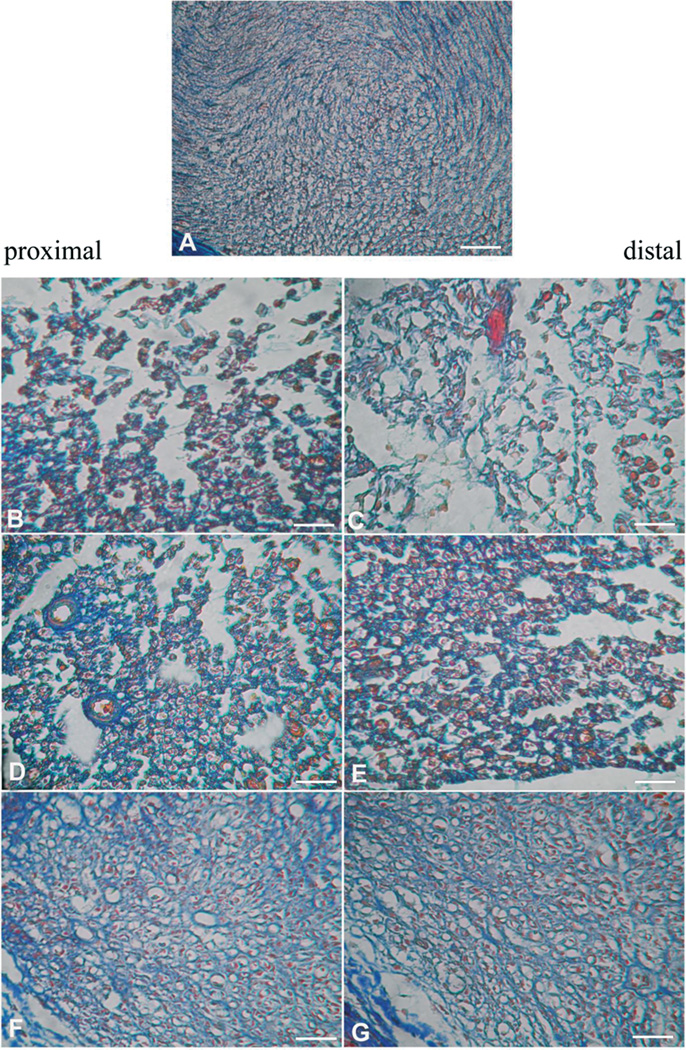
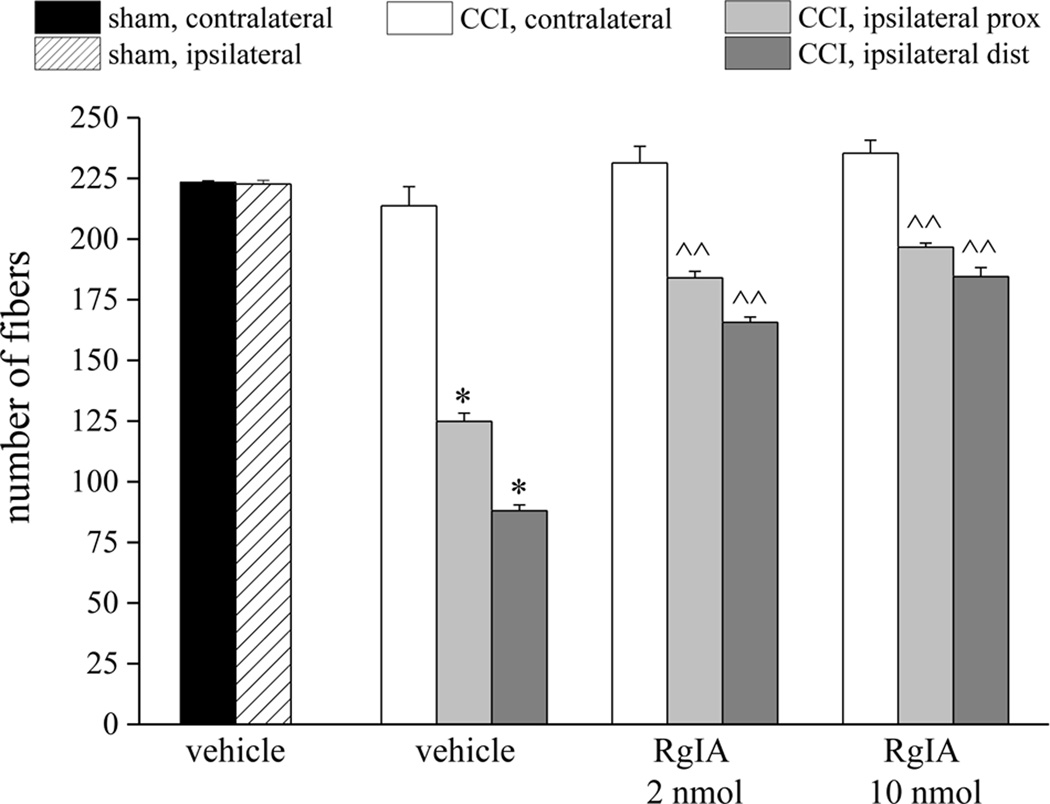
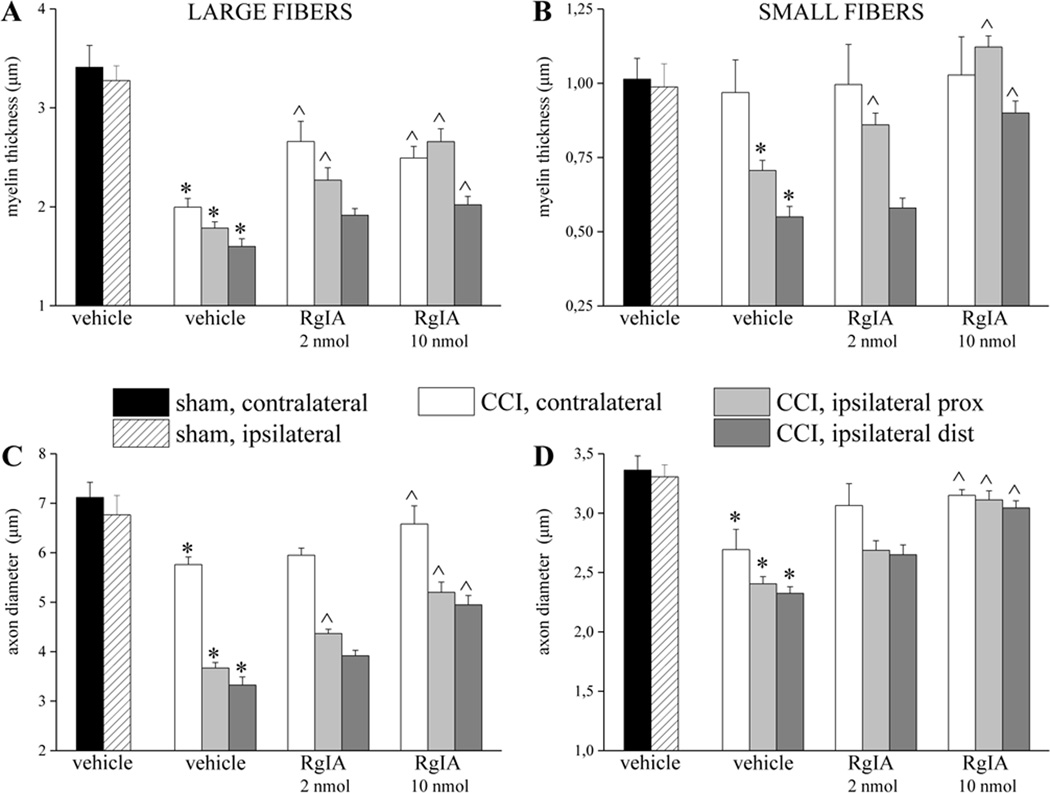
A morphological evaluation was performed on day-14 sciatic nerves, both proximal and distal to the site of ligation. Five µm sections of paraffin-embedded nerve, stained using the Azan-Mallory procedure (see Methods), revealed that CCI induced alterations in both the distal and proximal portions of the ipsilateral nerve. Micrographs show extensive demyelination, myelin degeneration, axonal damage, edema and inflammatory infiltrate (Figure 2), with more damage evident in the distal, than proximal, portion of the nerve. Repeated RgIA administrations, 2 and 10 nmol for 14 days, preserved the nerve morphology. RgIA-treated rats showed a higher number of fibers with respect to the vehicle-treated groups (Figure 3). Morphometric evaluation of osmium-fixed tissues allowed us to characterize alterations of the myelin sheet thickness and axonal diameter. CCI decreased the myelin thickness of both large and small fibers (diameters >6 and <6 µm for large and small fibers, respectively) in both distal and proximal portions of the ipsilateral nerve compared to sham (Figure 4A,B). Although primary damage from CCI occurs in the ipsilateral nerve, we and others have noted that secondary pathological changes may occur in the contralateral nerve [10,44]. Consistent with this, the myelin thickness of large fibers in the contralateral nerve was also decreased (Figure 4A). Small fibers showed a lesser reduction of myelin thickness in the ipsilateral nerve and no alteration of the contralateral nerve (Figure 4B, CCI + vehicle). CCI induced a decrease in axon diameter in both the distal and proximal portions of the ipsilateral nerve. Moreover, the contralateral nerve fibers of CCI + vehicle rats showed decreased axon diameter compared to the sham group (Figure 4C and 4D). Two nmol RgIA significantly reduced the