Abstract
IMPAACT P1097 was a multicenter trial to determine washout pharmacokinetics and safety of in utero/intrapartum exposure to raltegravir in infants born to HIV-infected pregnant women receiving raltegravir-based antiretroviral therapy. Twenty-two mother-infant pairs were enrolled; evaluable pharmacokinetic data was available from 19 mother-infant pairs. Raltegravir readily crossed the placenta, with median cord blood/maternal delivery plasma raltegravir concentration ratio 1.48 (range, 0.32–4.33). Raltegravir elimination was highly variable and extremely prolonged in some infants; [median t½ 26.6 hours (range 9.3–184 hours)]. Prolonged raltegravir elimination likely reflects low neonatal UGT1A1 enzyme activity and enterohepatic recirculation. Excessive raltegravir concentrations must be avoided in the neonate, since raltegravir at high plasma concentrations may increase the risk of bilirubin neurotoxicity. Sub-therapeutic concentrations, which could lead to inadequate viral suppression and development of raltegravir resistance, must be avoided as well. Two ongoing IMPAACT studies are investigating further the pharmacology of raltegravir in neonates.
Introduction
Safe and effective combination antiretroviral drug regimens for use in the first days of life are needed to prevent, treat and possibly cure HIV infection in neonates.[1] Administration of multiple antiretroviral drugs to neonates at high risk of acquiring HIV has been shown to reduce peripartum HIV transmission.[2] Only five antiretrovirals (zidovudine, lamivudine, emtricitabine, stavudine and nevirapine), are currently FDA approved for use in neonates less than 14 days of age. Raltegravir, a potent antiretroviral and the first HIV integrase inhibitor to be licensed and FDA approved for use in children, blocks the establishment of post-integration HIV latency and has the potential to play an important role in both prophylaxis and treatment of neonates.[3] Both raltegravir and bilirubin are metabolized by uridine diphosphate gluronosyl transferase (UGT) 1A1 and they compete for albumin binding sites.[4] This raises the possibility that raltegravir elimination could be prolonged in neonates and that elevated plasma raltegravir concentrations could increase the plasma concentration of free unconjugated bilirubin, posing an increased risk of acute bilirubin toxicity and its sequela, kernicterus.[5–7] The goal of this study was to describe the washout pharmacokinetics of raltegravir acquired through transfer across the placenta in infants born to mothers receiving raltegravir for treatment of their HIV infection during pregnancy.
Methods
The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1097 was a multicenter trial designed to describe the washout pharmacokinetics and safety of in utero/intrapartum exposure to raltegravir in full-term neonates born to HIV-infected pregnant women who received raltegravir-based antiretroviral therapy during pregnancy. The study was conducted at sites participating in the IMPAACT Network in the United States. HIV-infected mothers and their neonates were enrolled prior to delivery if the mother had received raltegravir 400 mg twice daily for at least two weeks prior to delivery as part of a combination antiretroviral regimen and had a singleton gestation. Local institutional review boards approved the protocol at all participating sites, and signed informed consent was obtained from the mothers of all study subjects before participation.
Maternal medical histories were abstracted from the medical record, and maternal plasma and cord blood for raltegravir assay were obtained at the time of delivery. Infant physical exams were performed shortly after birth and neonatal medical histories were obtained. Serial neonatal plasma samples were collected at 1–5, 8–14, 18–24 and 30–36 hours after birth if birth weight was above 2 kg, gestational age was over 37 weeks, and no medications that might induce UGT1A1 activity were received, in addition to the absence of any serious or life threatening medical condition. Dried blood spots were obtained from newborns for determination of UGT1A1 genetic polymorphisms. Study neonates had blood drawn for total and direct bilirubin, liver transaminases, and creatinine at 8–14 hours, 30–36 hours and 1–2 weeks after birth and for complete blood counts at 8–14 hours and 1–2 weeks after birth. Infants were monitored until 20 weeks after birth for signs of raltegravir toxicity. The target sample size was 15 infants with collection of 4 postnatal pharmacokinetic samples for raltegravir assay.
