Abstract
Magnetic resonance imaging (MRI) of macrophages in atherosclerosis requires the use of contrast-enhancing agents. Reconstituted lipoprotein particles that mimic native high density lipoproteins (HDL) are a versatile delivery platform for Gd-based contrast agents (GBCA) but require targeting moieties to direct the particles to macrophages. In this study, a naturally occurring methionine oxidation in the major HDL protein, apolipoprotein (apo) A-I, was exploited as a novel way to target HDL to macrophages. We also tested if fully functional GBCA-HDL can be generated using synthetic apo A-I peptides. The fluorescence and MRI studies reveal that specific oxidation of apo A-I or its peptides increases the in vitro macrophage uptake of GBCA-HDL by 2–3 times. The in vivo imaging studies using an apo E-deficient mouse model of atherosclerosis and a 3.0T MRI system demonstrate that this modification significantly improves atherosclerotic plaque detection using GBCA-HDL. At 24 h post-injection of 0.05 mmol Gd/kg GBCA-HDL containing oxidized apo A-I or its peptides, the atherosclerotic wall/muscle normalized enhancement ratios were 90% and 120%, respectively, while those of GBCA-HDL containing their unmodified counterparts were 35% and 45%, respectively. Confocal fluorescence microscopy confirms the accumulation of GBCA-HDL containing oxidized apo A-I or its peptides in intraplaque macrophages. Together, the results of this study confirm the hypothesis that specific oxidation of apo A-I targets GBCA-HDL to macrophages in vitro and in vivo. Furthermore, our observation that synthetic peptides can functionally replace the native apo A-I protein in HDL further encourages the development of these contrast agents for macrophage imaging.
Keywords: Macrophage, magnetic resonance imaging, lipoprotein nanoparticle, apolipoprotein A-I, biomimetic peptide, oxidation, gadolinium, atherosclerosis, vulnerable plaque
1. INTRODUCTION
Despite advances in cardiovascular care, atherosclerosis, the buildup of plaque in the artery walls, remains the leading cause of death in the United States (1). The majority of thromboembolic events such as stroke or myocardial infarction result from rupture or erosion of atherosclerotic plaques prone to rupture in the coronary arteries, so-called “high-risk” or “vulnerable” plaques (2), while stenosis by itself caused by these plaques is non-critical (3). Accurate in vivo tracking of plaque vulnerability and progression using non-invasive imaging approaches is important for early identification of high-risk patients and evaluation of the effectiveness of treatment. Thus, discrimination between stable and vulnerable plaques is of particular clinical importance. Currently, none of the available diagnostic methods for assessing atherosclerosis are able to detect and evaluate vulnerable plaques before disruption occurs (4–5).
Inflammation has a crucial role at all stages of atherosclerosis. Macrophages are involved in the pathogenesis of plaques (6–7) and high and active intraplaque macrophage content correlates strongly with plaque vulnerability (8–9). In coronary and carotid artery plaques, the macrophage-rich area, and the number of lipid-laden macrophages are significantly higher in vulnerable plaques as compared to stable plaques and this difference can be as high as 300–500% (9–11). These and other findings (12) suggest that the macrophage content can be used as a distinctive feature and a specific marker of vulnerable plaques for imaging purposes.
Magnetic resonance imaging (MRI) has emerged as one of the most powerful non-invasive methods for imaging atherosclerosis (13). The combination of MRI and contrast agents greatly enhances the possibility to detect the high macrophage content of vulnerable plaques and helps to discriminate them from stable counterparts (13–15). Furthermore, activated macrophages are reliable indicators of not only plaques, but also any inflamed tissues. This makes macrophage-targeted agents important for imaging evaluation of other pathologies (16–18). Therefore, macrophages are the most appealing targets for MRI contrast agents including the Gd-based contrast agents (GBCAs).
