Abstract
Given their capacity to regenerate cells lost through injury or disease, stem cells offer new vistas into possible treatments for degenerative diseases and their underlying causes. As such, stem cell biology is emerging as a driving force behind many studies in the field of regenerative medicine. This review focuses on our current understanding of the applications of stem cells in treating ailments of the human brain, with an emphasis on neurodegenerative diseases. Two types of neural stem cells are discussed: endogenous neural stem cells residing within the adult brain, and pluripotent stem cells capable of forming neural cells in culture. Endogenous neural stem cells give rise to neurons throughout life, but they are restricted to specialized regions in the brain. Elucidating the molecular mechanisms regulating these cells is key in determining their therapeutic potential, as well as finding mechanisms to activate dormant stem cells outside of these specialized microdomains. In parallel, patient-derived stem cells can be used to generate neural cells in culture, providing new tools for disease modeling, drug testing and cell-based therapies. Turning these technologies into viable treatments will require the integration of basic science with clinical skills in rehabilitation.
Overview
Recent developments in stem cell biology have contributed significantly to our understanding of brain development and maintenance. In this overview we summarize the ways in which they also show promise for rehabilitation and regenerative medicine.
This review focuses on two distinct populations of stem cells: endogenous neural stem cells in the adult brain, and pluripotent stem cell lines that can be differentiated into neural cells in culture. Dysfunction of endogenous stem cells or their niche - the specialized environment in which they grow – may underlie aspects of brain disease and aging. On the other hand, our ability to create new neurons and glia from patient-derived stem cells offers new hope for disease modeling, drug testing and cell-based therapy. In order for these insights to generate concrete advances in regenerative medicine we need to build a partnership between those performing rigorous mechanistic biology, others using human and animal models for preclinical studies, and clinician-scientists with a deep knowledge of patients’ needs and relevant outcome measures.
During development, pluripotent embryonic stem cells (ESCs) give rise to all brain cell types, often via multipotent precursor populations of more limited potential. Although in the adult brain generation of new cells is reduced compared to many other tissues, adult neural stem cells (NSCs) persist in two main areas: the ventricular-subventricular zone, where NSCs give rise to olfactory neurons, and the hippocampus, where new neurons involved in cognitive processes are generated. In both regions, the stem cells that give rise to neurons are specialized populations of astrocytes that maintain close interactions with the brain vasculature and can be activated by behavioral and pharmacological stimuli. Given the ability of NSCs to migrate to sites of injury, amplification of their capacity to generate neurons has therapeutic potential. The expected benefits of modulating endogenous NSCs would be even more widespread if astrocytes from other brain regions could be induced to adopt stem cell properties. Much research is therefore focused on the mechanisms underlying NSC differentiation and on the cellular and molecular characteristics of their niche.
To our knowledge, no drug has ever been tested for its effects on sick human neurons prior to initiation of clinical trials for neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD) or amyotrophic lateral sclerosis (ALS). The recent technology for creating induced pluripotent stem cells (iPSCs) from patient tissues has allowed the possibility to directly evaluate emerging drugs in cultured human disease-specific cells. It is now possible to generate multiple classes of neurons and glia from human ESCs or patient-derived iPSCs and to establish “disease in the culture dish” models that shed light on human disease mechanisms and allow for drug testing in vitro. Moreover, although replacement of neuronal circuits remains a distant goal, the grafting of stem cell-derived support cells to slow neuronal degeneration in specific regions of the brain or spinal cord has shown promise in animal models and is being tested in early human trials.
Despite its promise, neural stem cell biology still needs to cross several hurdles before its full clinical impact is realized. Potential implications of neural stem cell discoveries in the field of rehabilitation medicine over the coming decades may include improved treatments for neurodegenerative disease and stroke, among others. Taken together with the very active stem cell and bioengineering research being performed on bone, connective tissue and muscle, these avenues may lead to a radical change in our approach to patient rehabilitation and provide hope for significant clinical benefit.
Stem cell basics
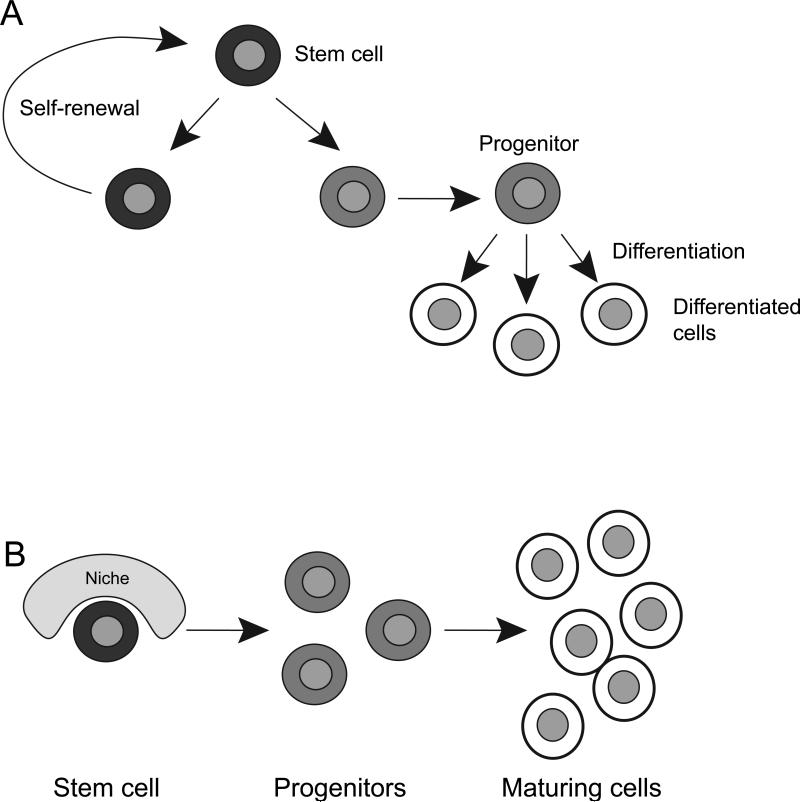
Stem cells have two essential properties: (1) They divide to give rise to another stem cell (self-renewal) and, (2) they undergo differentiation to form diverse cell types (Figure 1A). Stem cells play a central role both during development and in the adult. During embryogenesis, they give rise to all the different cell types that compose the human body. In addition, many (but not all) adult tissues retain pools of endogenous stem cells charged with replenishing cells lost through turnover (homeostasis), as well as regenerating the tissue following lesions. The property of self-renewal ensures that the stem cell pool is maintained throughout life. Stem cells often give rise to differentiated progeny via a short-lived rapidly dividing intermediate progenitors, thereby increasing the output of cells derived from a single stem cell (Figure 1A). Many adult stem cells are still capable of giving rise to different cell types albeit to a lesser extent than their embryonic counterpart1.