decreases in myelin thickness and axon diameter of the contralateral nerve and of the proximal portion of the ipsilateral nerve. The higher dose significantly decreased proximal and distal alterations in large fibers (Figure 4A,C) and led to a complete prevention of alterations in small fibers (Figure 4B,D).
Figure 2.
Light micrographs of 5-µm transverse sections of mouse sciatic nerve stained by Azan-Mallory, illustrating the effects of RgIA 14-days after CCI and chronic treatment. A) sham + vehicle; B) CCI + vehicle, proximal portion; C) CCI + vehicle, distal portion; D) CCI + RgIA 2 nmol, proximal portion; E) CCI + RgIA 2 nmol, distal portion; F) CCI + RgIA 10 nmol, proximal portion; G) CCI + RgIA 10 nmol, distal portion. Scale bar 100 µm.
Figure 3.
RgIA inhibits the reduction in number of sciatic nerve fibers induced by CCI. Five-µm nerve sections of osmium-fixed nerves were analyzed. The distal and proximal tracts of the ligated nerve (CCI) were compared with the contralateral nerve and nerves of sham-operated animals (sham). Effect of repeated administration of RgIA (2 and 10 nmol/day i.m. for 14 days) or vehicle on the number of nerve fibers are shown. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle.
Figure 4.
RgIA inhibits the reduction in myelin thickness and axon diameters of sciatic nerve induced over 14 days of CCI. Nerve sections (5 µm) of osmium fixed tissues were analyzed. The distal and the proximal tracts of the ipsilateral ligated nerve (CCI) were compared with the contralateral nerve and with the sciatic nerve of sham-operated animals (sham). Myelin thickness of A) large and B) small fibers of RgIA-treated (2 and 10 nmol/day i.m. for 14 days) rats in respect to saline-treated CCI and saline-treated sham animals. Axon diameter of C) large and D) small fibers. Quantitative analysis was performed evaluating 6 animals for each group. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle.