High density lipoproteins (HDL) is a group of native lipoproteins that transport cholesterol from the peripheral tissues to the liver (19). HDL can be readily reconstituted from lipids and apolipoproteins (apo) in vitro (20). Recently, GBCA-containing synthetic HDL (GBCA-HDL) have been reported to image the liver (21). The HDL complexes (both native and synthetic) have also been proposed to image plaques (22–28). However, native unmodified HDL are not normally uptaken by macrophages, leading to the need for targeting moieties. An example of a macrophage-targeted molecule is the apo E-derived lipopeptide, which improves contrast enhancement of atherosclerotic plaques upon incorporation into GBCA-HDL (22). On the other hand, this detergent-like, highly positively charged molecule was originally proposed to mediate drug transport to human cells expressing low density lipoprotein receptor such as brain capillary endothelial cells (29). In addition, this molecule can exert neurotoxic effects (30–31). To avoid the pitfalls and complications associated with this and other targeting moieties, novel approaches to target GBCA-HDL to macrophage-rich plaques are required.
It is well-established that two of the three methionines (Met-112 and Met-148) in apo A-I are susceptible to oxidation and both the oxidized and unoxidized forms of apo A-I occur in vivo (32–34). This modification has been demonstrated (35–36) to convert HDL from antiatherogenic to proatherogenic particles. Importantly, the oxidized apo A-I form has been found in human aortic lesions with its content correlating with increased disease severity (37).
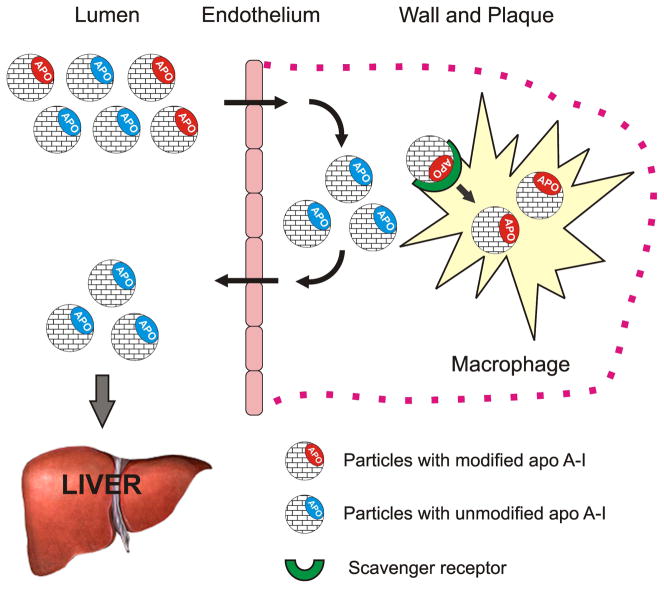
In this study, we hypothesized that oxidative modification of apo A-I in synthetic HDL may serve as a natural way to target HDL to macrophages and can be used to deliver incorporated GBCA to macrophage-rich areas of interest, such as vulnerable plaques (Fig. 1). We also hypothesized that the ability to target macrophages can be provided by not only the entire apo A-I protein, but also by synthetic 22-mer peptides that correspond to apo A-I amphipathic helixes 4 (H4) and 6 (H6), which contain methionines 112 and 148, respectively. The use of synthetic peptides in place of native apo A-I isolated from human plasma would help to avoid potential clinical complications, further encouraging the development of these contrast agents for macrophage imaging. We therefore aimed to: (1) test the ability of oxidized apo A-I and its peptides to promote the in vitro macrophage uptake of GBCA-HDL and (2) evaluate the efficacy of GBCA-HDL with oxidized apo A-I and its peptides for in vivo MRI of plaques using an apo E-deficient mouse model of atherosclerosis.
Figure 1.
Schematic representation of the proposed concept of targeting high density lipoproteins (HDL) to macrophages by using specific naturally occurring modifications of the HDL major protein, apolipoprotein (apo) A-I. In the human body, native unmodified HDL (depicted by blue) function to deliver excess cholesterol from peripheral tissues to the liver and are not normally uptaken by macrophages. In contrast, synthetic HDL containing oxidized (depicted by red) apo A-I (or apo A-I peptides) are uptaken by macrophages, thus delivering the incorporated agent(s) directly to the cells of interest. Macrophage uptake is illustrated using the example of intraplaque macrophages.