Figure 1. Stem cells self-renew and give rise to differentiated progeny.
A. Stem cells (black) divide to form another stem cell (self-renewal) and a progenitor cell (light grey). Progenitor cells divide to amplify their number, and in turn give rise to more differentiated progeny (white).
B. Stem cells reside in specialized niches, which provide important positional cues and regulatory elements that influence stem cell behavior. When a stem cell divides, the daughter cell that retains a stem cell identity is kept within the boundaries of the niche, while the other daughter cell loses the constraint on its phenotype. This second daughter cell now forms undifferentiated progenitors, which in turn give rise to more differentiated progeny.
Stem cells reside within specialized cellular environments, called niches, which modulate many aspects of their biology (Figure 1B). Elements within the niche provide positional cues important in determining which daughter cell retains stem cell identity and which daughter cell progresses down the lineage to form more differentiated progeny. Additionally, cells within the niche provide factors important for the survival of the stem cells and the differentiation of their progeny. Properties of both stem cells and their niche vary during development and according to the tissue in which they are found. Moreover, both are affected in human disease and aging. As one example, dysregulation of either stem cells or their niche can lead to cancer2-5. In the following sections, we review stem cells and their niche in the adult nervous system, how they are affected in disease, and how they might contribute to Central Nervous System (CNS) regeneration.
Endogenous adult neural stem cells and their niche
The CNS is composed of neurons, astrocytes and oligodendrocytes, as well as other non-neural cell types. Neurons, the main effector cells of the CNS, process the information entering and leaving the CNS. Astrocytes, a very diverse class of cells, are the main support cells of the CNS, with functions that range from regulating which molecules in the blood enter the brain to phagocytosing cellular debris in the parenchyma, and maintenance of brain homeostasis6,7. Oligodendrocytes form myelinating sheaths along axons, allowing the rapid propagation of action potentials by neurons. During embryonic brain development all three of these cell types arise from the same pool of stem cells: radial glial cells (RGCs)8.
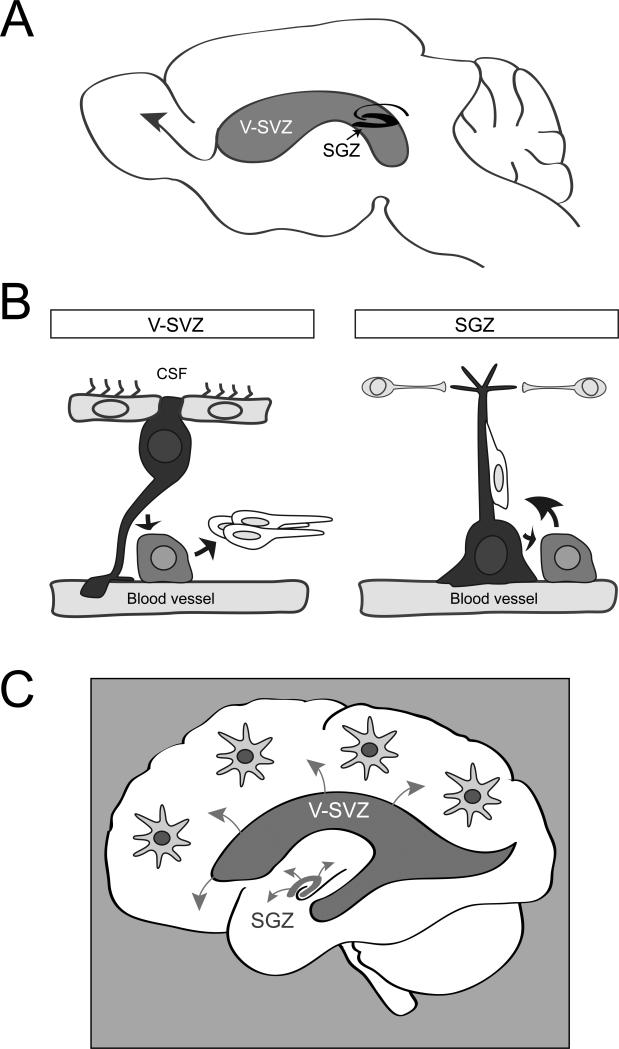
Most neurogenesis ends around birth. However, it is now clear that new neurons are continuously generated by stem cells in restricted brain regions of the adult mammalian brain throughout life. Adult neurogenesis occurs in two regions, the ventricular-subventricular zone (V-SVZ) of the lateral ventricles, which generates olfactory bulb neurons, and the subgranular zone (SGZ) in the hippocampal formation9 (Figure 2A). Some oligodendrocytes are also formed in both regions. Strikingly, in both adult neurogenic niches, the NSCs are specialized astrocytes, raising the question of whether astrocytes in other brain regions might also retain latent stem cell capacity.
Figure 2. Endogenous adult neural stem cells and their niche.
A. Schema of a sagittal section of an adult mouse brain showing the V-SVZ adjacent to the lateral ventricles and SGZ of the hippocampal formation. New neurons born in the V-SVZ migrate a long distance to their final destination in the olfactory bulb (arrow). In contrast, adult-born neurons in the SGZ integrate locally into the circuitry.
