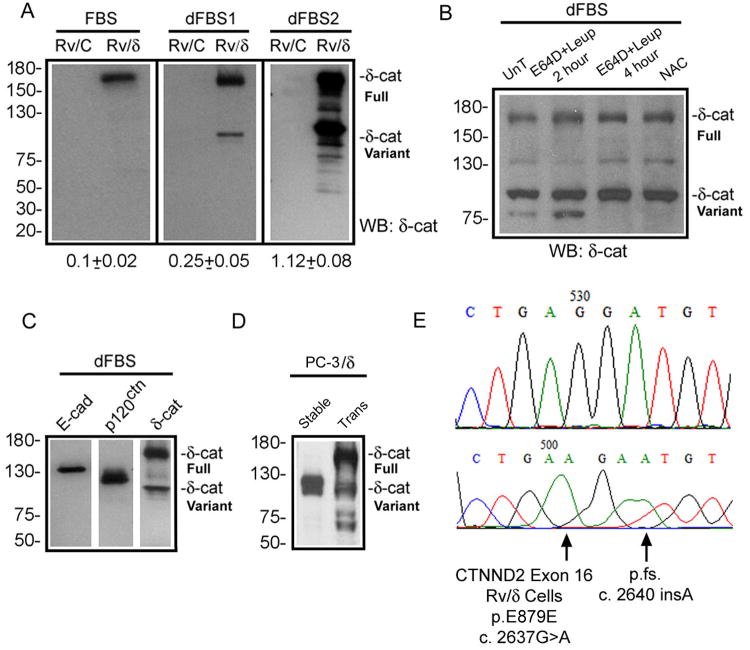
Figure 1. Mutations and formation of δ-catenin variants.
(A). Ectopically expressed full-length δ-catenin gradually changes to truncated variants in human prostate cancer xenograft CWR22-Rv1 in culture. FBS: fetal bovine serum with high glucose; dFBS1 and dFBS2: the first and second rounds of treatment with FBS and glucose deprivation. Rv/C: control cells; Rv/δ: cells expressing full-length δ-catenin. Numbers underneath the gels: ratio of truncated variant to full-length δ-catenin in Rv/δ cells. (B). Protease inhibition did not prevent the formation of δ-catenin variant. Rv/δ cells from dFBS2 culture were either untreated (UnT), treated with E64D+leipeptin for 2 or 4 hours, or treated with NAC for 4 hours. (C). Rv/δ cells from dFBS2 culture displaying fast migrating δ-catenin variant but not E-cadherin or p120ctn proteolysis. (D). Ectopically expressed full-length δ-catenin in transient (Trans) transfected human prostate cancer bone metastasis PC-3 cells (PC-3/δ) gave rise to truncated variants that migrated faster on SDS-gel after the δ-catenin overexpressing cells became stable cell lines (Stable) in culture. (E). Example of δ-catenin mutations in Rv/δ cells from dFBS2 culture. The lower panel indicates mutation and the upper panel indicates the normal control sequence. Molecular weights are on the left.