To those aims, we synthesized paramagnetic and fluorescent HDL containing either the oxidized or unmodified apo A-I protein (alternatively, a 1:1 mixture of either oxidized or unmodified peptides H4 and H6 was used) and performed comparative fluorescence and MRI studies of in vitro macrophage uptake using the well-established macrophage-like mouse cell line J774. The abdominal aortas of either apo E knockout (KO) mice kept on a Western diet or wild type (WT) mice kept on a normal chow diet were imaged using a 3.0T MRI system before and 24 h after injection of the synthesized contrast agents. In vivo MRI data were complemented by immunohistology, differential interference contrast (DIC) and fluorescence microscopy.
2. RESULTS
2.1. Oxidation of apo A-I or its peptides does not affect the size, composition, and relaxivity properties of GBCA-HDL
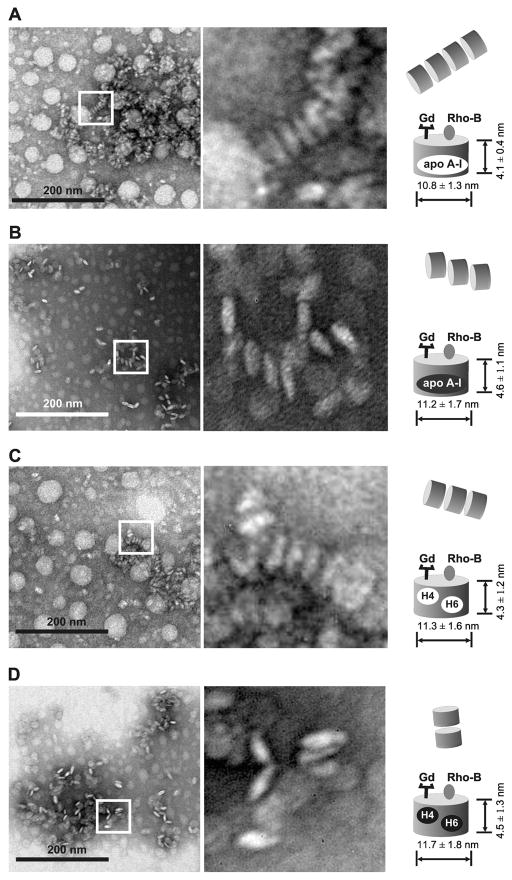
To test if oxidation of apo A-I or its peptides affects the size or composition of GBCA-HDL, we examined all synthesized GBCA-HDL by electron microscopy (EM). EM imaging showed no significant differences between different GBCA-HDL particles in terms of apparent particle size or shape. The GBCA-HDL particles reconstituted using unmodified apo A-I had a mean diameter of 10.8 ± 1.3 and a mean height of 4.1 ± 0.4 (Fig. 2A), which was not significantly different from the size of GBCA-HDL with oxidized apo A-I: 11.2 ± 1.7 and 4.6 ± 1.1, respectively (Fig. 2B). The GBCA-HDL complexes reconstituted using a 1:1 mixture of either unmodified or oxidized peptides H4 and H6 had similar morphology and particle size (Fig. 2C and 2D), suggesting that these peptides can functionally replace the native apo A-I protein with respect to their ability to assist in the self-assembly of HDL-like particles. All reconstituted GBCA-HDL complexes had a similar ability to arrange in characteristic stacks (Fig. 2). These observations are consistent with the results published before for reconstituted HDL and GBCA-HDL complexes (22,38). Analysis by inductively coupled plasma mass spectrometry (ICP-MS) showed that the average number of Gd atoms was 27 ± 3 per particle and did not significantly depend on the protein/peptide oxidation status. The longitudinal relaxivity values were 9.9 ± 0.5 and 10.4 ± 0.4 mM−1 s−1 for the GBCA-HDL complexes reconstituted using oxidized apo A-I and a 1:1 mixture of oxidized peptides H4 and H6, respectively. The r1 values for GBCA-HDL containing their unmodified counterparts were not significantly different (10.1 ± 0.2 and 10.1 ± 0.3 mM−1 s−1, respectively), suggesting that oxidation of apo A-I or its peptides does not affect relaxivity properties of GBCA-HDL. We have previously reported that oxidation of apo A-I does not significantly affect its ability to bind lipid or reconstitute into HDL-like particles (38). Consistent with these findings, we did not observe any significant difference in lipid and protein composition between the reconstituted GBCA-HDL particles with unmodified or oxidized apo A-I (data not shown). We have also found that unmodified or oxidized synthetic 22-mer peptides H4 and H6 may substitute apo A-I to accommodate GBCA-HDL without altering the overall lipid and protein composition of the particles (results are not shown).