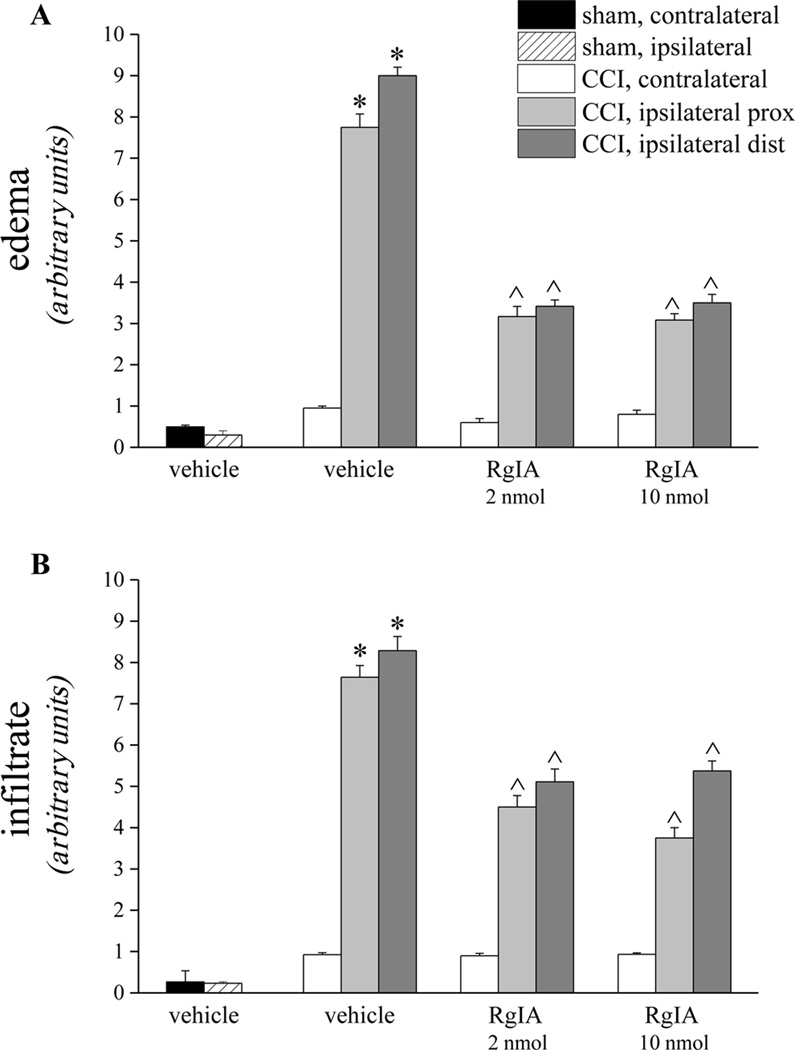
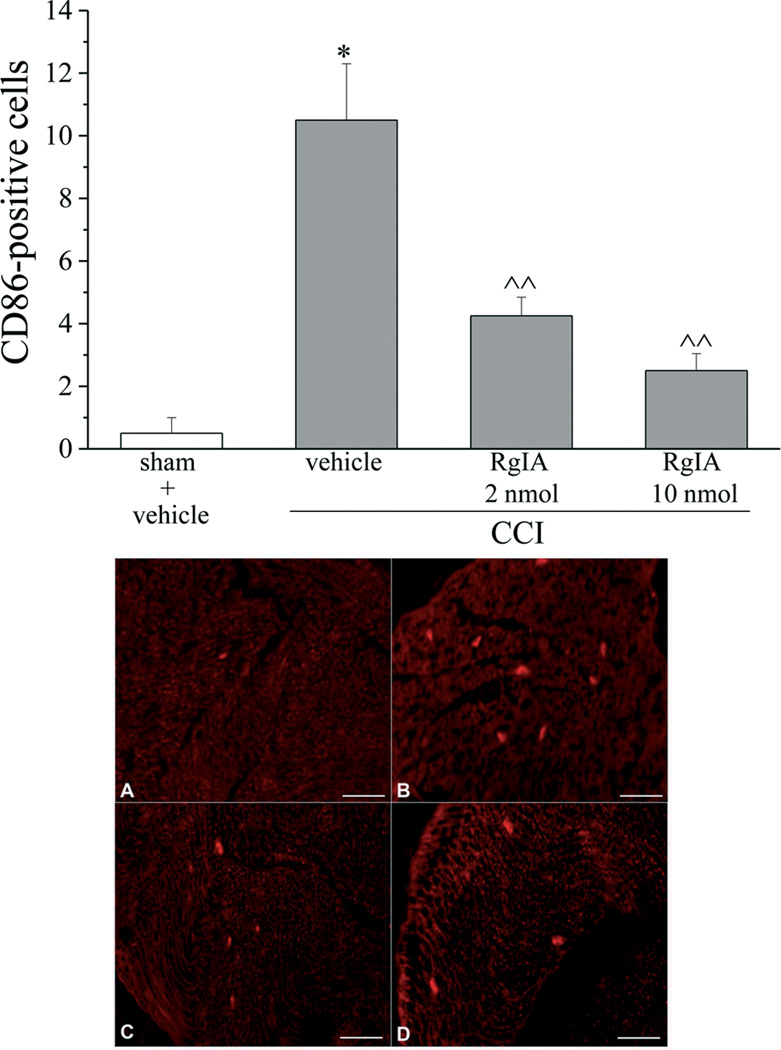
As shown in Figure 5A, 2 and 10 nmol RgIA reduced the presence of edema by approximately 60% in both the distal and the proximal portion of nerve from CCI animals. Moreover RgIA treatments significantly reduced the infiltrate diffusely distributed throughout the nerve (Figure 5B). In particular, the number of CD86 positive cells (characteristic of activated M1 macrophages [33]) decreased dose-dependently after RgIA treatments (Figure 6).
Figure 5.
RgIA reduces edema and infiltration of the sciatic nerve induced over 14 days of CCI. Five µm nerve sections of formalin-fixed nerve were Azan-Mallory stained. A) The presence of edema infiltrate was evaluated and quantified by an arbitrary scale starting from 1, mild edema up to 10, widespread edema. B) The presence of inflammatory infiltrate was evaluated and quantified by an arbitrary scale starting from 1, mild infiltrate up to 10, severe infiltrate. Effect of repeated treatment with RgIA (2 and 10 nmol i.m. daily for 14 days) was scored in the distal and the proximal tracts of the ipsilateral ligated nerve and compared with the contralateral nerve and with sciatic nerves of sham-operated animals. Semi-quantitative analysis was performed evaluating 6 animals for each group. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle
Figure 6.
RgIA reduces the number of CD86-positive cells in the distal portion of the chronically constricted sciatic nerve. Five-µm sections of the formalin-fixed distal part of the sciatic nerve were immunohistochemically stained for CD86. Effect of repeated i.m. treatment with RgIA, 2 and 10 nmol daily over 14 days, was evaluated in comparison with vehicle-treated animals. Quantitative analysis was performed evaluating 6 animals for each group (Upper panel). Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle. Lower panel: representative images of CD86 immunoreactivity. A) sham + vehicle; B) CCI + vehicle; C) CCI + 2 nmol RgIA; D) CCI + 10 nmol RgIA. Scale bar 100 µm.
