Abstract
Chronic pain following peripheral nerve damage is often accompanied by a reduction in prefrontal cortex (PFC)-related cognitive functions, which are regulated by noradrenaline, released from efferents originating in the locus coeruleus (LC). L5-L6 spinal nerve ligation (SNL) increased tissue content and extracellular concentrations of noradrenaline in microdialysates from the PFC, and impaired attentional level in the novel object recognition test. Systemic gabapentin, commonly used to treat chronic pain, impaired the novel object recognition task in normal, but not SNL animals. Accordingly, gabapentin increased c-fos expression in LC neurons and noradrenaline release in the PFC in normal animals, but in SNL animals, gabapentin failed to increase c-fos expression in LC neurons projecting to the PFC and failed to increase noradrenaline release in the PFC. In contrast, locally perfused gabapentin reduced noradrenaline release in the PFC in vivo and in PFC synaptosomes in vitro. SNL- and gabapentin-induced impairment of novel object recognition task was reversed by intraperitoneal injection of the α1-adrenoceptor antagonist prazosin. These results suggest that increase in noradrenergic tone, induced by nerve injury or gabapentin, impairs PFC functions possibly via α1-adrenoceptor-mediated mechanisms, that the net effect of gabapentin on noradrenaline release in the PFC would depend on sometimes opposing actions at different sites, and that nerve injury selectively impairs the response to gabapentin in PFC projecting neurons in the LC.
1. Introduction
Chronic pain due to traumatic, metabolic, or oncologic damage to peripheral nerves, is often accompanied by reduction in cognitive performance. As such, functions of the prefrontal cortex (PFC), including working memory and attention, are often impaired in patients with chronic pain [4], but the mechanisms of impaired PFC functions have not been well studied. Noradrenaline, released by efferents from the locus coeruleus (LC) to the PFC, plays an important role in regulation of PFC functions [3]. Moderate levels of noradrenaline under normal conditions strengthen PFC function via activation of α-2 adrenergic receptors with high affinity for noradrenaline, whereas greater release of noradrenaline during stress impairs PFC functions via activation of lower affinity α-1 and possibly β-1 adrenoceptors [23]. Since peripheral nerve injury increases content and basal release of noradrenaline in the spinal cord [11; 12], the first aim of the current study was to test whether L5-L6 spinal nerve ligation (SNL) also increases content and basal release of noradrenaline in the PFC, measured by tissue contents and in vivo microdialysis, and whether the increase in noradrenergic tone impairs PFC-related function, measured with the novel object recognition test [19], via α-1adrenoceptor-mediated mechanisms.
The antiepileptic agent, gabapentin, effectively treats acute postoperative and chronic pain but can also cause cognitive side effects [17; 18]. Although analgesic mechanisms of gabapentin have been extensively studied in the spinal cord, little is known about its supraspinal mechanisms in pain and cognition. We and others have proposed supraspinal actions of gabapentin by demonstrating in rodents after peripheral nerve injury and in patients with chronic pain that systemically administered gabapentin activates descending noradrenergic pathways to produce analgesia [10; 11; 24]. As such, systemically administered gabapentin activates noradrenergic neurons in the LC and induces noradrenaline release in the spinal dorsal horn in both normal and SNL rats [11]. Since both PFC and spinal cord receive noradrenergic innervation mainly from the LC, we speculated that PFC-related side effects of gabapentin are due to the excessive release of noradrenaline in the PFC mediated by gabapentin's action in the LC. Our second aim was to test whether systemic injection or LC-perfusion of gabapentin mimics the effects of SNL on noradrenergic tone and functions in the PFC in normal rats, and whether gabapentin further worsens the PFC function of SNL rats. We also examined the local effects of gabapentin on the noradrenaline release in the PFC, measured by microdialysis in vivo and synaptosomes in vitro.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN), weighing 200 to 280 g, were used. Animals were housed under a 12-hour light-dark cycle, with free access to food and water. All experiments were approved by the Animal Care and Use Committee at Wake Forest University (Winston Salem, NC).
2.2. Spinal nerve ligation
L5–L6 spinal nerve ligation (SNL) was performed as described previously [13]. Briefly, the animal was anesthetized with 2% isoflurane in oxygen, the right L6 transverse process was removed, and the right L5 and L6 spinal nerves were tightly ligated using 5–0 silk suture. In some animals, withdrawal threshold in their hindpaw was measured by application of von Frey filaments (Stoelting, Wood Dale, IL, USA) at 2 or 6 weeks after surgery, using an up–down statistical method [6].
2.3. Guide cannula implantation and microdialysis
Animals were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg), maintained with 0.5-1.0 % isoflurane. The animal was placed securely in a stereotaxic frame (KOPF, Tujunga, CA) and a sterile stainless steel guide cannula (Eicom Co., Kyoto, Japan) was implanted into the right medial PFC (3.0 mm anterior and 0.5 mm lateral to the bregma, and 5.0 mm ventral from the surface of the dura mater) or the right LC (9.8 mm posterior and 1.4 mm lateral to the bregma, and 6.5 mm ventral from the surface of the dura mater) according to the rat brain atlas [21]. Animals were allowed at least 5 days to recover from the surgery.
A microdialysis probe (OD = 0.22 mm, ID = 0.20 mm, length = 2 mm for the PFC and 1 mm for the LC, EICOM CO.) was inserted through the guide cannula and perfused with Ringer's solution at a constant flow rate (1 uL/min) using a syringe pump (ESP-64; EICOM CO.). The animal was then placed in the plastic cage (200*200*200 mm) and fractions were collected every 30 min. After 120 minutes of constant perfusion, 2 consecutive samples were collected to determine basal concentration. Then, some animals received either a single intraperitoneal injection or local LC-perfusion of gabapentin (Toronto Research Chemicals Inc., Toronto, Canada) for 2 hr. After the experiment, a methylene blue (1 uL) was injected into the PFC and/or LC, and animals were killed by intraperitoneal pentobarbital (150 mg/kg) injection. The brain was removed, post-fixed with 4% buffered formaldehyde, sectioned, and the placement of the cannula was verified visually. Noradrenaline content in the microdialysates was measured by a high pressure liquid chromatography system with electrochemical detection (HTEC-500, EICOM CO.) as we previously reported [11]. All microdialysis samples in the current study contained noradrenaline above the limit of detection (62 fg). This study included data from only animals (156 rats out of 186 rats) with staining in the LC and/or medial PFC and stable baseline noradrenaline concentrations, defined by less than 30% difference in noradrenaline concentrations between the first and second microdialysis samples from the PFC.
2.4. Novel object recognition test
Novel object recognition test was performed as previously described with modifications [19]. The light intensity (40 lux) was kept constant throughout the experiment. On the first day (Day0), the rat was habituated to the Plexiglas test cage for 15 min in the absence of objects. On the second day (Day1), two identical wood wheels (diameter 6 cm, height 2 cm, object A and A’) were placed into the test cage and the rat was allowed to explore for 10 min. The time spent for exploring each object was recorded. Exploration of each object was defined as facing the nose to the object at a distance of <1 cm or direct contact with the object. On the third day (Day2), the animal received intraperitoneal saline (2ml/kg), gabapentin (30 or 100 mg/2mL/kg), and/or prazosin hydrochloride (1mg/2mL /kg, Sigma-Aldrich Co., St. Louis, MO). One hour after injection, the animal was placed in the test cage, in which object A’ had been replaced with a plastic block (6*3*2 cm, object B). The animal was then allowed to explore for 10 min and the time spent exploring each object was recorded. A preference index, a ratio of the amount of time spent exploring the object B over the total time spent exploring both objects (A+B), was used to determine the novel object recognition. The person performing the test was blinded to treatments.