Raltegravir plasma concentrations were measured using a previously published validated, isocratic, reverse phase high performance liquid chromatography/tandem mass spectrometry method.[8] The linear calibration range was 10 to 10,000 ng/mL from a 200 μL plasma sample. Polymorphisms in UGT1A1 (rs5839491) were determined by real-time PCR on DNA extracted from dried blood spots using QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Descriptive statistics were determined for maternal delivery, cord blood, and neonatal raltegravir concentrations. Regression analysis was used to determine the neonatal raltegravir terminal elimination half-life (t1/2). The terminal t1/2 was calculated in Excel. Following natural log transformation, the last 2 to 3 measured concentration-time points were used to determine the slope of the elimination curve to estimate the elimination rate constant. The t1/2 was calculated as ln 2 divided by the rate constant. Seventeen participants had a t1/2 estimated; 8 of these datasets used three terminal phase points to determine the rate constant.
The relationship between raltegravir t1/2 and neonatal UGT1A1 polymorphisms [wild type (TA)6/(TA)6 vs. other three genotypes: heterozygous (TA)6/(TA)7 and (TA)5/(TA)6 or homozygous (TA)7/(TA)7 genotypes] was analyzed using the Wilcoxon Rank Sum test.
Infant safety data, including adverse birth outcomes, signs and symptoms, diagnoses and laboratory test results from evaluations specified in the protocol and additional evaluations done as part of the infant’s clinical care, were evaluated. In addition, the number and proportion of infants who received any therapy to treat elevated bilirubin were determined.
Results
Twenty-two mother-infant pairs were enrolled and all infants were included in the safety analyses. Maternal plasma and cord blood samples were available from 19 mothers. Nineteen infants had evaluable plasma collections. Of the 22 infants enrolled in the study, 6 (27%) were female, 13 (59%) were African American and 8 (36%) were Hispanic. The median gestational age at birth was 38 weeks (range: 37–40 weeks), and median birth weight was 3080 grams (range: 2200–4100 grams).
Median maternal raltegravir concentration at delivery was 540 ng/mL (range: 12–5809 ng/mL) collected at a median of 4.6 hours after dosing (range: 1.1–21.0 hours). Median cord blood raltegravir concentration was 957 ng/mL (range: 24–3974 ng/mL), and the median ratio of cord blood to maternal delivery concentration was 1.48 (range: 0.32–4.33). The relationship between the time interval from maternal dosing to delivery and maternal delivery concentration, cord blood concentration and the cord blood to maternal delivery concentration ratio are presented in Figure 1A and 1B.
Figure 1.
Figure 1A. Raltegravir concentrations in cord blood (crosses) and maternal plasma at delivery (open diamonds).
Figure 1B. Ratio of cord blood to maternal plasma delivery concentration (open triangles) plotted against time between maternal dosing and delivery.
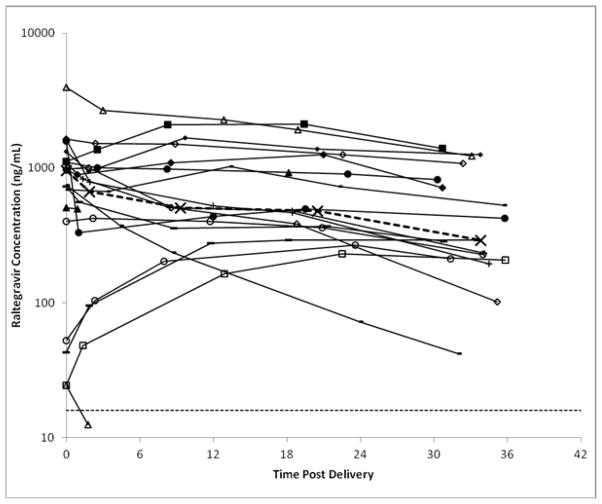
Individual infant raltegravir concentration time plots are presented in Figure 2 and individual raltegravir elimination t1/2, UGT1A1 genotype and need for phototherapy are presented in Table 1. Median (range) infant concentrations and the time of collection after birth were 671 (13 – 2672) ng/mL at 1.9 (0.9–4.4) hours, 507 (<10 – 2280) ng/mL at 9.3 (7.9–13.3) hours, 481 (<10 – 2106) ng/mL at 20.5 (18.1–23.9) hours, and 291 (<10 – 1402) ng/mL at 33.8 (30.3–35.8) hours. In 9 of 19 (47%) infants, raltegravir concentration increased over the initial 12 to 24 hours after birth before declining. Raltegravir concentrations remained above the IC95 (14 ng/mL) for wild-type HIV through the last time point for all but one infant, who was born within an hour of maternal dosing and had a low concentration of raltegravir detected only in the initial postnatal sample. Elimination t ½ could not be determined for another infant who had no decline in raltegravir concentration in the last three samples. For the 17 subjects for whom an elimination t1/2 could be determined, median elimination t½ was 26.6 hours, with a minimum of 9.3 hours, a maximum of 184 hours, and an interquartile range of 22.0 to 69.2 hours.