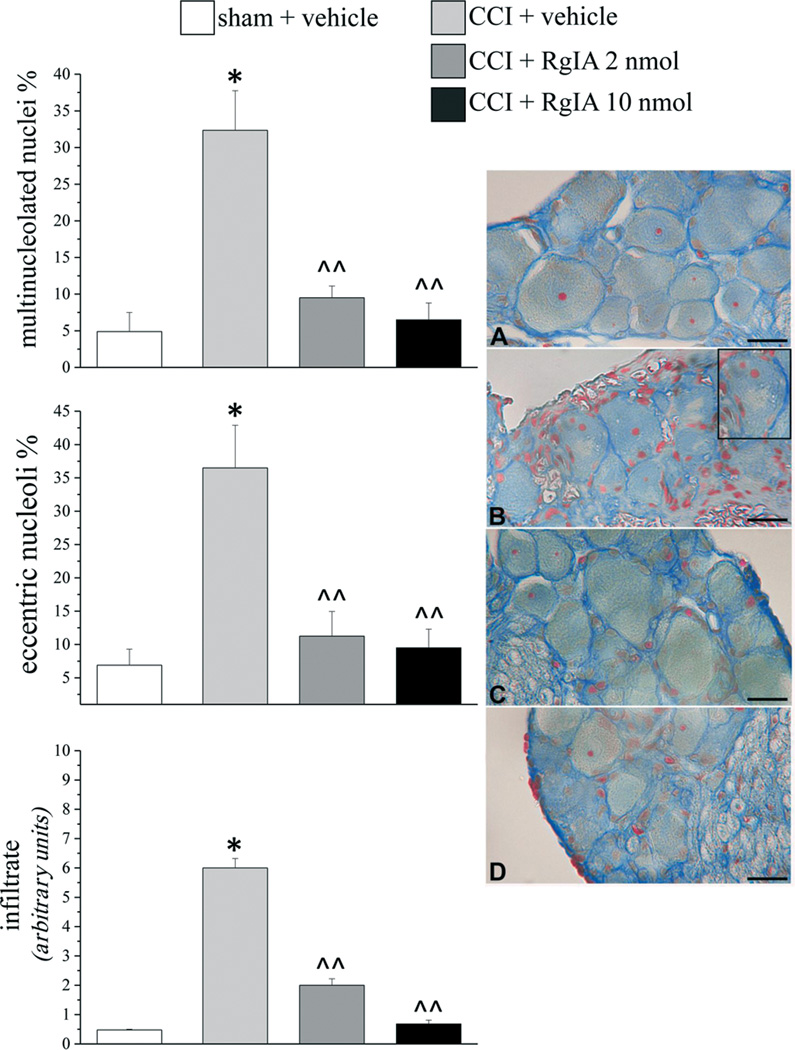
Effect of RgIA on morphological and morphometric derangement of L4–L5 DRGs
Damage to the sciatic nerve secondarily produces pathological changes in DRG [53]. Morphometric determinations were performed on ipsilateral L4–L5 DRGs to assess differences between the sham+ vehicle and the CCI + vehicle groups of animals (Figure 7, Azan-Mallory stain). Ten nmol RgIA significantly prevented the decrease in somatic area of neurons stratified in small, medium and large soma area (Table 1); the 2 nmol treatment did not differ from CCI + vehicle. CCI-induced damage, evidenced by general nucleolar eccentricity and the occurrence of multinucleolated nuclei of neurons, was almost completely prevented by RgIA treatments (Figure 7). The inflammatory infiltrate (Figure 7) was reduced by 70% after 2 nmol RgIA and by 90% after 10 nmol treatment. No evidence of edema was observed in DRGs of CCI rats. Glutamine synthetase expression was not altered in CCI + vehicle animals with respect to the sham + vehicle (Supplemental information, Figure S2).
Figure 7.
RgIA reduces CCI-induced morphological changes in nucleoli of neurons in L4–L5 DRGs. RgIA (2 and 10 nmol) were injected i.m. daily for 14 days. Five-µm cross sections were stained by the Azan-Mallory method. Left, percentages of neurons with eccentric nucleoli (top panel) and multinucleolated nuclei (middle panel). Lower panel: the presence of inflammatory infiltrate, evaluated and quantified by an arbitrary scale starting from 1, mild infiltrate up to 10, severe infiltrate. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle. Right panel: representative images of Azan Mallory-stainedsections of sciatic nerve. A) sham + vehicle; B) CCI + vehicle (inset shows slightly enlarged view of mid-left portion of parent image, illustrating a neuron with eccentric nucleoli and multinucleolated nucleus; C) CCI + 2 nmol RgIA; D) CCI + 10 nmol RgIA. Scale bar 25 µm.
Table 1.
Morphometric determinations performed on the soma area of ipsilateral DRG Neurons
| Soma area (µm2) | |||
|---|---|---|---|
| small neurons < 600 µm2 |
medium neurons 600–1200 µm2 |
large neurons > 1200 µm2 |
|
| sham + vehicle | 505.8 ± 15.1 | 863.6 ± 25.9 | 1685.1 ± 138.7 |
| CCI + vehicle | 445.6 ± 14.7* | 789.5 ± 17.8* | 1447.5 ± 44.6* |
| CCI + RgIA 2 nmol | 454.2 ± 14.6 | 788.48 ± 23.1 | 1566.3 ± 100.4 |
| CCI + RgIA 10 nmol | 496.3 ± 22.8^ | 899.5 ± 30.0^^ | 1607.2 ± 83.2^^ |
RgIA reduces CCI-induced morphological changes in the soma area of neurons in L4-L5 DRGs. RgIA (2 and 10 nmol) were injected i.m. daily for 14 days. Five-µm cross sections were stained by the Azan-Mallory method. The soma of small (<600 µm2), medium (600–1200 µm2) and large (>1200 µm2) neurons was measured and compared among treatments. Soma areas were computed measuring between 50 and 100 cells for each animal from several sections.