2.5 Noradrenaline content
Normal and SNL animals were killed by deep isoflurane anesthesia followed by decapitation. The brain was quickly removed and each side of the medial PFC was rapidly dissected on ice. After treatment with 0.1 M perchloric acid, the tissues were homogenized on ice and centrifuged. Supernatants were collected and noradrenaline content was measured by high-performance liquid chromatography with electrochemical detection as we described previously [11].
2.6 Noradrenaline release from synaptosomes
Under deep anesthesia with isoflurane, 5%, animals were killed by decapitation. The brain was quickly removed and placed in ice-cold sucrose (0.32 M)-HEPES (10 mM) buffer (pH 7.4). The brain slice (2-mm thickness) containing PFC was obtained and the right medial PFC was carefully dissected under the surgical microscope and homogenized in ice-cold sucrose-HEPES buffer. The initial homogenate was centrifuged at 1000 g for 5 min, and the resulting supernatant was centrifuged again at 10,000 g for 12 min. The supernatant was discarded, and the pellet was resuspended in Krebs buffer (in mM: NaCl 124, KCl 3, MgSO4 2, CaCl2 2, NaH2PO4 1.25, NaHCO3 25, and glucose 10, saturated with 95% O2-5% CO2, pH 7.4). After incubation with [3H]-noradrenaline and unlabeled noradrenaline (final concentration of 1 μCi/ml and 0.1 μM, respectively) for 20 min at 37°C, the synaptosome-containing solution was centrifuged at 10,000 g for 12 min, and the pellet was resuspended in Krebs buffer. Synaptosomes from one rat were divided into three equal aliquots and transferred to Whatman filters in temperature-controlled perfusion chambers (SF-12, Brandel, Gaithersburg, MD). Synaptosomes were perfused with Krebs buffer (0.67 ml/min) for 25 min to remove free radioactivity, and then fractions were collected every 5 min for 20 min. After a 10 min baseline collection, synaptosomes were perfused with vehicle or gabapentin (0.1 or 1 mM) for 1 min and then stimulated with 25 mM KCl-Krebs buffer (in mM: NaCl 102, KCl 25, MgSO4 2, CaCl2 2, NaH2PO4 1.25, NaHCO3 25, and glucose 10, pH 7.4) containing vehicle or gabapentin for 2 min. The inhibitor of noradrenaline transporters reboxetine (0.1 μM, Tocris Bioscience) was present during perfusion to inhibit reuptake and reverse-transport of noradrenaline. The amount of radioactivity of each fraction was measured by liquid scintillation spectrometry (LS6500, Beck- man Coulter, Fullerton, CA).
2.7. Retrograde tracing study
Animals were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg), maintained with 0.5-1.0 % isoflurane. The animal was placed securely in a stereotaxic frame and a sterile stainless steel injection cannula (Eicom CO.) was inserted into the right medial PFC (3.0 mm anterior and 0.5 mm lateral to the bregma, and 5.0 mm ventral from the surface of the dura mater). The red color fluorescent beads (0.3 μL, Fluosphere®, Life Technologies, Grand Island, NY) were injected at the speed of 0.1 μL/min. Seven days after tracer injection, animals received intraperitoneal saline (2 mL/kg) or gabapentin (100 mg/2mL/kg) 1 h prior to euthanasia and the brains were collected. Tissues were fixed with 4 % formalin in 0.1 M phosphate buffered saline (PBS), cryoprotected with 30 % sucrose in 0.1 M PBS, and then sectioned at a 16-mm thickness. After being pre-treated with 1.5% normal donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), the sections were incubated for with a rabbit anti-c-fos antibody (1:500, SC-52, Santa Cruz, Santa Cruz, CA) and a mouse monoclonal anti-dopamine-β-hydroxylase antibody (1:500, MAB144P; Millipore, Billerica, MA). Subsequently, the sections were incubated with AlexaFluor 647 conjugated donkey anti-rabbit (1:500; Jackson ImmunoResearch Laboratories Inc.) and Cy2 conjugated anti-mouse IgG (1:200; Jackson ImmunoResearch Laboratories Inc.), and then incubated with DAPI solution (4’,6-Diamidino-2-Phenylindole dihydrochloride, 1:40000; Molecular Probes, Eugene, OR, USA).
All images were taken at same time and the person performing image analysis was blinded to treatments or groups. For quantification, four to five brainstem sections were randomly selected from each rat and those images were captured using a digital CCD camera with a same setting. DβH immunoreactive cells containing fluorescent beads were defined as PFC projecting LC neurons. Then, DAPI-immunostained nuclei were selected using image analysis software (ImageJ, NIH, Bethesda, MA), and intensity of c-fos immunostaining was measured. DAPI negative cells are excluded from analysis.
2.8. Statistical Analysis
Data are presented as mean ± SEM. Microdialysis data were analyzed by two-way repeated-measures analysis of variance (ANOVA) followed by Turkey post hoc test using SigmaPlot software (Systat Software Inc, Chicago, IL). Other data were analyzed by one-way or two-way ANOVA followed by Turkey post hoc test. P < 0.05 was considered significant.
3. Results
Withdrawal threshold was measured in a total of 84 SNL rats. SNL resulted in mechanical hypersensitivity in the paw ipsilateral to injury and withdrawal threshold values did not differ between 2 weeks (1.6 ± 0.1 g, n=42) and 6 weeks (1.4 ± 0.1 g, n=42, p=0.10) after surgery.