B Schema of cell types and the anatomy of adult neurogenic niches. The V-SVZ niche (left) is composed of astrocyte NSCs (black) which contact the ventricular lumen between multiciliated ependymal cells, and which extend a radial process that contacts blood vessels. These cells give rise to amplifying progenitors (light grey), which in turn differentiate to migrating immature neurons (white). These immature neurons migrate to the olfactory bulbs, where they mature into interneurons. NSCs and progenitors often directly contact blood vessels at specialized sites that lack astrocyte end feet. The SGZ niche (right) is also composed of astrocyte NSCs (black) in contact with blood vessels, but these cells do not contact the ventricular lumen. SGZ NSCs also give rise to immature neurons through progenitors, and these neurons travel along the radial processes of the NSCs to integrate within the local circuitry. Local interneurons regulate SGZ NSCs.
C Schema showing adult neural stem cell niches in the human brain. Future mechanistic studies on the biology of both regions will provide important insight into how to harness these endogenous NSCs and exploit their therapeutic potential. By modulating molecular pathways that regulate adult NSCs, it may eventually be possible to stimulate astrocytes elsewhere in the brain to become NSCs.
Most of our knowledge about adult neurogenesis comes from studies in rodents. The VSVZ extends along the length of the lateral ventricles, and is the largest germinal region in the adult brain. Quiescent NSCs in the V-SVZ become activated to divide and generate intermediate transit amplifying progenitors, which in turn give rise to new neurons that migrate to the olfactory bulb10,11 (Figure 2A, B). NSCs in the V-SVZ have a polarized morphology and span different compartments of the stem cell niche12. NSCs extend a thin process between ependymal cells, which line the ventricles, and are thereby continuously bathed by cerebrospinal fluid (CSF)13-15 (Figure 2B). On the basal side, NSCs extend a long process to contact blood vessels within the V-SVZ niche. In recent years, the blood vessels in the V-SVZ have emerged as an important proliferative compartment of the niche16-18. Notably, the vasculature in this region of the brain has unique features. Dividing stem cells and their transit amplifying progeny frequently contact blood vessels directly at specialized sites lacking astrocyte end-feet and pericyte coverage16. Moreover, signals in the blood are able to directly access the V-SVZ16. Thus stem cells in this region are uniquely exposed to both contact-mediated and diffusible signals from the vasculature, as well as systemic signals in the circulation. Interestingly, long distance and local neurons also innervate the V-SVZ, suggesting a role for circuit regulation within the niche.
In the SGZ, NSCs also extend a radial process, and generate new neurons via a short-lived intermediate progenitor (Figure 2B). The newly generated neurons travel a short distance into the granule cell layer, where they integrate into the local circuitry. The vasculature is also an important niche compartment in the SGZ. However, there are interesting differences. Unlike the V-SVZ, the vasculature is angiogenic in the SGZ, with neurogenesis occurring near angiogenic foci19. The activity of local interneurons plays an important role in mediating NSC quiescence in the SGZ20 (Figure 2B). Importantly, alterations in natural physiological states can have potent effects on ongoing stem cell proliferation and adult neurogenesis. Exercise, pregnancy and enriched environments stimulate different aspects of NSC proliferation and survival of newly generated neurons. In contrast, stress and aging inhibit proliferation and neurogenesis21. As the molecular underpinnings mediating these effects are uncovered, it may be feasible to stimulate neurogenesis and oligodendrocyte formation.
In adult humans, NSCs are also present throughout life in both the V-SVZ and SGZ as specialized astrocytes22. While active neurogenesis occurs in the SGZ in humans23, the levels of neurogenesis in the V-SVZ declines dramatically after infancy24. Interestingly, infants also possess a second migratory route to the prefrontal cortex not observed in non-human mammals. Thus, in the adult human V-SVZ, NSCs are largely in a dormant state. However, as outlined below, they divide under pathological stimulation. As such, illuminating the pathways underlying stem cell quiescence and activation in rodents may shed light on how these stem cells are recruited upon injury or to form oligodendrocytes under baseline conditions.
Endogenous neural stem cells during disease and regeneration
Adult neurogenic niches harbor a pool of endogenous NSCs that could potentially be exploited for therapeutic purposes. Using fluorescence activated cell sorting, adult NSCs can be directly harvested from tissue samples. Indeed, evidence that adult NSCs persist into adulthood in humans comes from studies where these cells have been isolated from surgical specimens of brain tissue and grown in cell cultures22,25. Isolating these cells allows for the careful dissection of their molecular characteristics in vitro, providing insight into their regulation and potential for regeneration. In addition, the ability to grow these cells in isolation allows the elucidation of the mechanisms required for neurogenesis to successfully occur.
Changes to NSCs following disease and injury
Neural stem cells react strongly to pathologic conditions in the adult brain. In the V-SVZ niche there is increased proliferation following ischemic stroke in the adjacent striatum or overlying cortex, including in humans26-28. In mouse models of ischemic stroke newly-formed neuroblasts migrate to the sites of ischemic lesion and form synaptic connections with neurons in the vicinity28. In animal models of multiple sclerosis (MS) there is an increased influx of V-SVZ-derived cells into sclerotic lesions29,30 and there is evidence of this occurring in humans as well31,32. Determining whether these mechanisms contribute to healing, and whether they are conserved in humans, will be important in the search for better therapeutic strategies for these and other conditions.
Neurogenesis in the SGZ is associated with learning and memory, mood regulation and pattern separation. Changes in neurogenesis may be one pathophysiological cause of various affective disorders33,34. Moreover, certain psychological states (i.e., anxiety and stress) decrease the amount of hippocampal neurogenesis. Experimentally decreasing hippocampal neurogenesis in rodent models leads to behavioral effects similar to those seen in models of anxiety35.
Another important NSC-related change is that both V-SVZ and SGZ show a decrease in neurogenesis with aging36,37. It remains to be determined whether this is due to a loss of cells or a shift to a dormant (non-proliferative) state. In contrast, SGZ NSCs in humans seem to increase their rate of neurogenesis during chronic neurodegenerative diseases such as Alzheimer’s disease38.
Therapeutic strategies based on endogenous NSCs.