Figure 2.
Individual plots of raltegravir concentration vs. time after birth. Heavy dashed line represents median concentration at each sampling time point. Light dashed line represents the raltegravir IC95 for wild type virus of 14 ng/mL.
Table 1.
Raltegravir elimination t1/2, UGT1A1 phenotype, and need for phototherapy.
| Patient | Elimination t1/2 (hours) | UGT1A1 Genotype | Phototherapy |
|---|---|---|---|
| 1 | 69.2 | (TA)6/(TA)6 | No |
| 2 | 38.9 | (TA)6/(TA)6 | No |
| 3 | 22.3 | (TA)6/(TA)6 | No |
| 4 | 23.9 | (TA)6/(TA)6 | No |
| 5 | * | (TA)6/(TA)6 | No |
| 6 | 87.8 | (TA)6/(TA)6 | No |
| 7 | 26.6 | (TA)6/(TA)6 | No |
| 8 | 11.1 | (TA)5/(TA)6 | No |
| 9 | 88.7 | (TA)6/(TA)7 | No |
| 10 | 12.0 | (TA)6/(TA)7 | No |
| 11 | 183.9 | (TA)6/(TA)7 | No |
| 12 | 22.0 | (TA)6/(TA)7 | No |
| 13 | 75.4 | (TA)6/(TA)7 | Yes |
| 14 | 15.1 | (TA)6/(TA)7 | No |
| 15 | 59.7 | (TA)6/(TA)7 | No |
| 16 | 9.3 | NA | No |
| 17 | 23.1 | NA | No |
| 18 | 49.9 | NA | No |
| 19 | ** | (TA)7/(TA)7 | No |
No decline in raltegravir concentrations over sampling period
Raltegravir detected in only one postnatal sample
UGT1A1 genotyping was an optional evaluation in this study and was obtained for 17 out of 22 infants enrolled. Of the 17 with UGT1A1 genotyping, pharmacokinetic evaluations were performed on 16 infants. Eight infants (47.1%) were (TA)6/(TA)6 homozygotes, 7 (41.2%) were (TA)6/(TA)7 heterozygotes, 1 (5.9%) was a (TA)5/(TA)6 heterozygote, and 1 (5.9%) was a (TA)7/(TA)7 homozygote. There were no differences in median raltegravir concentrations at any time point or in elimination t1/2 when the (TA)6/(TA)6 infants were compared to the infants with other three UGT1A1 genotypes.
Five (22.7%) infants had grade 3 or 4 laboratory events (total bilirubin, creatinine, hemoglobin, neutrophil count and glucose), 2 (9.1%) had grade 3 or 4 signs and symptoms (fever and neonatal respiratory discomfort), and 1 (4.6%) had a grade 3 or 4 diagnosis (metabolic/endocrine disorder). There was 1 (4.6%) infant with low birth weight (2200 grams). No infant death or still birth was reported. None of the infant adverse events reported were determined to be related to maternal raltegravir use. Only one infant received phototherapy for treatment of hyperbilirubinemia. This infant was heterozygous (TA)6/(TA)7 and had a raltegravir t1/2 of 75.4 hours. None of the infants received an exchange transfusion therapy.