3.1. Effects of nerve injury on contents and basal extracellular concentrations of noradrenaline in the PFC
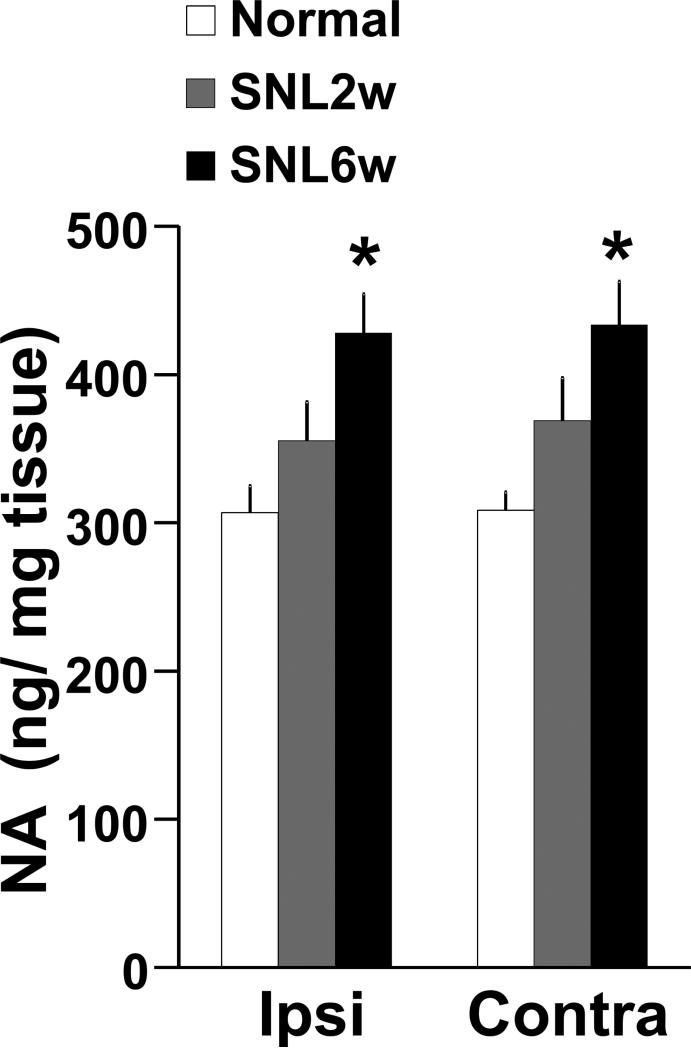
There was a significant main effect of nerve injury on noradrenaline content in the PFC (Fig. 1, F(2, 68) = 16.2, p <0.05), but no main effect of side (F(2, 68) = 0.14, p = 0.70) or nerve injury X side interaction (F(2.68) = 0.03, p=0.96). Post hoc testing revealed that rats 2 weeks after SNL (SNL2w) showed no significant difference in noradrenaline content in the PFC compared to normal rats (Ipsilateral: p=0.06, contralateral: p=0.14). Rats 6 weeks after SNL (SNL6w) showed significantly greater noradrenaline content bilaterally in the PFC compared to normal rats (p<0.05).
Figure 1.
Noradrenalin (NA) contents in the PFC. Tissue contents of NA in the medial PFC ipsilateral (Ipsi, right side) and contralateral (Contra, left side) to SNL surgery were measured in at 0 (Normal, n=13), 2 (SNL2w, n=12) and 6 (SNL6w, n=12) weeks after surgery. *p<0.05 vs normal.
Similarly, basal noradrenaline concentrations in microdialysates from the PFC ipsilateral to nerve injury were significantly greater in SNL6w rats (11.6 pg ± 1.2 / 30μL, n =65) than normal rats (8.4 pg ± 0.8 / 30μL, n= 74, p<0.05).
3.2. Effects of nerve injury on novel object recognition
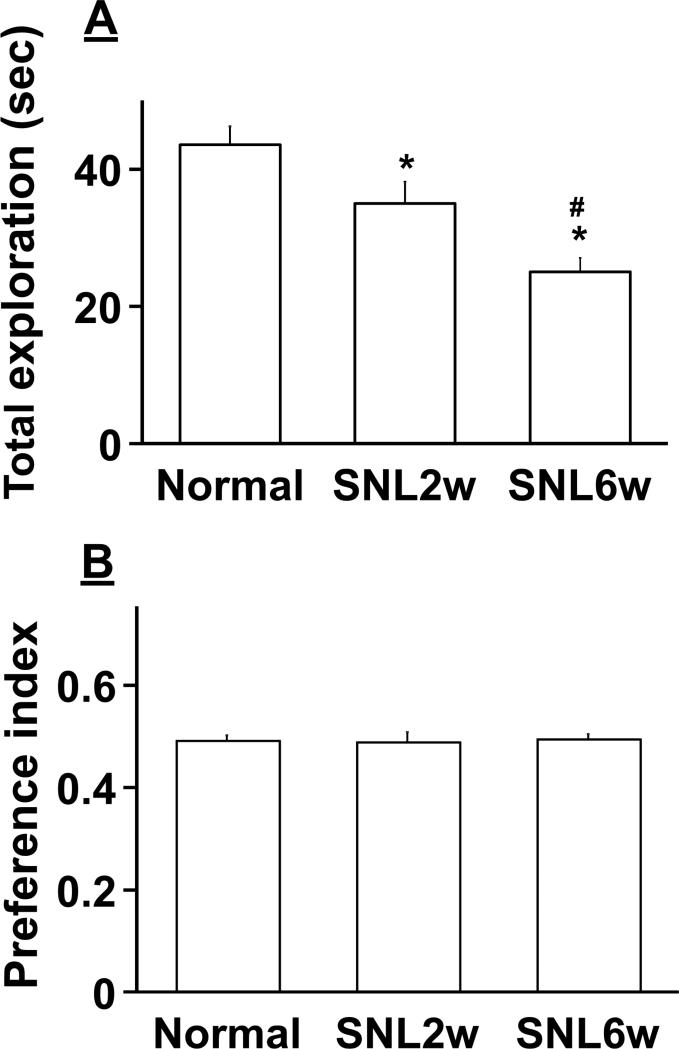
On Day1 of the novel object recognition test, animals explored two identical objects. SNL surgery resulted in a time-dependent decrease in the total exploration time compared to normal rats (Fig. 2A, F(2.149)=9.98, p<0.05). The preference index values were approximately 0.5 in all groups (Fig. 2B, F(2, 149)=0.09, p=0.90), indicating that normal and SNL animals had no preference for the side of objects and that total exploration times did not affect preference index values.
Figure 2.
Effects of SNL on the Day1 of novel object recognition test. Normal (n=73) and SNL rats at 2 (SNL2w, n=44) and 6 (SNL6w, n=35) weeks after surgery explored two identical objects (A and A’) without any treatment. A) Total exploration time of the two objects. *p<0.05 vs normal. #p<0.05 vs SNL2w. B) Preference index values are presented as the ratio of the exploration time of the object A’ over the total exploration time.
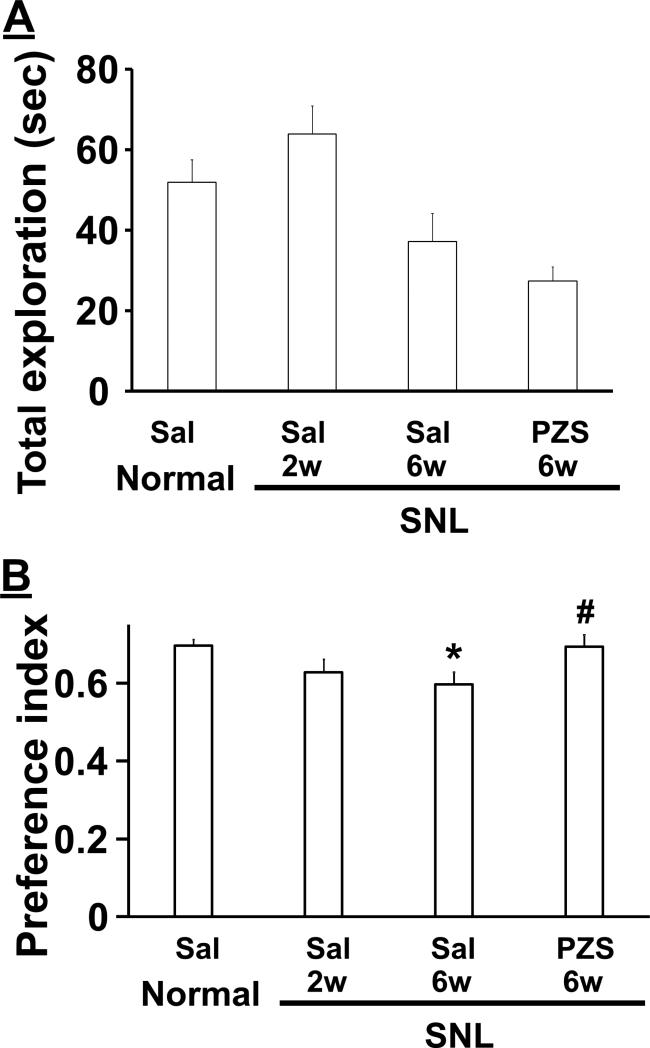
On Day2 of the novel object recognition test, animals explored one familiar object and one novel object. There was a significant main effect of group on total exploration time (Fig. 3A, F(3.54)=6.94, p<0.05), but post hoc testing showed no difference between saline treated normal and SNL2w rats (p=0.48), between saline treated normal and SNL6w rats (p=0.35), or between saline and prazosin treated SNL6w rats (p=0.70). There was a significant main effect of group on preference index (Fig. 3B, F(3.54)=3.74, p<0.05), and post hoc testing revealed that preference index was decreased in saline treated SNL6w rats compared to saline treated normal rats (p<0.05), and was increased in prazosin compared to saline treated SNL6w rats (p<0.05).