The ability of NSCs to generate new neurons in certain niches provides hope that if this process could be amplified and/or extended, it could serve as the basis for regenerative strategies in both neurovascular and neurodegenerative disease. Amplification of NSCs may already be part of standard clinical practice. Intriguingly, increased neurogenesis in the SGZ has been implicated in the therapeutic effects of SSRI antidepressants, potentially explaining the time needed for these drugs to take full effect33,34. The degree to which this can be generalized to human patients and other antidepressants remain to be determined. Importantly, physical activity increases neurogenesis in both V-SVZ and SGZ21, suggesting that non-pharmacological approaches could also be employed to target these cells. More generally, expanding the relatively limited potential of neural stem cells to generate other cell types could be important. Another still speculative therapeutic possibility is that some of the molecular mechanisms involved in differentiation of NSCs could be used to activate astroglial cells elsewhere in the brain (Figure 2C). Indeed, following traumatic brain injuries, glial cells in the vicinity of the lesion proliferate and form a glial scar. This glial scar has been proposed to create a negative environment for neurogenesis and proper wound healing. By further understanding the niche elements in the V-SVZ and SGZ that permit such abundant neurogenesis to occur it might be possible to find ways to turn the environment in these glial scars, and elsewhere in the brain, into a more permissive one. Elucidating this will be key in understanding the therapeutic potential of these cells and other astroglial cells in the brain.
Making neurons from stem cells in the culture dish
The preceding sections focused on production of neurons from stem cells in situ, and how this might be modulated in the therapeutic context. This is a rapidly developing field but more emphasis still is being put on the potential uses of neurons and glia generated from stem cells in the laboratory, either to model diseases in vitro as a basis for drug testing, or for direct cell replacement strategies.
Human stem cell-derived neurons open new avenues.
Both aspects of this approach rely on the ability to generate neurons from human stem cells, through methods discussed in more detail below. This possibility is bringing about a sea change in our approaches to many neurological and psychiatric diseases, and in particular to neurodegenerative disease. As one example, in patients with amyotrophic lateral sclerosis (ALS), degeneration and death of cortical and spinal motor neurons leads to progressive muscle paralysis, often starting in the distal limbs and progressing to the respiratory muscles39. However, the sole FDA-approved drug for ALS, riluzole, confers only modest clinical benefit40. There is therefore a pressing need for disease- modifying treatments. One major obstacle to a successful therapy for ALS is the near-absence of validated targets, molecular events in the disease pathway whose inhibition would slow onset or progression. Genes such as superoxide dismutase 1 (SOD1) whose mutation can lead to ALS may be considered to be validated targets, but familial forms of the disease collectively represent only 10% of all cases. Therapeutic targets applicable to the 90% of sporadic cases would likely be genes acting early in the disease pathway. If such targets could be identified, they would provide a solid foundation for targeted drug discovery programs.
A great majority of studies on the mechanisms of ALS have focused on mouse models expressing disease-triggering mutant forms of SOD141. These mice develop a disease that strikingly mimics not only familial but also sporadic ALS, including selective resistance of oculomotor and slow spinal motor neurons42. However, even in this model, there are few validated targets other than the SOD1 gene itself. Moreover, concerns have been raised that results obtained in mSOD1 mice may not translate well to the clinic. Although this may in part reflect underpowered mouse preclinical studies, there is a need for human models to discover and validate novel candidate disease modifiers. Strikingly, none of the many drugs that have undergone clinical trials in ALS was ever first tested on the cells affected in this disease: human motor neurons. The best strategy would appear to be to evaluate candidate treatments in two systems in parallel: human motor neurons from familial and sporadic patients in vitro and the mouse neuromuscular system in vivo.
Making motor neurons from pluripotent stem cells.
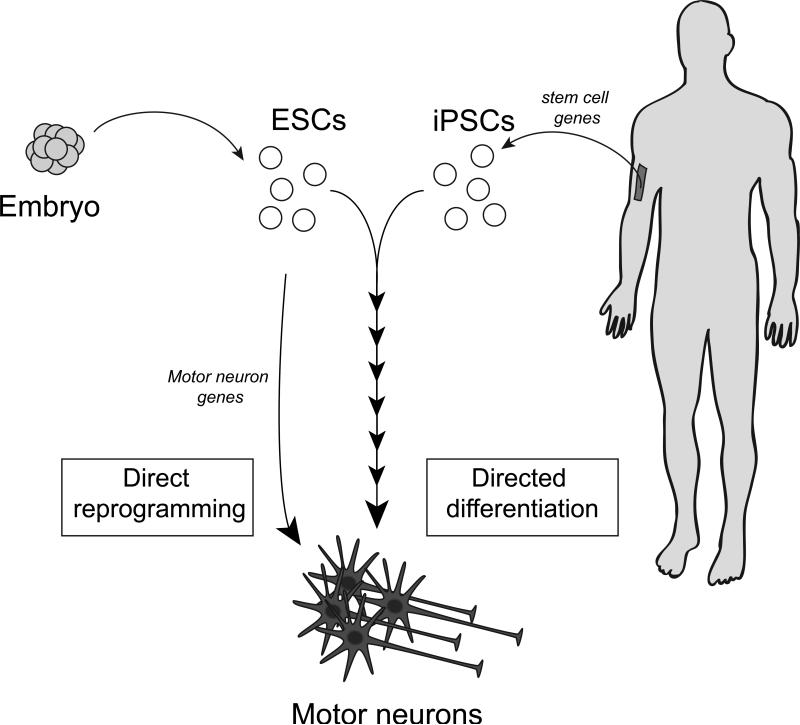
For this reason, we and others have begun to use motor neurons and other cell types generated from human induced pluripotent stem cells (hiPSCs) to model ALS in the culture dish. The first step was to devise protocols by which stem cells can be coaxed into losing their stem cell properties and adopting those of differentiated, post-mitotic neurons. We first achieved this using mouse embryonic stem cells (ESCs), by carefully mimicking the stepwise process in the embryo through which external factors guide stem cells through a series of intermediate stages to become motor neurons43 (Figure 3). This process is very robust, and generates billions of cells that are “real” motor neurons by many molecular and functional criteria.
Figure 3. Making motor neurons from stem cells.