Discussion
Previously published data on peripartum raltegravir pharmacokinetics are limited to several case reports describing cord blood and maternal delivery raltegravir concentrations in a total of seven term and preterm infants.[9–11] While these reports suggest that raltegravir readily crosses the placenta, they provide limited data describing the postnatal elimination of transplacentally-acquired raltegravir in the infant. One preterm infant has been described who had raltegravir concentrations present one month after delivery, suggesting very prolonged elimination.[11]
The current study to our knowledge is the first to describe the elimination of transplacentally-acquired raltegravir in infants. In this study, we found that raltegravir readily crossed the placenta so that by 3 hours after maternal dosing umbilical cord raltegravir concentration equaled or exceeded maternal plasma concentration at the time of delivery. In the first 12–24 hours after delivery, raltegravir concentrations increased in almost half of study infants, even though they received no postnatal raltegravir doses. Once raltegravir concentrations started to decline, the rate of elimination was highly variable. Although several of our subjects had elimination t1/2 in the range similar to that reported in adults (7–12 hours), most demonstrated very slow elimination, with the longest t1/2 of 184 hours.[5] As a result of the excellent placental transport and slow neonatal elimination of raltegravir, infant raltegravir concentrations remained above the IC95 (14ng/mL) for wild-type HIV through 30–36 hours post-delivery, the time of collection of the last sample, in all but one of the study infants.
A limitation of our study is the limited pharmacokinetic sampling time. We did not anticipate that the half-life would be so prolonged and that the plasma concentrations would continue to increase in the absence of raltegravir dosing in some infants. Since these were healthy full-term infants delivered to pregnant women on cART, many were discharged from the hospital within a few days. The study was designed to be completed prior to discharge from the hospital so as to inconvenience the study families as little as possible. Another limitation of our study is that no raltegravir was directly administered to study neonates, so we can provide no data describing neonatal raltegravir absorption. Follow-up IMPAACT studies now underway include direct administration of raltegravir to neonates and more extensive pharmacokinetic sampling.
Raltegravir is primarily eliminated via hepatic glucuronidation by UGT1A1 followed by biliary and renal excretion of the glucuronide conjugate.[3] Bilirubin is also primarily metabolized by UGT1A1 and its metabolism is known to be slow immediately after birth and to increase dramatically over the first weeks of life. It is therefore not surprising that raltegravir elimination was found to be prolonged in most neonates in the first days of life.[6] Genetic variations in the number of TA repeats in the promoter region of the UGT1A1 gene are associated with reduced gene expression and enzyme activity, leading to elevated bilirubin concentrations in the neonate and later in life.[12] The wild-type allele (UGT1A1*1) has six TA repeats in the promoter of UGT1A1. Alleles with five or seven TA repeats are associated with decreased gene transcription and expression of UGT1A1 and reduced enzyme activity.[12] In our small group of infants, no relationship was found between raltegravir concentrations or elimination when infants with the wild type (TA)6/(TA)6 genotype were compared to those with other UGT1A1 genotypes. It is likely that immaturity of neonatal UGT1A1 enzyme activity accounts for much of the variability in the observed raltegravir pharmacokinetics rather than the infant genotype. The relationship between UGT1A1 genotype and neonatal raltegravir pharmacokinetics and bilirubin metabolism will be explored further in the enrolling IMPAACT follow-up studies.
Nearly half of the study subjects demonstrated an initial increase in raltegravir concentration over the first 12–24 hours after birth in the absence of any directly administered infant raltegravir dosing. This increase is likely explained by enterohepatic recirculation, where beta-glucuronidase present in the brush border of the fetal and neonatal gut breaks down luminal substrate-glucuronide complexes and allows intestinal reabsorption of the now unconjugated substrates.[13] It is likely that immediately after birth when gut motility and raltegravir metabolism are slow, raltegravir glucuronide present in meconium undergoes deconjugation followed by reabsorption of the unconjugated raltegravir resulting in the initial increases in raltegravir plasma concentrations seen in nearly half of the study infants.