Figure 2.
Oxidation of apo A-I or synthetic apo A-I peptides does not affect HDL size and size distribution. EM images of paramagnetic and fluorescent HDL either with unmodified (A) and oxidized (B) apo A-I or unmodified (C) and oxidized (D) apo A-I peptides. Similar disc-shaped particles often in characteristic rouleaux (depicted by rectangles and zoomed in the right panels) are found in HDL particles containing apo A-I or synthetic apo A-I peptides in either unmodified or oxidized form. Unmodified apo A-I protein/peptides: black letters on a white background; oxidized apo A-I protein/peptides: white letters on a black background. Abbreviations: apo, apolipoprotein; high density lipoproteins, HDL; Gd, Gd-based contrast agent; Rho-B, fluorescently labeled lipid.
2.2. Oxidation of apo A-I or its peptides enhances in vitro macrophage uptake of paramagnetic and fluorescent HDL
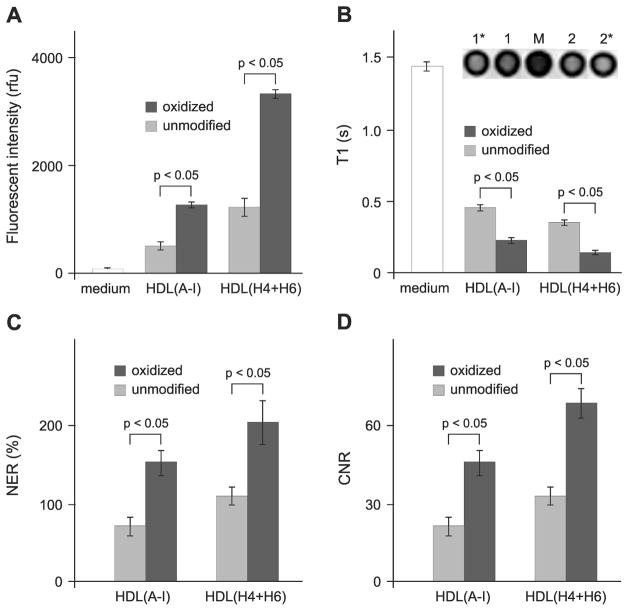
In order to study in vitro macrophage uptake, we tested the paramagnetic and fluorescent HDL formulations containing Gd- and rhodamine B-labeled lipids using the established J774 macrophage line. Fluorescent intensities were measured in J774 cell lysates after a 4 h incubation with the HDL formulations and the values were normalized to cell protein content (Fig. 3A). Macrophage uptake of HDL containing oxidized apo A-I or a 1:1 mixture of oxidized peptides (both H4 and H6) is significantly higher as compared to their unmodified counterparts (Fig. 3A). This data not only suggests that synthetic peptides H4 and H6 can substitute apo A-I for fully functional HDL, but also indicates that oxidation of methionines within the protein/peptide components targets HDL to macrophages. In addition, higher macrophage uptake (p < 0.05) was observed at 4 h incubation for either unmodified or oxidized peptide-containing particles as compared to the corresponding apo A-I-containing particles (Fig. 3A). The observed difference becomes smaller and eventually disappears with increasing incubation times up to 24 h, suggesting different kinetic parameters. The results of in vitro MRI of J774 macrophages incubated with paramagnetic and fluorescent HDL demonstrate that T1 values are significantly lower for macrophages incubated with HDL containing oxidized apo A-I or a 1:1 mixture of oxidized peptides H4 and H6 as compared to cells incubated with their unmodified counterparts (Fig. 3B). Accordingly, the mean normalized enhancement ratios (NER) and contrast-to-noise ratios (CNR) calculated from the corresponding T1-weighted images relative to cells incubated with medium only (Fig. 3C and 3D) are significantly higher for cells incubated with HDL containing oxidized protein/peptide components as compared to their unmodified counterparts. Together with the data from the fluorescence studies, these findings confirm that: 1) specific oxidation of apo A-I significantly improves the in vitro macrophage uptake of HDL and 2) similar to native apo A-I, synthetic peptides H4 and H6 not only assist in the self-assembly of the reconstituted HDL particle, but also can target particles to macrophages upon oxidation of key methionine residues.