Embryonic stem cell lines (ESCs) are generated from early human embryos, whereas differentiated skin cells can be turned into induced pluripotent stem cells (iPSCs) by upregulating specific stem cell genes. Both ESCs and iPSCs can be directed to differentiate into motor neurons through a multi-step process by culturing them for weeks in the presence of factors that mimic normal neuronal development (“directed differentiation”). A more rapid process (days) is the “direct reprogramming” of ESCs (or fibroblasts, not shown) into motor neurons directly by forced expression of motor neuron genes.
We and others subsequently used adaptations of the same protocol to generate motor neurons from human ESCs44-46. This approach made living human motor neurons widely available for the first time. However, hESC-derived motor neurons have at least one significant drawback for disease modeling: existing lines were derived from embryos representative of healthy controls. There are essentially two ways to overcome this. First, it is possible to introduce into human ESCs copies of mutant genes known to cause familial forms of ALS or other neurodegenerative diseases, and to study their effect on motor neuron survival and regeneration. Second, a more far-reaching change occurred when Yamanaka and his colleagues demonstrated the possibility of reprogramming differentiated cells to induced pluripotent stem cells (iPSCs)47. They showed that following the introduction of 3-4 stem cell genes into skin fibroblasts cultured following biopsy, not only do the cells adopt a stem cell phenotype, they nearly completely “forget” their skin cell origin. We used iPSCs from human ALS patients and controls to generate patient-specific motor neurons with high yield45,48 (Figure 3). Although some differences between these and the gold-standard hESCs certainly exist, they are relatively minor in most cases and iPS cells have generated enormous excitement due to their potential applications in disease modeling and regenerative therapy. Directed differentiation of iPS cells provides the first patient-specific access to living preparations of many tissue types. By capturing patient-specific genetic background, iPS cells enable modeling of poorly understood complex genetic disorders, creation of humanized disease models that may be used to study disease modifiers and other correlations with clinical data, and immunologically matched tissue for eventual cell-replacement therapy.
Despite these many advantages, the derivation of iPS lines and their subsequent differentiation can be a lengthy process. More recently, different groups have shown it is possible to take a shortcut by direct reprogramming of skin fibroblasts or ESCs into motor neurons (Figure 3). In this case it is motor neuron genes, not stem cell genes, that are introduced as part of the reprogramming process49-51. This type of approach allows mouse motor neurons to be generated in as little as two days from ESCs, but both techniques have their advantages and will likely coexist.
Disease Modeling Using Human iPSC-Derived Neurons
The past years have seen the development of a number of hiPSC-based neurodegenerative and neurological disease models. The basic “disease in the culture dish” paradigm of these models involves producing iPS cells from groups of patients, differentiating them into disease-relevant cell types, and assessing the derivatives for changes in gene expression, survival, protein localization/aggregation, sensitivity to exogenous stressors, or physiologic activity. Disease-related phenotypes have been observed in iPS-derived neurons from patients with schizophrenia, Huntington’s disease, Parkinson’s disease, familial dysautonomia, Rett syndrome, spinal muscular atrophy, and ALS52-57. Mechanistic characterization of such phenotypes is nascent, but promising. Another critical feature needed for rigorous analysis is to show that any “clinical phenotype” observed in the culture dish is truly due to the disease gene, and not to inter-individual differences in genetic background between the patient and control samples. The best way of doing this is to “correct” the mutation in a given iPSC line so as to create a gene-corrected (isogenic) control line which only differs from the patient-derived line by this single change58. However, this has been performed in very few published studies. Some examples of diseases modeled using iPS cells are given below.
Rett syndrome
iPS cells derived from female patients with Rett syndrome, another neurodevelopmental disorder, can be efficiently differentiated to neurons. However, Rett iPS-neurons exhibit decreased synaptic connectivity, spine density, and soma size, as well as reduced spontaneous action potential firing in culture54. These deficits are consistent across multiple RettiPS cell lines, and can be rescued by repletion of mutant protein or treatment with IGF-1. However even in this system, genetic and epigenetic instability have proven to be concerns59.
Schizophrenia
iPS-neurons derived from adult-onset schizophrenic (SCZD) patients also display decreased synaptic connectivity, spine density, and soma size in culture, but show no apparent differences in spontaneous action potential55. Expression profiling reveals a large number of differences between SCZD neurons and controls, some of which have been observed in previous work performed on post-mortem patient samples. Treatment with Loxapine, but not other antipsychotic drugs, was able to reverse some putative SCZD-iPS disease phenotypes, including select expression changes55.
Parkinson's disease
Sensitivity to environmental stressors has also been observed in disease-specific iPS-neurons. Dopaminergic neurons derived from a single PD patient were more sensitive to 6-OHDA induced cell death than those derived from a control iPS or hES line57. Although mixed cultures of dopaminergic neurons derived from a PD patient were also more sensitive to the oxidative stressor hydrogen peroxide, the tyrosine hydroxylase-positive dopaminergic neurons themselves were not. More recently, iPS models of PD have been used to address a potential drawback of such culture systems for studying late-onset adult diseases. By expressing progerin, a gene associated with premature aging, it was possible to enhance the PD- related phenotypes in the culture model60.
Spinal muscular atrophy (SMA)
SMA patients show a characteristic loss of spinal motor neurons. SMA iPS-derived motor neurons exhibit a dearth of nuclear SMN aggregates, a pathological hallmark of the disease52. This effect can be partially rescued by treatment with drugs known to increase SMN in other tissues. Furthermore, SMA iPS-MNs are less abundant in culture than control iPS-MNs following 6 weeks of differentiation, despite being present at similar levels two weeks prior to this time-point52. The numbers of motor neurons can be increased either by genetic correction of the deficit61 or by blocking apoptosis62.