In addition to sharing major elimination pathways, raltegravir and bilirubin also compete for plasma albumin binding sites. Circulating unconjugated bilirubin binds to plasma albumin, so that at low bilirubin concentrations there is minimal free unbound bilirubin in the circulation. If the unconjugated bilirubin concentration rises above the plasma albumin binding capacity, then circulating unbound bilirubin is available to cross the blood brain barrier and cause bilirubin associated central nervous system toxicity. Administration of a competitor to bilirubin binding to albumin may enhance the potential for bilirubin toxicity by decreasing bilirubin-albumin binding. In the 1950’s, administration of sulfisoxazole, which at therapeutic concentrations displaces bilirubin from albumin binding sites, to low birth weight infants resulted in an increased incidence of kernicterus and mortality.[14] Since raltegravir also competes with bilirubin for albumin binding sites, its use in neonates poses the risk of a similar effect. However, the binding affinity of bilirubin to albumin is much greater that than of raltegravir, and in an in vitro study in neonatal serum, raltegravir was shown to significantly displace bilirubin from albumin only at concentrations 50- to 100-fold higher than typical therapeutic concentrations.[4]
Our data suggest that a safe and effective neonatal raltegravir dosing regimen must take into account developmental changes in raltegravir elimination over the first weeks of life. Excessive raltegravir concentrations must be avoided in the neonate, since raltegravir at high plasma concentrations may increase the risk of bilirubin neurotoxicity. Sub-therapeutic concentrations, which could lead to inadequate viral suppression and development of raltegravir resistance, must be avoided as well. Raltegravir administration to preterm infants poses heightened risk of toxicity, since raltegravir and bilirubin elimination are prolonged, plasma albumin binding reduced, and the blood-brain barrier more permeable in preterm infants. As a result, administering raltegravir to preterm infants is likely to carry a greater risk of causing central nervous system bilirubin toxicity and should be avoided until raltegravir has been well-studied in term and preterm infants.
Two ongoing IMPAACT studies will investigate the safety of raltegravir administered directly to term infants and washout raltegravir pharmacokinetics in low birth weight infants. IMPAACT P1110 is a dose-finding pharmacokinetic study to evaluate the safety and tolerability of raltegravir oral granules for suspension when administered during the first 6 weeks of life with standard PMTCT ARV prophylaxis to HIV-1 exposed infants at high risk of HIV infection. P1110 will enroll an initial cohort receiving two single doses approximately one week apart, followed by a second cohort receiving daily dosing. The study design allows for adjustment of cohort 2 dosing based on analysis of the cohort 1 data. Version 2 of P1097 will investigate raltegravir washout pharmacokinetics in low birth weight infants, and P1110 may be expanded to include low birth weight infants. The goal of these studies is to provide the data necessary to develop regimens that will allow for the safe administration of raltegravir in the first month of life for prevention, treatment and possibly cure of HIV infection.
Acknowledgments
Sources of Support:
This protocol was supported by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) [U01 AI068632], the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) [AI068632]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9- 001/HHSN267200800001C).
Source of Funding:
Hedy Teppler, Matthew Rizk, Carolee Welebob are employees of Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc., and may own stock and/or stock options in the company.
In addition to the authors, members of the IMPAACT 1097 protocol team include: Elizabeth Hawkins, M.A., Social Scientific Systems, Silver Springs, MD; Bobbie Graham, B.S., Frontier Science and Technology Research Foundation, Amherst, NY; John Gaeddert, MPH, Frontier Science and Technology Research Foundation, Amherst, NY; Linda Marillo, B.A., Frontier Science and Technology Research Foundation, Amherst, NY; John Gaeddert, MPH, Frontier Science and Technology Research Foundation, Amherst, NY; Terence Fenton, Ed. D., Statistical and Data Analysis Center, Harvard School of Public Health, Boston, MA; Derek Weibel, B.S., Frontier Science and Technology Research Foundation, Amherst, NY; Catherine Kneut, CPNP, M.S., Boston Children’s Hospital, Boston, MA.; Debra McLaud, R.N., Boston Medical Center, Boston, MA; Larissa Wenning, Ph.D., Merck Research Laboratories, North Wales, PA; Elizabeth Rhee, M.D., Merck Research Laboratories, Kenilworth, NJ.
The authors would like to acknowledge the expertise of the P1097 protocol team members.
We wish to thank the women and infants who participated in the protocol and the staff of the participating International Maternal Pediatric Adolescent AIDS Clinical Trials centers. This article is dedicated to Catherine Kneut, field representative for the protocol, who provided compassionate care for many children with HIV and their families.
Footnotes
Presented in part at:
Clarke DF, Acosta EP, Bryson Y, et al. Raltegravir (RAL) pharmacokinetics (PK) and safety in neonates: Washout PK of transplacental RAL (IMPAACT P1097). Abstract presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; 16–18 April 2012, Barcelona, Spain. Abstract O_22.