Figure 3.
Effects of SNL on the Day2 of novel object recognition test. At Day 2, normal (n=15) and SNL rats at 2 (SNL2w, n=17) and 6 (SNL6w, n=13 in saline treated. n=14 in prazosin treated) weeks received an intraperitoneal injection of saline (Sal) or prazosin (PZS, 1 mg/kg) 1 hour prior to the test and explored the familiar and novel objects. A) Total exploration time of the two objects. B) Preference index values are presented as the ratio of the exploration time of the novel object over the total exploration time. *p<0.05 vs normal-sal. #p<0.05 vs SNL-6w-sal.
3.3. Effects of systemic gabapentin on novel object recognition
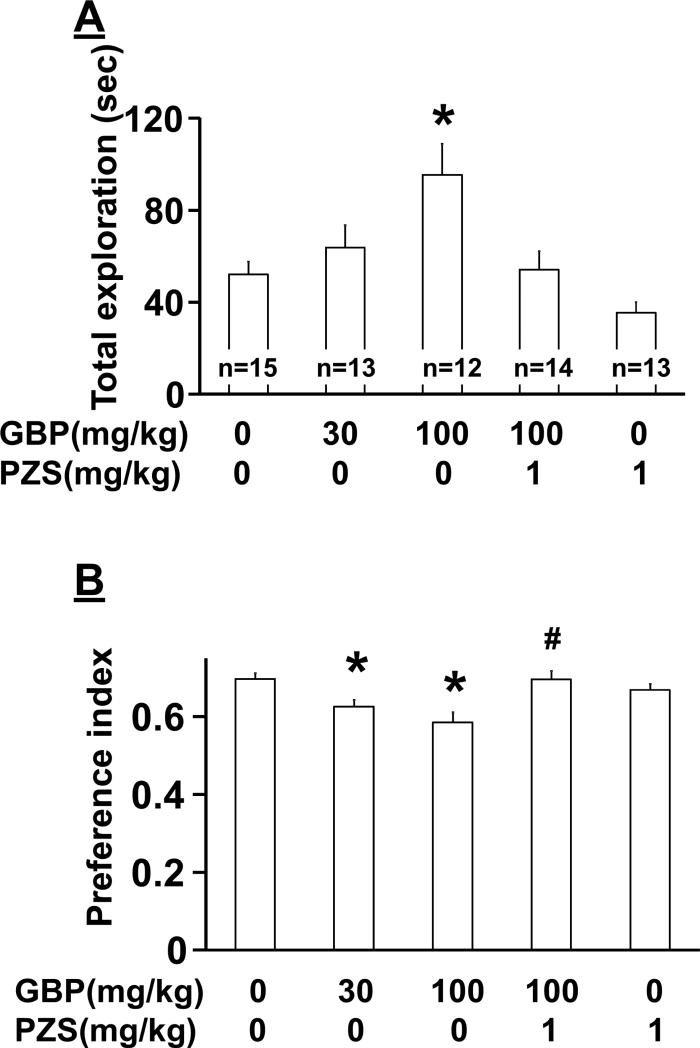
In normal rats, there was a significant main effect of group on total exploration time (Fig. 4A, F(4, 62)=6.30, p<0.05), and post hoc testing revealed that gabapentin (100 mg/kg) increased total exploration time compared to saline (p<0.05). There was a significant main effect of group on preference index (Fig. 4B, F(4, 62)=6.32, p<0.05), and post hoc testing revealed significant differences between saline and gabapentin (30 and 100 mg/kg) (p<0.05), and between gabapentin (100 mg/kg) alone and gabapentin with prazosin (p<0.05). Prazosin alone did not affect total exploration time (p=0.73) or preference index (p=0.90) compared to saline.
Figure 4.
Effect of gabapentin (GBP) on novel object recognition in normal rats. At Day2 of novel object recognition test, animals received an intraperitoneal injection of either saline, GBP (30-100 mg/kg), prazosin (PZS, 1 mg/kg), or their combination 1 hr prior to the test and explored the familiar and novel objects. A) Total exploration time of the two objects. *p<0.05 vs saline only. B) Preference index values are presented as the ratio of the exploration time of the novel object over the total exploration time. *p<0.05 vs saline only. #p<0.05 vs GBP 100 mg/kg only.
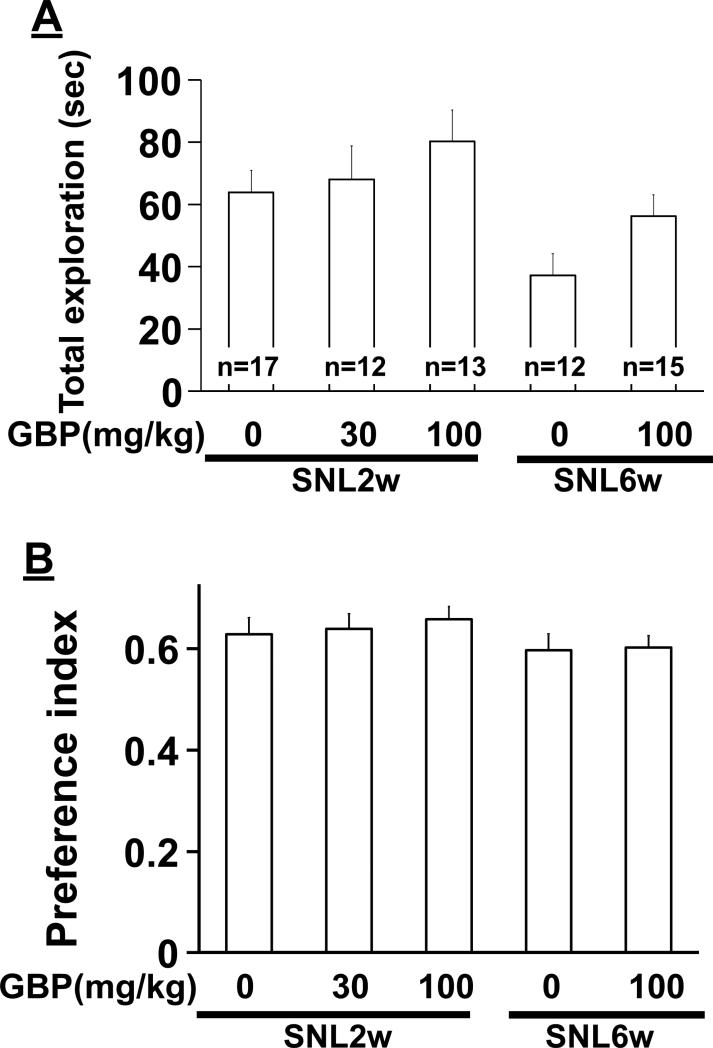
In SNL2w rats treated with intraperitoneal saline or gabapentin (30 and 100 mg/kg), there was no significant main effect of group on total exploration time (Fig. 5A, F(2, 39) = 0.88, p=0.42) and preference index (Fig. 5B, F(2, 39) = 0.24, p=0.79). Similarly, in SNL6w rats, saline and gabapentin (100 mg/kg) treated animals did not differ in total exploration time (p=0.06) and preference index (p=0.90).