Amyotrophic lateral sclerosis
ALS was the first disease to be modeled using patient-derived iPS cells48. Since then, multiple attempts have been made to generate a model of “ALS in the culture dish” that is clearly relevant to the human disease. Perhaps unsurprisingly for a late-onset disease such as this, motor neurons from ALS patients do not show spontaneous degeneration in the few weeks they can be maintained in culture. Instead, in several cases, iPSMNs derived from familial forms of the disease do exhibit some of the molecular hallmarks of human pathology56. Motor neurons derived from patients with the most frequent familial mutation, in C9ORF72, show characteristic accumulations (foci) of mutated RNA63,64. Even this is not alone sufficient to trigger neurodegeneration, and it is necessary to stress the ALS neurons with high levels of glutamate in order to observe exacerbated cell death. This vulnerability can be reversed using DNA antisense oligonucleotides targeting the disease gene63.
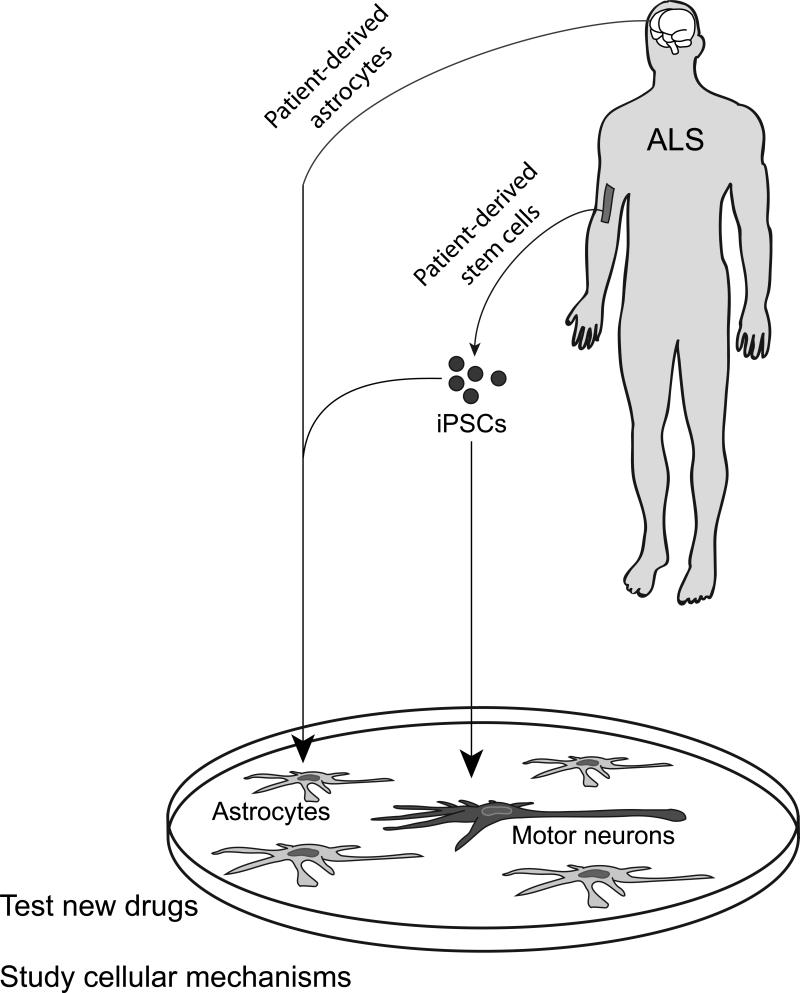
In addition to artificial aging or exposure to stressors, another approach to disease modeling is to more fully recreate the cellular environment of motor neurons within the spinal cord. Astrocytes normally provide a supportive “niche” for motor neurons within the adult CNS. However, in both mouse and human models, it has been reported that ALS astrocytes instead become toxic for motor neurons, secreting factors that can trigger neuronal death65-67. The availability of stem cell-derived motor neurons and astrocytes68, in some cases in combination with astrocytes harvested from post-mortem CNS with familial or even sporadic forms of the disease69, has made it possible to generate co-culture systems in which this toxic non-cell-autonomous interaction leads to spontaneous motor neuron degeneration in the culture dish. The immediate goal will be to create a fully humanized model of ALS in the culture dish that shows spontaneous neurodegeneration using astrocytes of both familial and sporadic ALS origin (Figure 4).
Figure 4. Human ALS in the culture dish.
To date, human ALS astrocytes have been shown to be toxic for mouse ESC-derived motor neurons, as are mouse ALS astrocytes for human ESC-derived motor neurons. The ideal system would be a completely humanized model as depicted here, in which astrocytes (light grey) are derived from post mortem brain or ALS ESCs/iPSCs and motor neurons (dark grey) are generated from ESCs/iPSCs. If human astrocytes lead to spontaneous motor neuron degeneration in this simplified culture system it should be possible to test drugs for their efficacy in preventing ALS-related motor neuron cell death, as well as study the cellular and molecular mechanisms underpinning disease progression.
The emergence of humanized disease models of ALS has opened the door to their use for screening collections of small molecules for neuroprotective drug candidates. For technical reasons, it is not yet feasible to perform screening on human models on a large scale. But arguably, all candidate disease treatments should now be evaluated in such systems, hopefully increasing the predictive value of preclinical studies.
Cell-based therapy in the nervous system
In parallel with attempts to slow neurodegeneration using drugs developed with the help of stem cell models, a future strategy would be to replace lost neurons. In reality, this is an extraordinarily challenging goal. In a disease such as ALS, it would be necessary not only to get grafted motor neurons to establish themselves in the spinal cord and send out axons to distant target muscles, but also for the new motor neurons to become integrated in the circuits involved in motor control. At present, this prospect must be considered distant. Even for the dopaminergic neurons lost focally in PD patients, no robust technology yet exists to generate neurons in sufficient quantity and quality, but this will likely be the neurological disease where the first neuronal replacement trials are carried out.