Clarke DF, Acosta EP, Rizk M, et al. Raltegravir Pharmacokinetics and Safety in Neonates: IMPAACT P1097 (2013). Abstract presented at Conference on Retroviruses and Opportunistic Infections (CROI). Atlanta, GA.
Conflicts of Interest
All other authors report no potential conflicts.
All authors have submitted the ICMJE form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Diana F. Clarke, Section of Pediatric Infectious Diseases, Boston Medical Center, Boston, Massachusetts
Edward P. Acosta, Division of Clinical Pharmacology, University of Alabama at Birmingham, Birmingham, Alabama
Matthew L. Rizk, Merck Research Laboratories, Whitehouse Station, New Jersey
Yvonne J. Bryson, Pediatric Infectious Diseases, David Geffen School of Medicine at University of California at Los Angeles, Los Angeles, California
Stephen A. Spector, Department of Pediatrics, University of California, San Diego and Rady Children’s Hospital San Diego, La Jolla, California
Lynne M. Mofenson, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland
Edward Handelsman, Division of AIDS, National Institute of Health, Bethesda, Maryland
Hedy Teppler, Merck Research Laboratories, North Wales, Pennsylvania
Carolee Welebob, Merck Research Laboratories, North Wales, Pennsylvania
Deborah Persaud, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland
Mae P. Cababasay, Statistical and Data Analysis Center, Harvard School of Public Health, Boston, Massachusetts
JiaJia Wang, Statistical and Data Analysis Center, Harvard School of Public Health, Boston, Massachusetts
Mark Mirochnick, Department of Pediatrics, Boston University School of Medicine, Boston, Massachusetts
References
- 1.Persaud D, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–35. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen-Saines K, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366(25):2368–79. doi: 10.1056/NEJMoa1108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger DM. Raltegravir: a review of its pharmacokinetics, pharmacology and clinical studies. Expert Opin Drug Metab Toxicol. 2010;6(9):1151–60. doi: 10.1517/17425255.2010.513383. [DOI] [PubMed] [Google Scholar]
- 4.Clarke DF, et al. Raltegravir in vitro effect on bilirubin binding. Pediatr Infect Dis J. 2013;32(9):978–80. doi: 10.1097/INF.0b013e31829044a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto M, et al. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin Pharmacol Ther. 2008;83(2):293–9. doi: 10.1038/sj.clpt.6100281. [DOI] [PubMed] [Google Scholar]
- 6.Onishi S, et al. Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem J. 1979;184(3):705–7. doi: 10.1042/bj1840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlfors CE. Bilirubin-albumin binding and free bilirubin. J Perinatol. 2001;21(Suppl 1):S40–2. doi: 10.1038/sj.jp.7210631. discussion S59–62. [DOI] [PubMed] [Google Scholar]
- 8.Long MC, Bennetto-Hood C, Acosta EP. A sensitive HPLC-MS-MS method for the determination of raltegravir in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867(2):165–71. doi: 10.1016/j.jchromb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Hegazi A, et al. Raltegravir in the prevention of mother-to-child transmission of HIV-1: effective transplacental transfer and delayed plasma clearance observed in preterm neonates. AIDS. 2012;26(18):2421–3. doi: 10.1097/QAD.0b013e32835a9aeb. [DOI] [PubMed] [Google Scholar]
- 10.McKeown DA, et al. High neonatal concentrations of raltegravir following transplacental transfer in HIV-1 positive pregnant women. AIDS. 2010;24(15):2416–8. doi: 10.1097/QAD.0b013e32833d8a50. [DOI] [PubMed] [Google Scholar]
- 11.Clavel-Osorio C, et al. One-month transplacental pharmacokinetics of raltegravir in a premature newborn after short-course treatment of the HIV-1-infected mother. Antimicrob Agents Chemother. 2013;57(12):6393–4. doi: 10.1128/AAC.01349-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miners JO, McKinnon RA, Mackenzie PI. Genetic polymorphisms of UDP-glucuronosyltransferases and their functional significance. Toxicology. 2002;181–182:453–6. doi: 10.1016/s0300-483x(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics Subcommittee on, H. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 14.Andersen DH, et al. A difference in mortality rate and incidence of kernicterus among premature infants allotted to two prophylactic antibacterial regimens. Pediatrics. 1956;18(4):614–25. [PubMed] [Google Scholar]