Figure 5.
Effects of gabapentin (GBP) on novel object recognition and extracellular noradrenaline concentrations in the PFC in SNL rats. At Day2 of novel object recognition test, SNL rats at 2 (SNL2w) and 6 (SNL6w) weeks after surgery received an intraperitoneal injection of saline or GBP (30-100 mg/kg) 1 hr prior to the test and explored the familiar and novel objects. A) Total exploration time of the two objects. *p<0.05 vs SNL2w. B) Preference index values are presented as the ratio of the exploration time of the novel object over the total exploration time.
3.4 Effects of systemic and intra-LC perfused gabapentin on extracellular concentrations of noradrenaline in PFC
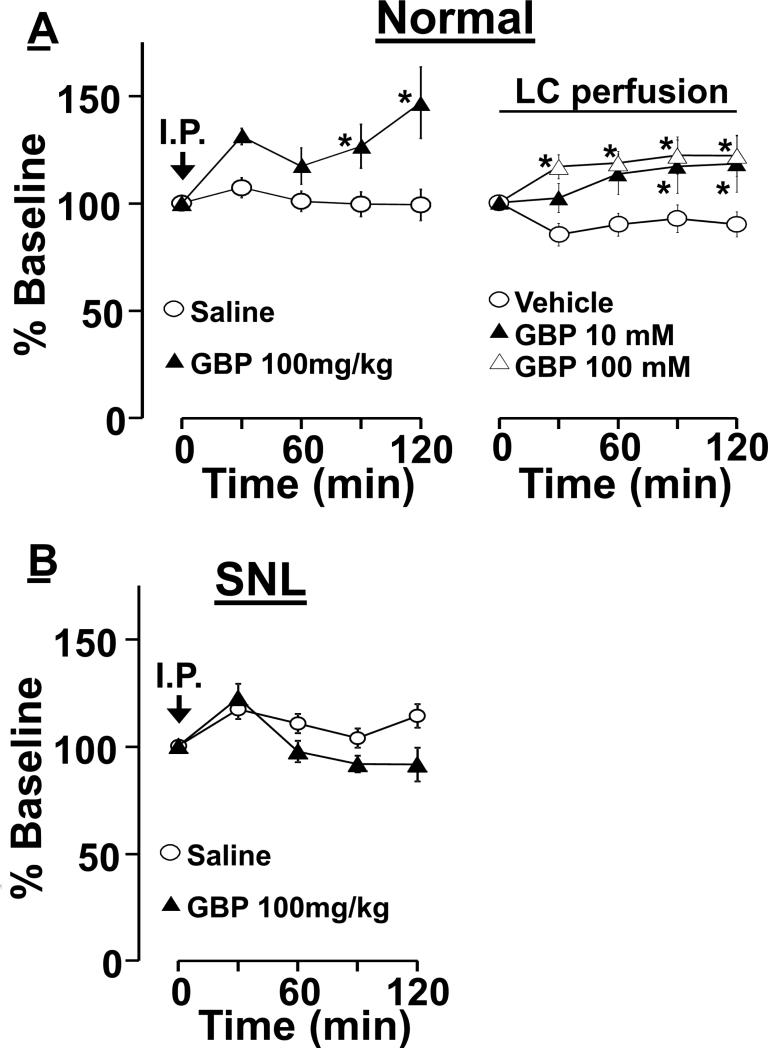
In normal animals, noradrenaline concentrations in microdialysates from the PFC following an intraperitoneal administration of gabapentin (100 mg/kg) increased compared to saline administration (Fig. 6A, treatment: F(1, 84) = 8.0, time: F(4, 84) = 3.8, treatment X time interaction: F(4, 84) = 3.7, p<0.05 in all F values). Post hoc testing revealed that intraperitoneal gabapentin significantly increased PFC noradrenaline concentrations compared to saline at 90 and 120 min after administration (p<0.05).
Figure 6.
Effect of gabapentin (GBP) on extracellular noradrenaline concentrations in the PFC. Changes in noradrenaline concentrations in microdialysates from the right PFC are presented over time as percentage of baseline. A) Normal rats received either an intraperitoneal (I.P.) injection of saline (n=12) or GBP (100 mg/kg, n=12), or a local perfusion of vehicle (n=13), GBP 10mM (n=10), or GBP 100 mM (n=13) into the right LC though microdialysis probe. *P<0.05 vs. saline or vehicle. B) SNL rats at 6 weeks after surgery received an intraperitoneal injection of saline (n= 12) or GBP (n=12).
Similarly, noradrenaline concentrations in microdialysates from the PFC following a local LC-perfusion of gabapentin (10 and 100mM) increased in normal rats compared to vehicle treatment (treatment: F(2, 128) = 7.0, time; F(4, 128) = 4.2, treatment X time interaction; F(8, 128) = 2.7, p<0.05 in all F values). Post hoc testing revealed that LC-perfusion of gabapentin significantly increased PFC noradrenaline concentrations compared to vehicle at 90 and 120 min at both gabapentin infusion concentrations in normal rats (p<0.05).
In contrast, in SNL6w rats, noradrenaline concentrations in microdialysates from the PFC following an intraperitoneal administration of gabapentin (100 mg/kg) did not differ from vehicle (Figure 6B, treatment; F(1, 96) = 2.8, p=0.10, time; F(4, 96) = 10.7, p<0.05, treatment X time interaction; F(4, 96) = 4.3, p<0.05).