Figure 3.
Oxidation of apo A-I or synthetic apo A-I peptides enhances macrophage uptake of paramagnetic and fluorescent HDL in vitro. (A) Mean fluorescence intensities of cell lysates normalized to cell protein content (mean ± SD, n = 3): J774 macrophages were incubated with medium only (white bars) or with 0.05 mM Gd paramagnetic and rhodamine B-labeled HDL(A-I) or HDL(H4+H6) for 4 h at 37°C. (B) Mean T1 values of cell pellets (mean ± SD, n = 3): J774 macrophages were incubated with medium only (white bars) or with medium containing 0.05 mM Gd paramagnetic and Rho B-labeled HDL(A-I) or HDL(H4+H6) for 4 h at 37°C. The inset: exemplary T1-weighted images of cell pellets after incubation with medium only (M), HDL(A-I) with either unmodified (1) or oxidized (1*) apo A-I, and HDL(H4+H6) with either unmodified (2) or oxidized (2*) peptides. (C) Normalized enhancement ratio (NER) of cell pellets: values (mean ± SD, n = 3) are calculated from the corresponding T1-weighted images and are relative to cells incubated with medium only. (D) Contrast-to-noise ratio (CNR) values of cell pellets: values (mean ± SD, n = 3) are calculated from the corresponding T1-weighted images and are relative to cells incubated with medium only. Abbreviations: apo, apolipoprotein; Gd, Gd-based contrast agent; high density lipoproteins, HDL; HDL(A-I), HDL with apo A-I; HDL(H4+H6), HDL with a 1:1 mixture of synthetic peptides H4 and H6 that correspond to apo A-I helixes 4 and 6, respectively. Unmodified and oxidized protein/peptide species are depicted by light and dark gray bars, respectively.
2.3. Oxidation of apo A-I or its peptides enables targeted in vivo imaging of macrophages in aortic atherosclerotic lesions
To evaluate if oxidation of apo A-I or its peptides enables targeted delivery to macrophages in vivo, we utilized apo E KO mice, which are known to develop vulnerable, macrophage-rich atherosclerotic lesions when fed a high fat, high cholesterol Western diet. For a control, we utilized age-matched WT mice that were fed a normal chow diet. After at least 5 months on either a Western diet (apo E KO experimental mice, n = 4) or a normal chow diet (WT control mice, n = 2), mice were injected intravenously with a single dose of paramagnetic and fluorescent HDL containing apo A-I or peptides H4 and H6 in either oxidized or unmodified form at a dose of 0.05 mmol Gd/kg. All injections as well as the MRI procedures were well-tolerated by the animals and no visible side-effects were observed.