P<0.01 with respect to the sham + vehicle treated rats;
P<0.05 and
P<0.01 versus CCI + vehicle group
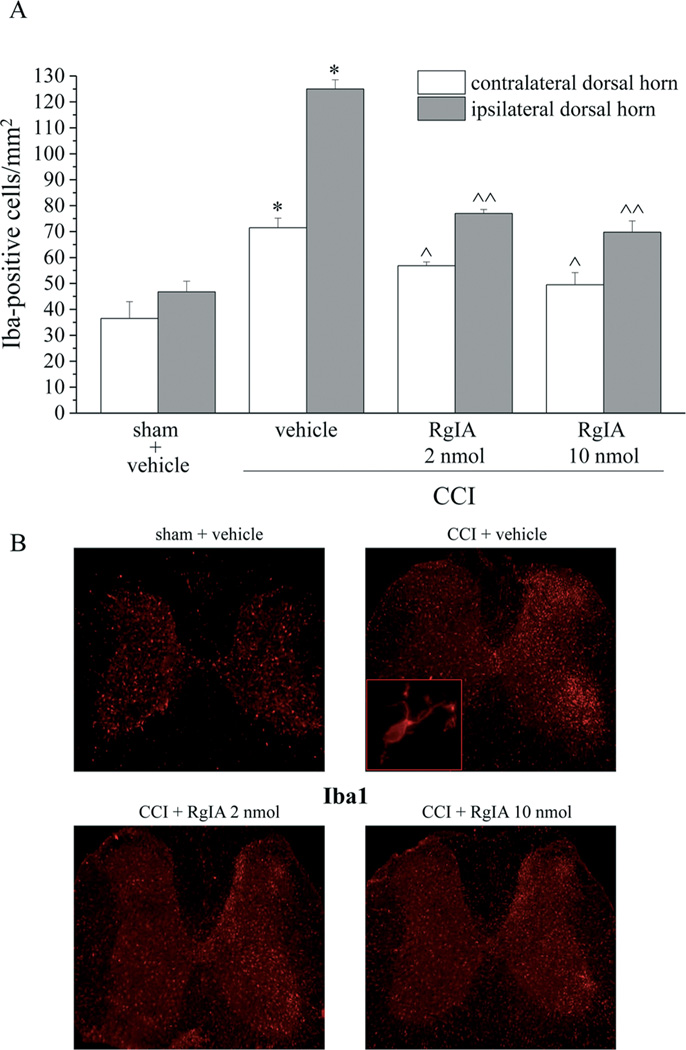
Effect of RgIA on CCI-induced activation of glia cells in the spinal cord
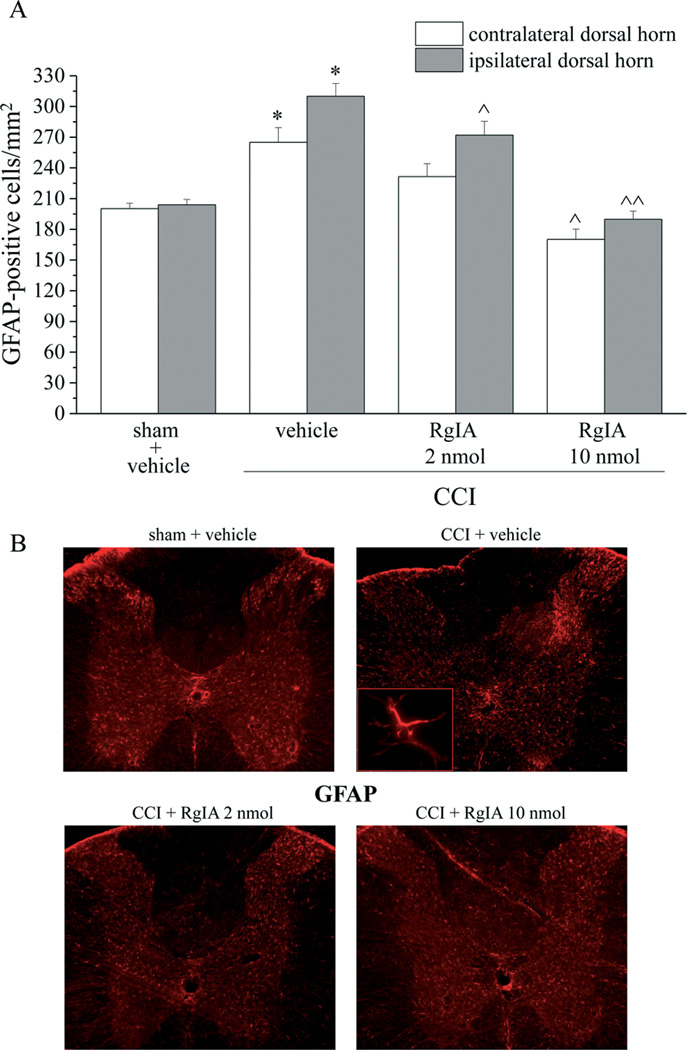
Damage to the sciatic nerve also causes secondary pathological changes in the dorsal horn of the spinal cord [53,38]. To that end, the spinal cord was analyzed to assess the effect treatment with RgIA had on glial cell reorganization. Microglia were labeled immunohistochemically with antibodies against Iba1. Microglia cell density increased by approximately 100% in the ipsilateral dorsal horn of the spinal cord of CCI + vehicle rats (a cell density increase was also evident in the anterior horn) and by 50% in the contralateral side (Figure 8A and 8B). Both dosages of RgIA also significantly decreased the Iba1 positive cell density in the ipsilateral and contralateral horns (Figure 8A and 8B). The density of astrocytes (GFAP positive cells) increased particularly in superficial laminae of CCI + vehicle rats (55% increase in the ipsilateral dorsal horn and 35% in the contralateral side). Ten nmol RgIA fully prevented the increase in astrocyte density (Figure 9A and 9B). No morphological alteration was detectable in microglia or astrocytes per se (insets of Figure 8B and 9B imaged with 40× objective). Additional 20× representative images are shown in the Supplemental information (Figure S3 and S4).
Figure 8.
RgIA inhibits the CCI-induced increase in the density of Iba1-positive cells in the dorsal horn of the lumbar tract 14 days after ligation. RgIA (2 and 10 nmol) was administered daily by i.m. injections starting on the day of ligation. A) Quantitative analysis of cellular density was performed evaluating 6 animals for each group. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle. B) transverse sections of spinal cord imaged with 2.5× objective; insert shows representative morphological characteristics of a microglial cell obtained with 40× objective.
Figure 9.
Glial profile in spinal cord scored with GFAP-positive cells in the dorsal horn of the lumbar tract 14 days after surgery. RgIA (2 and 10 nmol) was daily administered i.m. starting on the day of ligation. A) Quantitative analysis of cellular density was performed evaluating 6 animals for each group. Each value represents the mean ± SEM of 6 rats per group, performed in 2 different experimental sets. *P<0.01 versus sham + vehicle; ^P<0.05 and ^^P<0.01 versus CCI + vehicle. B) transverse sections of spinal cord imaged with 2.5× objective; insert shows morphological characteristics of a representative astrocyte imaged with 40× objective.
Discussion
Neuropathic pain syndromes are a complex combination of symptoms that originate from nervous tissue injuries; the resulting pain is secondarily amplified by maladaptive changes in injured sensory neurons and along the entire nociceptive pathway within the central nervous system [30]. The absence of medications that prevent pathological progression limits the control of pain and leaves an estimated 80% of patients worldwide without adequate treatment [55].
In this report, we showed that RgIA was able to control pain by inhibiting its progression during a persistent noxious condition. As a pivotal characteristic, this compound prevented damage to the nervous system. Thus, RgIA exerts effects on the origins of neuropathic pain and offers a means to directly modulate nervous signaling and to prevent maladaptive alterations.