3.5. Local effects of gabapentin on noradrenaline release in the PFC
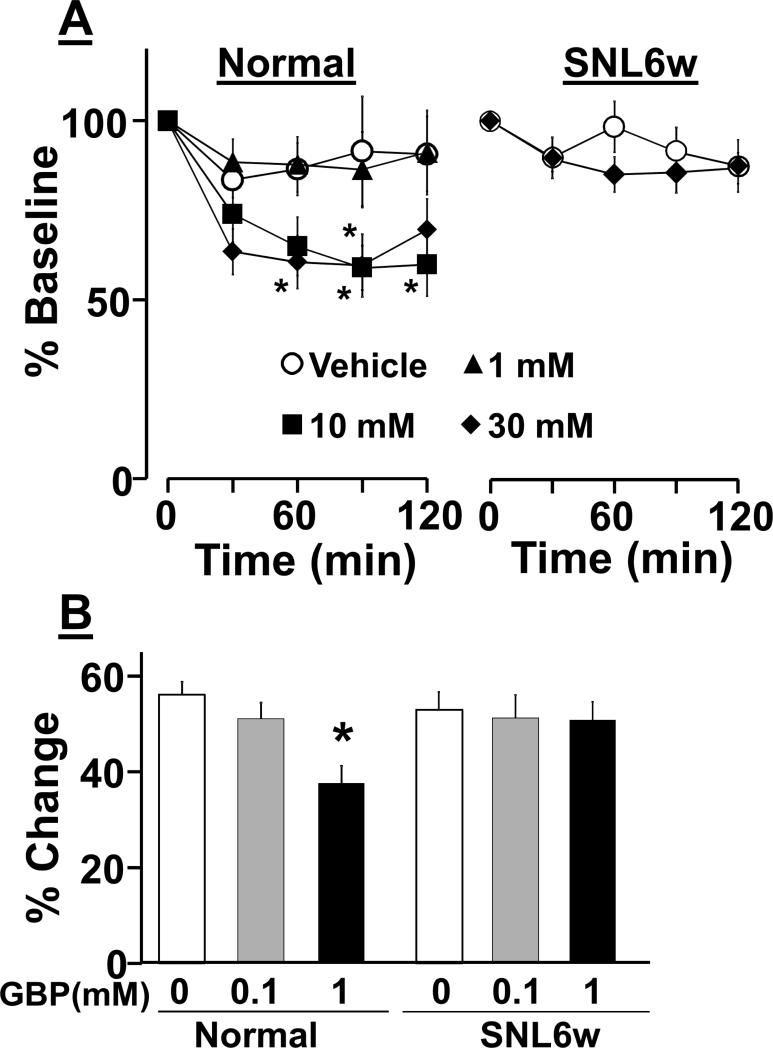
In normal rats, local PFC-perfusion of gabapentin (1-30 mM) concentration-dependently reduced extracellular noradrenaline concentrations in microdialysates from the PFC (Fig. 7A, treatment; F(3, 160) = 3.6, p<0.05, time; F(4, 160) = 13.6, p <0.05, treatment X time interaction; F(12, 160) = 1.6, p = 0.07). Post hoc testing revealed that local perfusion of gabapentin (10 and 30 mM) significantly reduced PFC noradrenaline concentrations compared to vehicle at 90 min (10 mM, p<0.05) or at 60, 90, and 120 min (30 mM, p<0.05). In contrast, in SNL6w rats, local perfusion of gabapentin (30 mM) failed to affect noradrenaline release in microdialysis (Fig. 7A, treatment; F(1, 104) = 0.4, p=0.50, time; F(4, 104) = 4.2, p<0.05, treatment × time interaction; F(4, 104) = 1.3, p = 0.24)
Figure 7.
Local effects of gabapentin (GBP) on noradrenaline release in the PFC in normal and SNL rats at 6 weeks after surgery (SNL6w). A) Animals received a local perfusion of vehicle or GBP (1-30 mM) into the right PFC though microdialysis probe. Changes in noradrenaline concentrations in microdialysates from the right PFC are presented over time as percentage of baseline. Normal groups (vehicle: n=15, GBP 1mM: n=11, GBP 10 mM: n=10, GBP 30 mM: n=14). SNL6w groups (vehicle: n=14, GBP 30 mM: n=14) *P<0.05 vs. vehicle. B) Synaptosomes prepared from the right medial PFC were perfused with vehicle or GBP (0.1-1 mM) for 1 min and then stimulated with 25 mM KCl containing vehicle or gabapentin for 2 min. Data are presented as percentage change from baseline release. N=16 in each group. *P<0.05 vs. vehicle.
Synaptosomes were prepared from the PFC of normal and SNL rats and treated with vehicle or gabapentin (0.1 and 1 mM) (Fig. 7B). There were significant main effect of treatment (F(2, 82) = 5.0, p<0.05) and treatment X nerve injury interaction (F(2, 82) = 3.7, p<0.05), but no main effect of nerve injury (F(1, 82) = 1.5, p = 0.21). Post hoc testing revealed that gabapentin (1 mM) significantly inhibited KCL-evoked [3H]-noradrenaline release from PFC synaptosomes in normal rats (p<0.05) but not SNL rats (p=0.91) compared to vehicle.
3.6. Effect of systemic gabapentin on c-fos expression in LC neurons
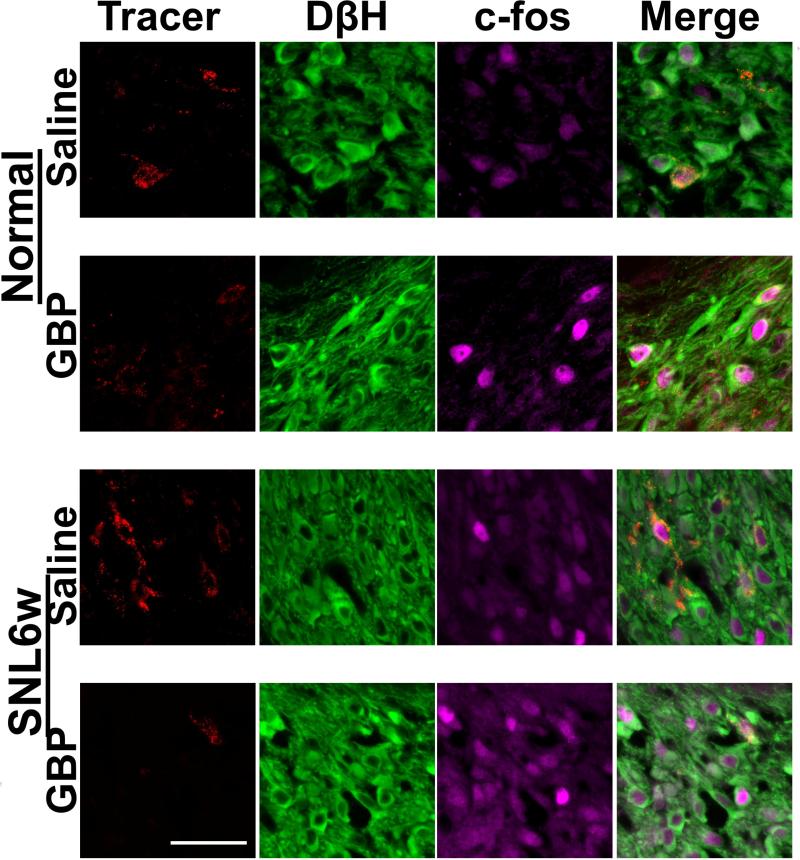
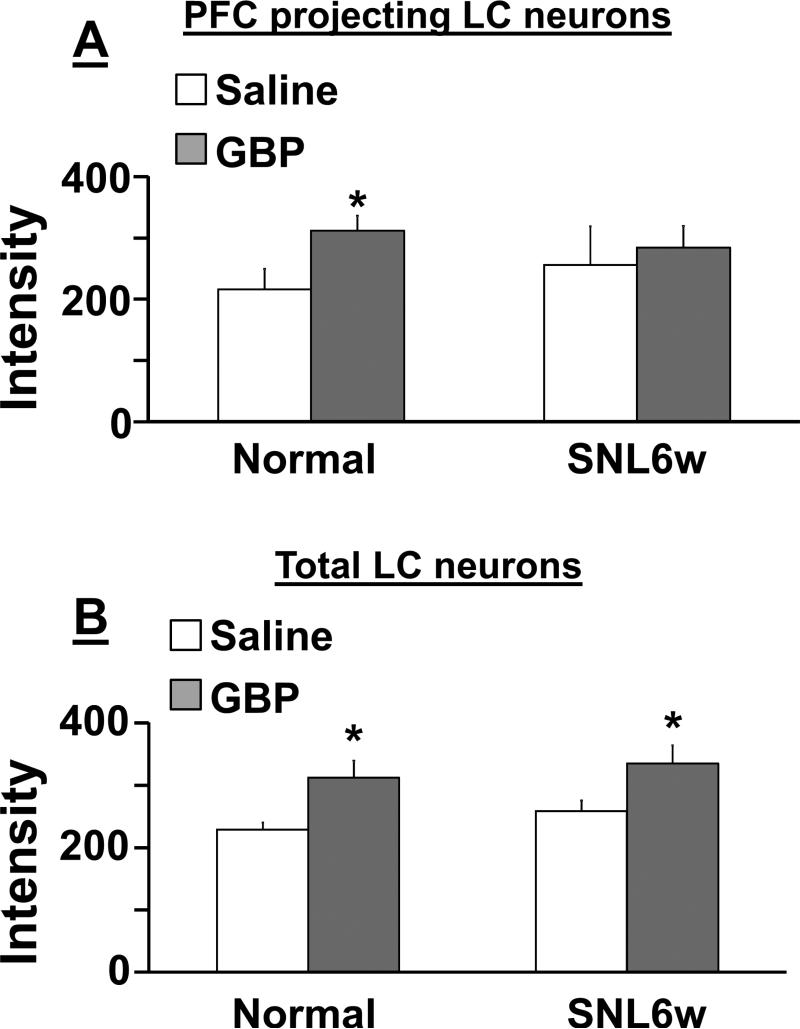
Photomicrographs in Figure 8 depict c-fos immunoreactivity in PFC-projecting LC neurons, identified by the retrograde tracer-positive cells with DβH-immunoreactivity, in normal and SNL6w rats following an intraperitoneal injection of saline or gabapentin (100mg/kg). In PFC-projecting LC neurons, there were significant main effect of treatment (F(1, 18) = 15.0, p<0.05) and treatment X nerve injury interaction (F(1, 18) = 4.4, p <0.05), but no main effect of nerve injury (F(1, 18) = 0.08, p = 0.76) (Fig. 9A). Post hoc testing revealed that gabapentin significantly increased c-fos expression in PFC-projecting LC neurons in normal (p<0.05) but not in SNL6w rats (p=0.83) compared to saline. In all LC neurons, there was a significant main effect of treatment (F(1, 18) = 12.6, p<0.05), but no main effect of nerve injury (F(1, 18) = 1.0, p = 0.31) and treatment X nerve injury interaction (F(1, 18) = 0.01, p = 0.90) (Fig. 9B). Post hoc testing revealed that gabapentin significantly increased c-fos expression in PFC-projecting LC neurons in both normal and SNL6w rats compared to saline (p<0.05).
Figure 8.
Photomicrographs of effect of systemic administered gabapentin (GBP) on cfos expression in the PFC-projecting LC neurons in normal and SNL rats at 6 weeks after surgery (SNL6w). Animals received a local injection of retrograde fluorescent tracer (300 nl, red) into the right PFC. At 1 week after the tracer injection, their brainstems were collected 1 hr following an intraperitoneal injection of saline or GBP (100 mg/kg) and immunostained for DβH (green) and c-fos (purple). Tracer-positive cells with DβH-immunoreactivity were defined as PFC-projecting LC neurons. Scale bar = 50 μm.
Figure 9.