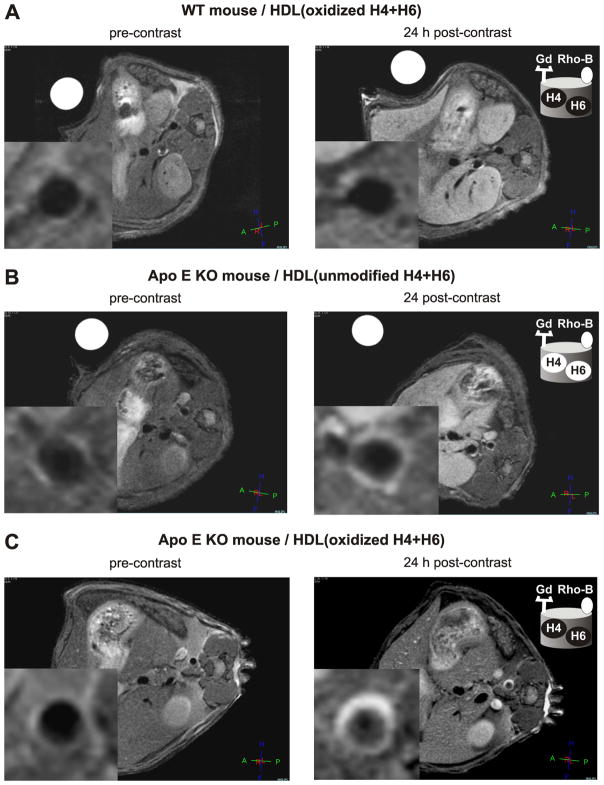
Axial T1-weighted images of the abdominal aorta were taken before as well as 24 h after contrast agent administration and the signal enhancement of the aortic wall was analyzed. Figure 4 shows representative images of aorta of a WT control mouse (Fig. 4A) and an apo E KO mouse (Fig. 4B, C), pre- and post-injection of GBCA-HDL containing a 1:1 mixture of either oxidized (Fig. 4A, C) or unmodified (Fig. 4B) peptides H4 and H6. As expected, no significant signal enhancement of the aortic wall of a WT mouse was observed 24 h following injection of GBCA-HDL with apo A-I or peptides H4 and H6 in either oxidized (Fig. 4A) or unmodified (data not shown) form. In contrast, injection of these agents enhances the signal of the aortic wall of an apo E KO mouse and this enhancement is much more pronounced for GBCA-HDL containing oxidized peptides (Fig. 4C) as compared to their unmodified counterparts (Fig. 4B). In apo E KO mice, the atherosclerotic wall/muscle NER values were 120% and 45% 24 h following the injection of GBCA-HDL with oxidized and unmodified peptides H4 and H6, respectively (Fig. 4C, B; Fig. 5A). Similar NER values of 90% and 35% were observed for particles containing oxidized and unmodified apo A-I, respectively (Fig. 5A). As shown in Figure 5B, the CNR values also were significantly higher 24 h post-injection of particles containing oxidized peptide/protein components as compared to their unmodified counterparts.
Figure 4.
Oxidation of synthetic apo A-I peptides enables targeted in vivo imaging of macrophages in aortic atherosclerotic lesions. Representative axial T1-weighted images of wild type (WT) and apo E knockout (KO) mice, as collected before (left panels) and 24 h after (right panels) contrast agent injection: WT (A) and apo E KO (B, C) mice were administered with the equivalent of 0.05 mmol Gd/kg of paramagnetic and fluorescent HDL containing either oxidized (A, C) or unmodified (B) peptides H4 and H6. The insets: original images were cropped to select the aorta. Abbreviations: apo, apolipoprotein; high density lipoproteins, HDL; HDL(H4+H6), HDL with a 1:1 mixture of synthetic peptides H4 and H6 that correspond to apo A-I helixes 4 and 6, respectively; Gd, Gd-based contrast agent; Rho-B, fluorescently labeled lipid. Unmodified peptides: black letters on a white background; oxidized peptides: white letters on a black background.
Figure 5.