Hyperalgesia is an increased painful response to suprathreshold stimulation; allodynia is a painful response to a stimulus that does not normally provoke pain. RgIA was able to significantly reduce CCI-induced pain, when evaluated as an increase in suprathreshold stimulation (a hyperalgesia-related measure) or as a decrease in pain threshold (an allodynia-related measure). RgIA also reduced postural unbalance, a feature of neuropathy progression, as measured by hind limb weight bearing alterations. This measure, in particular, may asses the somatosensory component of neuropathy highlighting spontaneous, non-evoked pain. The effects of RgIA on altered pain threshold was dose-dependent and free from analgesic effects assessed on the contralateral paw and on sham animals. As previously described [52] a single RgIA administration was able to decrease the CCI-dependent pain, but the present data show that the pain-reliever effect progressively increased during treatment suggesting a rescue mechanism that protects nervous tissue from the damages that result in chronic pain.
CCI induced morphometric alterations of the sciatic nerve, consistent with previous reports [44,15]. Treatment with RgIA preserved the number of nerve fibers and significantly prevented the reduction in both myelin sheet thickness and axonal diameter. RgIA also limited the propagation of degeneration in DRG and prevented the decrease of soma area in small, medium and large neurons of L4–L5 DRGs. Moreover, RgIA reduced nucleolar alterations that are a common characteristic of different types of neuropathies [49,16]. To our knowledge this is the first evidence of a neuroprotective effect of RgIA.
CCI-mediated derangement of nerve morphology is accompanied by a profound local inflammatory reaction that includes edema, infiltration of hematogenous immune cells and the induction of various soluble factors such as cytokines and chemokines [13,44]. In particular, the presence of CD86 positive cells is characteristic of nerve trauma [15]. CD86 is a phenotypic marker of “classically activated” M1 macrophages stimulated by proinflammatory cytokines, such as IFNγ, or by lipopolysaccharide; these macrophages are typically recruited after nervous system trauma 33]. M1 macrophages produce high levels of oxidative metabolites (e.g., nitric oxide and superoxide) and proinflammatory cytokines that are essential for host defense and tumor-cell killing but also cause collateral tissue damage [19]. Treatment with RgIA attenuated the degree of peripheral nerve inflammation, reducing edema and infiltrate suggesting synergy between the anti-inflammatory and the neuroprotective effects of this compound. The same parallelism between improved morphometry and decreased infiltrate was also produced in DRG suggesting a tissue restoration which could improve the signaling of primary sensory neurons. In addition, damaged sensory neurons evoke pain-mediating pro-inflammatory and neurotoxic signals in the CNS [34]. Electrical and chemical stimuli promote complex cascades in intracellular transduction pathways that participate in alteration of neuronal and glial functions and subsequently lead to central sensitization [20]. This form of long-lasting synaptic plasticity facilitates nociceptive processes [56] and generates clinical pain hypersensitivity and makes neuropathic pain an autonomous disease state [53]. Plasticity of spinal cord and brain areas are partly due to the activation of glial cells that recently have been recognized as powerful modulators of pain [48]. Growing evidence implicates glial cell activation in the production of neuronal hyperexcitability and chronic inflammation. In the initial phases of neuropathic pain, a pivotal role has been imputed to spinal microglia, whereas astrocytes may be involved in pain maintenance [48]. Substantial damage to the nervous system evokes morphological changes in glial cells [48] while the effect of slight alteration may be highlighted by increased expression of specific glial antigens or increase in cell density [16]. The present results revealed an inhibitory effect of RgIA on microglia and astrocytes in the dorsal horn of the spinal cord with decreases in the number of both cell types. Since RgIA is a charged peptide, it is unlikely to cross the blood brain barrier (BBB) in significant quantity suggesting an indirect effect on central glial cells by preventing peripheral damage. However, the spinal inflammatory reaction triggered by nerve injury alters the integrity of the BBB and increases its permeability [21] which could result in RgIA penetration.
Adverse effects of RgIA were not evidenced during the repeated treatment. Animals showed a normal behavior and a macroscopic neurological evaluation did not reveal alterations. Body weight constantly increased similarly to vehicle treated animals. Analogous results were obtained in the previous study [52] where no adverse effects of RgIA administration were noted. RgIA did not alter normal locomotion (gait) or the stepping reflex, indicating a lack of activity at muscle subtype nAChRs. α9α10 nAChRs are present in cochlear hair cells of the inner ear [24]. If the peptide was able to cross the cochlear-blood barrier [43], block of these nAChRs might alter susceptibility to noise-induced hearing loss [39,23]. α9α10 have not been found in the CNS or at the neuromuscular junction [29,23]. This restricted expression may explain the lack of observed motor or neurological side effects. Moreover Vc1.1, another of the three natural conotoxins [40], was tested in human clinical trials [36]. In a phase 1 safety study, there was no evidence of systemic drug-related adverse effects from single or multiple doses [41].
α9 and α10 subunits form a distinct and perhaps primordial branch within the nAChR gene family [22,24]. RgIA is a potent, selective antagonist of α9α10 nAChRs [25]. The pain relieving effects of RgIA and a related conotoxin, Vc1.1, have been related to block of α9α10 nAChRs [52]. CCI increases the number of lymphocytes able to produce and release ACh providing, at the site of nerve injury, a source of ACh able to stimulate the localized immune response [32]. The α9α10 antagonists RgIA and Vc1.1 significantly reduce the number of choline acetyltransferase positive lymphocytes and the number of macrophages (CD68 positive cells) in the neural and perineural area of chronic constriction injured nerves [52]. Consistent with this, Vc1.1 was previously shown to accelerate functional recovery of injured neurons as measured by an indirect stimulation of the peripheral nerve terminals [47]. Whereas α9α10 block of nAChRs is anti-inflammatory, stimulation of α7 nAChRs is anti-inflammatory [51]. In addition, recent data suggest that α7 nAChRs down regulate after a nerve injury [17]. Thus, the nAChR subtype selectivity of compounds is critical. It is essential, therefore, to assess the activity of therapeutic drug candidates on both nAChR subtypes. Separately, recent evidence indicates that RgIA is able to inhibit high voltage-activated N-type calcium channel currents in rat DRG via activation of GABAB receptors [11], though this effect is not replicated in all systems [40]. GABAB agonist activity was not observed in spinal cord neurons [42]. The efficacy of the prototypical GABAB agonist baclofen in neuropathic pain is debated [27]. Furthermore, in contrast to RgIA, the analgesic effects of baclofen appear centrally mediated [28] and are associated with drug tolerance [26]. Thus we feel that the mechanism for the preventive and neuroprotective effects of RgIA is more likely a result of block of α9α10 nAChRs. Finally, in the present research RgIA was administered at the onset of the injury; the study of the protective properties of RgIA on more established damage remains an intriguing future perspective.