Quantification of c-fos-immunoreactivity in LC neurons from normal and SNL at 6 weeks after surgery (SNL6w) rats following systemic injection of saline (n=5 in normal, n=6 in SNL) or gabapentin (GBP, 100 mg/kg, n=5 in normal, n=6 in SNL). See conditions in figure 7. A) Data are presented as the mean intensity values of c-fos immunostaining in tracer positive cells with DβH-immunoreactivity. B) Data are presented as the mean intensity values of c-fos immunostaining in cells with DβH-immunoreactivity.*P<0.05 vs. saline.
4. Discussion
Noradrenaline is thought to produce an inverted U shape dose-response in PFC function, whereby either too little or too much noradrenaline release impairs PFC function. Moderate concentrations of noradrenaline under normal conditions preferentially activate α2-adrenoceptors to increase delay-related firing in PFC neurons via inhibition of cAMP, thereby strengthening PFC-regulation of behavior , while high concentrations of noradrenaline under stress condition activate α1-adrenoceptors to decrease delay-related firing via activation of phosphatidylinositol protein kinase C signaling [3; 23]. The current study confirms previous observations in animals and humans that chronic pain reduces PFC-related attentional processes [4; 19; 20], and extends these by demonstrating that peripheral nerve injury or a single administration of gabapentin increases noradrenergic tone in the PFC and impairs attentional process, and that peripheral nerve injury reduces the response to gabapentin selectively in PFC-projecting LC neurons. Consistent with α1-adrenoceptor-mediated impairment of PFC function [3; 23], nerve injury- and gabapentin-induced impairment of attentional behavior was reversed by intraperitoneal injected prazosin. Although systemic administered prazosin has been shown to affect PFC function in rats [8], further study would be required to determine if this is a direct effect on PFC neurons or an indirect one by affecting other circuits.
SNL produced a significant and stable mechanical hypersensitivity during the testing period, associated with an increase in noradrenergic tone in the PFC and decrease in novel object recognition at 6 weeks but not 2 weeks after nerve injury with no relation to total exploration time. Similarly, Alba-Delgado et. al. recently demonstrated a delayed appearance of affective signs after nerve injury, including anxiety- and depression-like behaviors, associated with increase in bursting activity of LC neurons [1]. We and others previously demonstrated that unilateral peripheral nerve injury bilaterally activates LC neurons, measured by neuronal activation markers, with no difference between sides of LCs [5; 11; 15]. These results suggest that bilateral neuroplasticity in the LC may contribute to the delayed appearance of cognitive dysfunction after unilateral nerve injury. Although both spinal cord and PFC receive noradrenergic axons from the LC, we previously observed that SNL increases noradrenaline content in the spinal dorsal horn 3 days after nerve injury [12]. On the other hand, other studies demonstrated in rats that chronic constriction of sciatic nerve did not alter extracellular concentrations of noradrenaline in the PFC up to 4 weeks after nerve injury [1; 2]. These results suggest that time course, location, and degree of noradrenergic neuroplasticity after nerve injury would depend on the type of nerve injury. Unlike postganglionic sympathetic noradrenergic neurons, which express tropomyosine receptor kinase A (trkA) and respond to nerve growth factor, noradrenergic neurons in the LC express trkB and respond to brain-derived neurotrophic factor (BDNF) [14]. Since peripheral nerve injury up-regulates the expression of BDNF to increase noradrenergic axon density in the spinal cord and since a local injection of BDNF into the spinal dorsal horn increases noradrenergic axon density in normal rats [12], one could argue that local up-regulation of BDNF after peripheral nerve injury increases noradrenergic tone in the PFC. However, animals and humans with depression, which is often concomitant with chronic pain, show lower rather than higher level of BDNF in the PFC compared to normal subjects [22; 25]. Further studies are required to clarify which factors contribute to this delayed increase in noradrenergic tone in the PFC after peripheral nerve injury.