An alternative, parallel approach involves the development of paracrine therapies. One possibility, as in models of epilepsy or neuropathic pain, is to reintroduce local interneurons that can correct the properties of dysfunctional neural circuits70,71. Stem cells and their glial derivatives have the potential to serve as local “factories” of growth factors that support the repair of nearby tissues72. Thus placement of stem cells in a damaged brain may facilitate repair through these paracrine effects, rather than relying on direct replacement of missing cells. The cell populations evaluated for this type of approach range from mesenchymal stem cells to more targeted glial precursor populations73. Cells may be administered either locally, using cutting-edge neurosurgery, or more systemically74. The former approach is more invasive, but the latter poses problems of specificity and quantity of cells delivered. Perhaps the most promising approaches are those that use stem cell derivatives not only as supportive cells in their own right but also as “Trojan horses” for the delivery of recombinant proteins with known neuroprotective activity75. Collectively, these approaches certainly deserve more development, but need to surmount the hurdles of relatively weak efficacy in preclinical models (where evaluated), quantity and quality of cells for human administration, and the paucity of relevant outcome measures during the early phases of clinical trial.
Putting stem cell biology to work in a rehabilitation department
How will stem cell biology and regenerative medicine impact rehabilitation over the coming decade? There a multiple challenges ahead, including that of managing excessive expectations. The strategy we have adopted within the Department of Rehabilitation and Regenerative Medicine at Columbia University Medical Center is a long-term one, based on supporting cutting-edge basic research in close and frequent contact with the clinicians who understand patients’ problems and can help to define areas that present the most promising opportunities for future intervention. The Columbia Stem Cell Initiative (CSCI; External link http://www.ColumbiaStemCell.org) has its home within the Department of Rehabilitation and Regenerative Medicine, while extending to >100 laboratories in all corners of the two campuses. To enhance stem cell focus within the department, we have created a Division of Regenerative Medicine which houses stem cell scientists in our own laboratory space, and organize research meetings and collaborations involving both basic and clinical faculty.
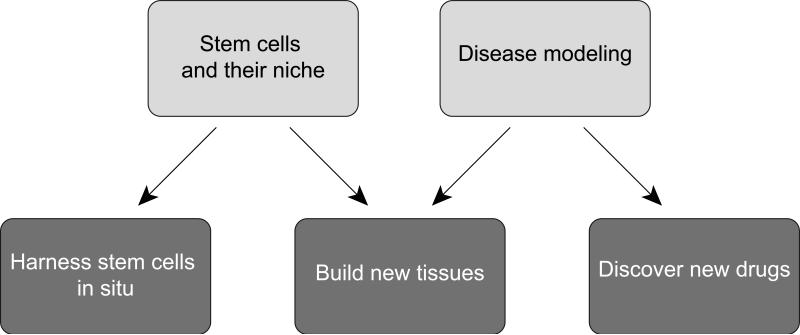
The topic of this review – Stem Cells in the Nervous System – encompasses only a fraction of the approaches that will be needed for successful rehabilitation. Strategically, therefore, it will be critical to integrate our approaches across the whole range of pathologies encountered in rehabilitation medicine. We plan to do this by implementing the two main research areas outlined in this text – the biology of stem cells and their niche, and disease modeling – and to use these as a solid basis for new therapeutic approaches based on pharmacological interventions, bioengineering and cell-based therapy (Figure 5).
Figure 5. Tapping the potential of stem cells for human health.
A parallel focus on the two main areas of research outlined in this review – endogenous stem cells and their niche, and disease modeling – provides a basis for three novel areas of progress toward clinical application and regenerative medicine.
ACKNOWLEDGEMENTS
Work in the Columbia Stem Cell Initiative and the authors’ laboratories was supported by NYSTEM, the Leona M. and Harry B. Helmsley Charitable Trust, the Vidda Foundation, Project A.L.S., P2ALS, Target ALS, the Jerry and Emily Spiegel Laboratory for Cell Replacement Therapies, and the National Institute of Neurological Disorders and Stroke (NINDS) award numbers F31NS079057 (A.R.M.S.) and 5R01NS074039-03 (F.D.).
References
- 1.Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3(5):480–483. doi: 10.1016/j.stem.2008.10.007. doi:10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Evers P, Lee PP, DeMarco J, et al. Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer. 2010;10:384. doi: 10.1186/1471-2407-10-384. doi:10.1186/1471-2407-10-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457(7229):603–607. doi: 10.1038/nature07589. doi:10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masui K, Suzuki SO, Torisu R, Goldman JE, Canoll P, Iwaki T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58(9):1050–1065. doi: 10.1002/glia.20986. doi:10.1002/glia.20986. [DOI] [PubMed] [Google Scholar]
- 5.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. doi:10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 6.Molofsky AV, Krencik R, Krenick R, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26(9):891–907. doi: 10.1101/gad.188326.112. doi:10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. doi:10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. doi:10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming G-L, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. doi:10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264(5162):1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 12.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23(6):935–942. doi: 10.1016/j.conb.2013.09.004. doi:10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. doi:10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokovay E, Wang Y, Kusek G, et al. VCAM1 Is Essential to Maintain the Structure of the SVZ Niche and Acts as an Environmental Sensor to Regulate SVZ Lineage Progression. Cell Stem Cell. 2012;11(2):220–230. doi: 10.1016/j.stem.2012.06.016. doi:10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Doetsch F, García-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96(20)::11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavazoie M, der Veken Van L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. doi:10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. doi:10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokovay E, Goderie S, Wang Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7(2):163–173. doi: 10.1016/j.stem.2010.05.019. doi:10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10(6):698–708. doi: 10.1016/j.stem.2012.05.012. doi:10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vukovic J, Blackmore DG, Jhaveri D, Bartlett PF. Activation of neural precursors in the adult neurogenic niches. Neurochem Int. 2011;59(3):341–346. doi: 10.1016/j.neuint.2011.04.003. doi:10.1016/j.neuint.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427(6976):740–744. doi: 10.1038/nature02301. doi:10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 23.Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. doi:10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011 doi: 10.1038/nature10487. doi:10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4(6):576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 26.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26(50):13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. doi:10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martí-Fàbregas J, Romaguera-Ros M, Gómez-Pinedo U, et al. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74(5):357–365. doi: 10.1212/WNL.0b013e3181cbccec. doi:10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- 28.Kahle MP, Bix GJ. Neuronal restoration following ischemic stroke: influences, barriers, and therapeutic potential. Neurorehabil Neural Repair. 2013;27(5):469–478. doi: 10.1177/1545968312474119. doi:10.1177/1545968312474119. [DOI] [PubMed] [Google Scholar]