In summary, RgIA was able to control pain and prevent alterations of both the peripheral and central nervous system induced by nerve injury. Chronic neuropathic pain is a manifestation of pathological neural plasticity [53]. α9α10 antagonists may offer a dual protective approach against etiological factors and resulting maladaptative plasticity.
Supplementary Material
RgIA has not an analgesic effect. Peripheral neuropathy was induced by chronic constriction injury (CCI) of the right sciatic nerve (ipsilateral), and RgIA (2 and 10 nmol) was injected daily by i.m. into the ipsilateral paw starting on the day of surgery. Pain threshold of the contralateral paw was measured to evaluate analgesic effects. A) Sensitivity to a noxious mechanical stimulus as measured by the paw-pressure test. B) Pain threshold to a non-noxious mechanical stimulus as measured by the Von Frey test. All behavioral tests were performed 7 and 14 days after operation: either 24 hours after the last treatment (chronic) or 30 minutes after a new i.m. administration (acute). Control animals were subjected to sham surgery and treated with vehicle. Each value represents the mean ± SEM of 12 rats per group, performed in 2 different experimental sets
Lack of satellite glia activation illustrated in sections of L4–L5 DRGs immunohistochemically stained for glutamine synthetase. Upper panel, representative images of glutamine synthetase immunoreactivity in sham + vehicle and CCI + vehicle groups, scale bar 25 m. Lower panel, values are expressed as the mean ± SEM of 6 rats per group, performed in 2 different experimental sets
RgIA inhibits the CCI-induced increase in the density of Iba1-positive cells in the dorsal horn of the lumbar tract 14 days after ligation. RgIA (2 and 10 nmol) was administered daily by i.m. injections starting on the day of ligation. Transverse sections of spinal cord imaged with 20× objective.
Glial profile in spinal cord scored with GFAP-positive cells in the dorsal horn of the lumbar tract 14 days after surgery. RgIA (2 and 10 nmol) was administered daily i.m. starting on the day of ligation. Transverse sections of spinal cord imaged with 20× objective.
Acknowledgement
This research was funded by the Italian Ministry of Instruction, University and Research (MIUR) and by the University of Florence and NIH GM48677 and GM103801. The University of Utah holds patents on α-conotoxin RgIA on which J. Michael McIntosh is listed as an inventor. We thank Doju Yoshikami for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflict of interest.
References
- 1.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backonja MM. Neuropathic pain therapy: from bench to bedside. Semin Neurol. 2012;32:264–268. doi: 10.1055/s-0032-1329204. [DOI] [PubMed] [Google Scholar]
- 3.Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly Roberts D, Puttfarcken PS, Bitner RS, Diaz A, Dickenson AH, Porsolt RD, Williams M, Arneric SP. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Bartolini A, Di Cesare Mannelli L, Ghelardini C. Analgesic and antineuropathic drugs acting through central cholinergic mechanisms. Rec Pat CNS Drug Disc. 2011;6:119–140. doi: 10.2174/157488911795933901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL. Nicotine and postoperative management of pain. Anesth Analg. 2008;107:739–741. doi: 10.1213/ane.0b013e3181813508. [DOI] [PubMed] [Google Scholar]
- 8.Bordet T, Pruss RM. Targeting neuroprotection as an alternative approach to preventing and treating neuropathic pain. Neurotherap. 2009;6:648–662. doi: 10.1016/j.nurt.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis and Cartilage. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 10.Bullens P, Daemen M, Freling G, Kitslaar P, Van den Wildenberg F, Kurvers H. Motor dysfunction and reflex sympathetic dystrophy. Bilateral motor denervation in an experimental model. Acta Orthop Belg. 1998;64:218–223. [PubMed] [Google Scholar]
- 11.Callaghan B, Adams DJ. Analgesic α-conotoxins Vc1.1 and RgIA inhibit N-type calcium channels in sensory neurons of α9 nicotinic receptor knockout mice. Channels. 2010;4:51–54. doi: 10.4161/chan.4.1.10281. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan M-I, Fishbain DA, Foley K-M, Fudin J, Gilson A-M, Kelter A, Mauskop A, O'Connor P-G, Passik S-D, Pasternak G-W, Portenoy R-K, Rich B-A, Roberts R-G, Todd K-H, Miaskowski C. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 14.Di Cesare Mannelli L, Ghelardini C, Calvani M, Nicolai R, Mosconi L, Toscano A, Pacini A, Bartolini A. Neuroprotective effects of acetyl-L-carnitine on neuropathic pain and apoptosis: a role for the nicotinic receptor. J Neurosci Res. 2009;87:200–207. doi: 10.1002/jnr.21815. [DOI] [PubMed] [Google Scholar]
- 15.Di Cesare Mannelli L, D'Agostino G, Pacini A, Russo R, Zanardelli M, Ghelardini C, Calignano A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediators Inflamm. 2013a;2013:328797. doi: 10.1155/2013/328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Cesare Mannelli L, Pacini A, Bonaccini L, Zanardelli M, Mello T, Ghelardini C. Morphological features and glia involvement in rat oxaliplatin-dependent neuropathic pain. J Pain. 2013b;14:1585–1600. doi: 10.1016/j.jpain.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Di Cesare Mannelli L, Pacini A, Matera C, Zanardelli M, Mello T, De Amici M, Dallanoce C, Ghelardini C. Involvement of α7 nAChR subtype in rat oxaliplatin-induced neuropathy: effects of selective activation. Neuropharmacology. 2013c;79C:37–48. doi: 10.1016/j.neuropharm.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Di Cesare Mannelli L, Zanardelli M, Ghelardini C. Nicotine is a pain reliever in trauma- and chemotherapy-induced neuropathy models. Eur J Pharmacol. 2013d;711:87–94. doi: 10.1016/j.ejphar.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 20.Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M. SOCS3-Mediated Blockade of JAK/STAT3 Signaling Pathway Reveals Its Major Contribution to Spinal Cord Neuroinflammation and Mechanical Allodynia after Peripheral Nerve Injury. J Neurosci. 2010;30:5754–5766. doi: 10.1523/JNEUROSCI.5007-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Echeverry S, Shi XQ, Rivest S, Zhang J. Peripheral Nerve Injury Alters Blood–Spinal Cord Barrier Functional and Molecular Integrity through a Selective Inflammatory Pathway. J Neurosci. 2011;31(30):10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. alpha9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 23.Elgoyhen AB, Katz E, Fuchs PA. The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol. 2009;78:712–719. doi: 10.1016/j.bcp.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellison M, Haberlandt C, Gomez-Casati ME, Watkins M, Elgoyhen AB, McIntosh JM, Olivera BM. Alpha-RgIA: a novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry. 2006;45:1511–1517. doi: 10.1021/bi0520129. [DOI] [PubMed] [Google Scholar]