Gabapentin is effective in a wide range of animal pain models and in patients with acute postoperative and chronic pain, and is a first line treatment for chronic pain. The adverse events associated with gabapentin are well described – somnolence, dizziness and decreased learning and attention [16-18], yet there is extremely little known about the mechanisms by which gabapentin produces these therapy limiting side effects. We and others proposed that supra-spinal actions of gabapentin are important to its analgesic mechanisms, by demonstrating that noradrenergic neurons in the LC are excited by gabapentin, coincident with noradrenaline release in the spinal cord and behavioral antihypersensitivity in rodents after peripheral nerve injury [11; 24], and that oral gabapentin, in a dose that produces postoperative analgesia, increases noradrenaline concentration in cerebrospinal fluid in patients with chronic pain [10]. These results suggest that gabapentin activates spinally projecting LC neurons to induce noradrenaline release in the spinal cord, and thereby reducing hypersensitivity after nerve injury. The current study demonstrates that gabapentin's excitatory actions in the LC may also cause its impairment of PFC-related attentional function by demonstrating that a single systemic or intra-LC administration of gabapentin activates LC neurons to induce noradrenaline release in the PFC and reduced attentional level in normal rats. This effect is opposite to local action of gabapentin on PFC neurons, since a locally perfused gabapentin reduced noradrenaline release in vivo and in the synaptosomes in vitro in the PFC. Since α2δ subunits of voltage-gated calcium channels are expressed in forebrain regions including the PFC [7], this local effect of gabapentin is likely due to its well-known inhibitory action on the neurotransmitter release via α2δ interactions. These results suggest that the net effect of gabapentin on noradrenaline release in the PFC would depend on sometimes opposing actions at different sites.
In SNL rats, systemically administered gabapentin activated LC neurons, consistent with our previous observation [11]. However, gabapentin failed to alter novel object recognition in SNL rats. Since several studies in animals with chronic pain show that a single treatment with gabapentin and other analgesics improves cognitive functions [9; 19], one could argue that gabapentin's analgesic efficacy cancels its inhibitory effects on PFC function in SNL rats. Against this argument is the current observation in SNL rats that gabapentin failed to activate PFC-projecting LC neurons and alter noradrenaline release in the PFC. On the other hand, gabapentin induces similar degrees of overall LC neuronal activation and spinal noradrenaline release in normal and SNL rats [11]. The current results also show that locally perfused gabapentin did not affect noradrenaline release in vivo and in the synaptosomes in vitro in the PFC of SNL rats. Although the mechanisms of LC neuronal activation by gabapentin are under the investigation, these results suggest that nerve injury selectively impairs the response to gabapentin in PFC-projecting noradrenergic neurons in the LC and their axon terminals in the PFC. Based on this, one would speculate that the attention-memory effects produced by gabapentin would be less when it is used in the treatment of established chronic pain than acute postoperative pain.
In summary, the current study demonstrates that peripheral nerve injury induces impairment of PFC-related function via increasing noradrenergic tone in the PFC. We also demonstrates that the mechanism of gabapentin's side effects on attention may be related to its excitatory action in the LC and depend on plasticity of PFC-projecting LC neurons after nerve injury.
Acknowledgements
This work was supported by grants DA27690 to KH from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have no conflicts of interest to declare.
References
- 1.Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sanchez-Blazquez P, Meana JJ, Berrocoso E. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biological psychiatry. 2013;73(1):54–62. doi: 10.1016/j.biopsych.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Alba-Delgado C, Mico JA, Sanchez-Blazquez P, Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: implications for neuropathic pain. Pain. 2012;153(7):1438–1449. doi: 10.1016/j.pain.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Arnsten AF. Catecholamine influences on dorsolateral prefrontal cortical networks. Biological psychiatry. 2011;69(12):e89–99. doi: 10.1016/j.biopsych.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Pain. Vol. 154. International Association for the Study of Pain; 2013. Evidence for working memory deficits in chronic pain: A systematic review and meta-analysis. pp. 1181–1196. [DOI] [PubMed] [Google Scholar]
- 5.Brightwell JJ, Taylor BK. Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 2009;160(1):174–185. doi: 10.1016/j.neuroscience.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 7.Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, Gu G. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. The Journal of comparative neurology. 2005;491(3):246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- 8.Do Monte FH, Souza RR, Wong TT, Carobrez Ade P. Systemic or intra-prelimbic cortex infusion of prazosin impairs fear memory reconsolidation. Behavioural brain research. 2013;244:137–141. doi: 10.1016/j.bbr.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire S, Michaud V, Chapuy E, Eschalier A, Ardid D. Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain. 2012;153(8):1657–1663. doi: 10.1016/j.pain.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida K-i, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106:557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Hayashida K-i, Obata H, Nakajima K, Eisenach JC. Gabapentin Acts within the Locus Coeruleus to Alleviate Neuropathic Pain. Anesthesiology. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashida K, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136(3):348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 14.King VR, Michael GJ, Joshi RK, Priestley JV. trkA, trkB, and trkC messenger RNA expression by bulbospinal cells of the rat. Neuroscience. 1999;92(3):935–944. doi: 10.1016/s0306-4522(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 15.Mao J, Mayer DJ, Price DD. Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J Neurosci. 1993;13(6):2689–2702. doi: 10.1523/JNEUROSCI.13-06-02689.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, Gilliam F, Faught E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321–321. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- 17.Martin R, Meador K, Turrentine L, Faught E. Comparative cognitive effects of carbamazepine and gabapentin in healthy senior adults. 2001:1–8. doi: 10.1046/j.1528-1157.2001.33300.x. [DOI] [PubMed] [Google Scholar]
- 18.Meador KJ, Loring DW, Ray PG, Murro AM, King DW, Nichols ME, Deer EM, Goff WT. Differential Cognitive Effects of Carbamazepine and Gabapentin. Epilepsia. 1999;40:1279–1285. doi: 10.1111/j.1528-1157.1999.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 19.Millecamps M, Etienne M, Jourdan D, Eschalier A, Ardid D. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain. 2004;109(3):214–224. doi: 10.1016/j.pain.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Progress in Neurobiology. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. p. 402. [DOI] [PubMed] [Google Scholar]
- 22.Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN. Abnormal Retinoid and TrkB Signaling in the Prefrontal Cortex in Mood Disorders. Cerebral cortex. 2013 doi: 10.1093/cercor/bht203. [DOI] [PubMed] [Google Scholar]
- 23.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacology & Therapeutics. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanabe M, Takasu K, Kasuya N, Shimizu S, Honda M, Ono H. Role of descending noradrenergic system and spinal alpha2-adrenergic receptors in the effects of gabapentin on thermal and mechanical nociception after partial nerve injury in the mouse. British journal of pharmacology. 2005;144(5):703–714. doi: 10.1038/sj.bjp.0706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yulug B, Ozan E, Gonul AS, Kilic E. Brain-derived neurotrophic factor, stress and depression: a minireview. Brain research bulletin. 2009;78(6):267–269. doi: 10.1016/j.brainresbull.2008.12.002. [DOI] [PubMed] [Google Scholar]