- 29.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. doi:10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11(12):4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 31.Nait-Oumesmar B, Picard-Riera N, Kerninon C, et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc Natl Acad Sci USA. 2007;104(11):4694–4699. doi: 10.1073/pnas.0606835104. doi:10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin RJM, Gallo V. The translational biology of remyelination: Past, present, and future. Glia. 2014 doi: 10.1002/glia.22622. doi:10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- 33.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. doi:10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 35.Saxe MD, Battaglia F, Wang J-W, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. doi:10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couillard-Després S. Hippocampal neurogenesis and ageing. Curr Top Behav Neurosci. 2013;15:343–355. doi: 10.1007/7854_2012_232. doi:10.1007/7854_2012_232. [DOI] [PubMed] [Google Scholar]
- 37.Conover JC, Shook BA. Aging of the subventricular zone neural stem cell niche. Aging Dis. 2011;2(1):49–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. doi:10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2(11):806–819. doi: 10.1038/35097565. doi:10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 40.Ginsberg G, Lowe S. Cost effectiveness of treatments for amyotrophic lateral sclerosis: a review of the literature. Pharmacoeconomics. 2002;20(6):367–387. doi: 10.2165/00019053-200220060-00002. [DOI] [PubMed] [Google Scholar]
- 41.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85(1):94–134. doi: 10.1016/j.pneurobio.2008.01.001. doi:10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS ONE. 2012;7(4):e34640. doi: 10.1371/journal.pone.0034640. doi:10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 44.Amoroso MW, Croft GF, Williams DJ, et al. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci. 2013;33(2):574–586. doi: 10.1523/JNEUROSCI.0906-12.2013. doi:10.1523/JNEUROSCI.0906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):279–286. doi: 10.1038/nbt.1783. doi:10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X-J, Du Z-W, Zarnowska ED, et al. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. doi:10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. doi:10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. doi:10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 49.Mazzoni EO, Mahony S, Closser M, et al. Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nat Neurosci. 2013;16(9):1219–1227. doi: 10.1038/nn.3467. doi:10.1038/nn.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer K, Ferraiuolo L, Miranda CJ, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA. 2014;111(2):829–832. doi: 10.1073/pnas.1314085111. doi:10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Son EY, Ichida JK, Wainger BJ, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. doi:10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebert AD, Yu J, Rose FF, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–280. doi: 10.1038/nature07677. doi:10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461(7262):402–406. doi: 10.1038/nature08320. doi:10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchetto MCN, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–539. doi: 10.1016/j.cell.2010.10.016. doi:10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473(7346):221–225. doi: 10.1038/nature09915. doi:10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitne-Neto M, Machado-Costa M, Marchetto MCN, et al. Downregulation of VAPB expression in motor neurons derived from induced pluripotent stem cells of ALS8 patients. Hum Mol Genet. 2011;20(18):3642–3652. doi: 10.1093/hmg/ddr284. doi:10.1093/hmg/ddr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen HN, Byers B, Cord B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8(3):267–280. doi: 10.1016/j.stem.2011.01.013. doi:10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byrne JA. Generation of isogenic pluripotent stem cells. Hum Mol Genet. 2008;17(R1):R37–41. doi: 10.1093/hmg/ddn053. doi:10.1093/hmg/ddn053. [DOI] [PubMed] [Google Scholar]
- 59.Dajani R, Koo S-E, Sullivan GJ, Park I-H. Investigation of Rett syndrome using pluripotent stem cells. J Cell Biochem. 2013;114(11):2446–2453. doi: 10.1002/jcb.24597. doi:10.1002/jcb.24597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller JD, Ganat YM, Kishinevsky S, et al. Human iPSC-Based Modeling of Late-Onset Disease via Progerin-Induced Aging. Cell Stem Cell. 2013;13(6):691–705. doi: 10.1016/j.stem.2013.11.006. doi:10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corti S, Nizzardo M, Simone C, et al. Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Sci Transl Med. 2012;4(165):165ra162. doi: 10.1126/scitranslmed.3004108. doi:10.1126/scitranslmed.3004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sareen D, Ebert AD, Heins BM, McGivern JV, Ornelas L, Svendsen CN. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS ONE. 2012;7(6):e39113. doi: 10.1371/journal.pone.0039113. doi:10.1371/journal.pone.0039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donnelly CJ, Zhang P-W, Pham JT, et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80(2):415–428. doi: 10.1016/j.neuron.2013.10.015. doi:10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sareen D, O'Rourke JG, Meera P, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5(208):208ra149. doi: 10.1126/scitranslmed.3007529. doi:10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. doi: 10.1016/j.stem.2008.09.017. doi:10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3(6):649–657. doi: 10.1016/j.stem.2008.10.001. doi:10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. doi: 10.1038/nn1876. doi:10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roybon L, Lamas NJ, Garcia-Diaz A, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4(5):1035–1048. doi: 10.1016/j.celrep.2013.06.021. doi:10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haidet-Phillips AM, Hester ME, Miranda CJ, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29(9):824–828. doi: 10.1038/nbt.1957. doi:10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bráz JM, Sharif-Naeini R, Vogt D, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74(4):663–675. doi: 10.1016/j.neuron.2012.02.033. doi:10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci. 2013;16(6):692–697. doi: 10.1038/nn.3392. doi:10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lepore AC, Rauck B, Dejea C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11(11):1294–1301. doi: 10.1038/nn.2210. doi:10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meamar R, Nasr-Esfahani MH, Mousavi SA, Basiri K. Stem cell therapy in amyotrophic lateral sclerosis. J Clin Neurosci. 2013;20(12):1659–1663. doi: 10.1016/j.jocn.2013.04.024. doi:10.1016/j.jocn.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 74.Riley J, Glass J, Feldman EL, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74(1):77–87. doi: 10.1227/NEU.0000000000000156. doi:10.1227/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 75.Krakora D, Mulcrone P, Meyer M, et al. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther. 2013;21(8):1602–1610. doi: 10.1038/mt.2013.108. doi:10.1038/mt.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]