- 26.Enna SJ, Bowery NG. GABA(B) receptor alterations as indicators of physiological and pharmacological function. Biochem Pharmacol. 2004;68:1541–1548. doi: 10.1016/j.bcp.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Franek M, Vaculin Š, Rokyta R. GABAB Receptor Agonist Baclofen Has Non-Specific Antinociceptive Effect in the Model of Peripheral Neuropathy in the Rat. Physiol Res. 2004;53:351–355. [PubMed] [Google Scholar]
- 28.Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RWt, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci. 2004;113:32–42. doi: 10.1016/j.autneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Jonnala RR, Buccafusco JJ. Relationship between the increased cell surface alpha7 nicotinic receptor expression and neuroprotection induced by several nicotinic receptor agonists. J Neurosci Res. 2001;66:565–572. doi: 10.1002/jnr.10022. [DOI] [PubMed] [Google Scholar]
- 32.Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A Critical Role of Toll-like Receptor 2 in Nerve Injury-induced Spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 35.Leighton GE, Rodriguez RE, Hill RG, Hughes J. k-opioid agonist produce antinociception after i.v. and i.c.v. but not intrathecal administration in the rat. Br J Pharmacol. 1988;93:553–560. doi: 10.1111/j.1476-5381.1988.tb10310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livett BG, Sandall DW, Keays D, Down J, Gayler KR, Satkunanathan N, Khalil Z. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon. 2006;48:810–829. doi: 10.1016/j.toxicon.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Loram LC, Taylor FR, Strand KA, Maier SF, Speake JD, Jordan KG. Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats. J Pain. 2012;13:1162–1171. doi: 10.1016/j.jpain.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, Rossi FS, Lazzari P, Pani L, de Novellis V, Malcangio M, Maione S. 1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol Dis. 2010;37:177–185. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci. 2002;22:10838–10846. doi: 10.1523/JNEUROSCI.22-24-10838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem Pharmacol. 2009;78:693–702. doi: 10.1016/j.bcp.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metabolic’s neuropathic pain drug, ACV1 - Clinical trials update. 2006 http://www.asx.com.au/asxpdf/20061129/pdf/3zv2c96tyh1nx.pdf. [Google Scholar]
- 42.Napier IA, Klimis H, Rycroft BK, Jin AH, Alewood PF, Motin L, Adams DJ, Christie MJ. Intrathecal α-conotoxins Vc1.1, AuIB and MII acting on distinct nicotinic receptor subtypes reverse signs of neuropathic pain. Neuropharmacol. 2012;62:2202–2207. doi: 10.1016/j.neuropharm.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J Assoc Res Otolaryngol. 2013;14:175–185. doi: 10.1007/s10162-012-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini C. Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy. Pain. 2010;150:542–549. doi: 10.1016/j.pain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 45.Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146:245–252. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Sakurai M, Egashira N, Kawashiri T, Yano T, Ikesue H, Oishi R. Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain. 2009;147:165–174. doi: 10.1016/j.pain.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Satkunanathan N, Livett B, Gayler K, Sandall D, Down J, Khalil Z. Alphaconotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurones. Brain Res. 2005;1059:149–158. doi: 10.1016/j.brainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 49.Somer C. In: Pain in Peripheral Nerve Diseases. Reichmann H, editor. Vol. 13. Karger press; 2001. p. 18. [Google Scholar]
- 50.Takarada T, Nakamichi N, Kawagoe H, Ogura M, Fukumori R, Nakazato R, Fujikawa K, Kou M, Yoneda Y. Possible neuroprotective property of nicotinic acetylcholine receptors in association with predominant upregulation of glial cell line-derived neurotrophic factor in astrocytes. J Neurosci Res. 2012;90:2074–2085. doi: 10.1002/jnr.23101. [DOI] [PubMed] [Google Scholar]
- 51.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 52.Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 2006;103:17880–17884. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wala EP, Crooks PA, McIntosh JM, Holtman JR., Jr Novel small molecule α9α10 nicotinic receptor antagonist prevents and reverses chemotherapy-evoked neuropathic pain in rats. Anesth Analg. 2012;115:713–720. doi: 10.1213/ANE.0b013e31825a3c72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. Access to Controlled Medications Programme. Framework WHO/PSM/QSM/2007.2. 2007 http://www.who.int/medicines/areas/quality_safety/Framework_ACMP_withcover.pdf.
- 56.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RgIA has not an analgesic effect. Peripheral neuropathy was induced by chronic constriction injury (CCI) of the right sciatic nerve (ipsilateral), and RgIA (2 and 10 nmol) was injected daily by i.m. into the ipsilateral paw starting on the day of surgery. Pain threshold of the contralateral paw was measured to evaluate analgesic effects. A) Sensitivity to a noxious mechanical stimulus as measured by the paw-pressure test. B) Pain threshold to a non-noxious mechanical stimulus as measured by the Von Frey test. All behavioral tests were performed 7 and 14 days after operation: either 24 hours after the last treatment (chronic) or 30 minutes after a new i.m. administration (acute). Control animals were subjected to sham surgery and treated with vehicle. Each value represents the mean ± SEM of 12 rats per group, performed in 2 different experimental sets
Lack of satellite glia activation illustrated in sections of L4–L5 DRGs immunohistochemically stained for glutamine synthetase. Upper panel, representative images of glutamine synthetase immunoreactivity in sham + vehicle and CCI + vehicle groups, scale bar 25 m. Lower panel, values are expressed as the mean ± SEM of 6 rats per group, performed in 2 different experimental sets
RgIA inhibits the CCI-induced increase in the density of Iba1-positive cells in the dorsal horn of the lumbar tract 14 days after ligation. RgIA (2 and 10 nmol) was administered daily by i.m. injections starting on the day of ligation. Transverse sections of spinal cord imaged with 20× objective.
Glial profile in spinal cord scored with GFAP-positive cells in the dorsal horn of the lumbar tract 14 days after surgery. RgIA (2 and 10 nmol) was administered daily i.m. starting on the day of ligation. Transverse sections of spinal cord imaged with 20× objective.