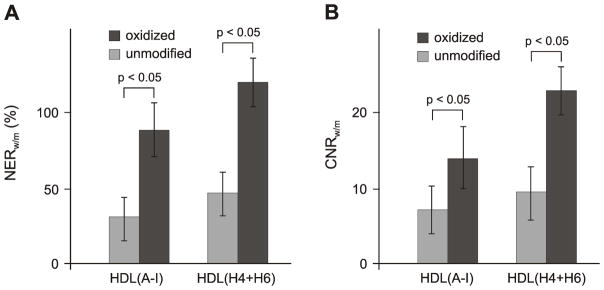
Oxidation of apo A-I or synthetic apo A-I peptides enhances intraplaque macrophage uptake of paramagnetic and fluorescent HDL in vivo. (A) Normalized enhancement ratio (NER) of the apo E KO mouse aortic wall 24 h following injection of the equivalent of 0.05 mmol/kg Gd of paramagnetic and fluorescent HDL(A-I) or HDL(H4+H6): values (mean ± SD, n=4) are calculated from the corresponding T1-weighted images and are relative to muscle. (B) Contrast-to-noise ratio (CNR) of mouse aorta wall 24 h following injection of the equivalent of 0.05 mmol/kg Gd of paramagnetic and fluorescent HDL(A-I) or HDL(H4+H6): values (mean ± SD, n=4) are calculated from the corresponding T1-weighted images and are relative to muscle. Abbreviations: apo, apolipoprotein; Gd, Gd-based contrast agent; high density lipoproteins, HDL; HDL(A-I), HDL with apo A-I; HDL(H4+H6), HDL with a 1:1 mixture of synthetic peptides H4 and H6 that correspond to apo A-I helixes 4 and 6, respectively; KO, knockout. Unmodified and oxidized protein/peptide species are depicted by light and dark gray bars, respectively.
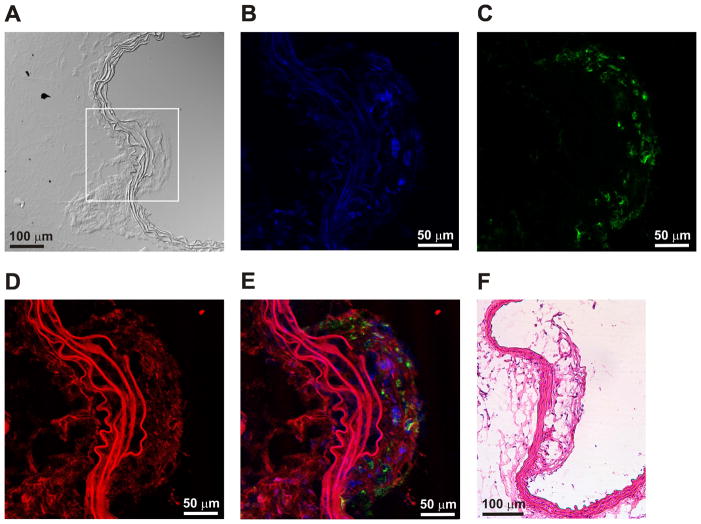
In order to confirm that specific oxidation of apo A-I or synthetic peptides H4 and H6 targets HDL complexes to macrophages, WT and apo E KO mice were sacrificed after the 24 h post-contrast MRI. Aortas were excised from both WT and apo E KO mice and aortic sections were prepared for further optical and histological studies. Illustrated in Figure 6 is a representative confocal microscopy image of sizeable areas of aortic plaques from animals injected with paramagnetic and fluorescent HDL containing a 1:1 mixture of oxidized peptides H4 and H6. The confocal images convincingly reveal the colocalization of rhodamine B-labeled GBCA-HDL (red) with intraplaque macrophages as detected by immunohistochemical staining for a human macrophage-associated antigen CD68 (green). No GBCA-HDL particles were found in the aortas of WT mice (not shown).
Figure 6.
Paramagnetic and fluorescent HDL with oxidized peptides H4 and H6 colocalize with macrophages in an atherosclerotic plaque. (A) Differential interference contrast (DIC) microscopy image of an aorta section from an apo E KO mouse 24 h following injection of the equivalent of 0.05 mmol/kg Gd of paramagnetic and fluorescent HDL containing a 1:1 mixture of oxidized peptides H4 and H6. Confocal laser scanning microscopy of a zoomed-in area (depicted by rectangle) of the section shown in (A) and stained with DAPI (blue) (B) and an antibody against a macrophage surface marker, CD68, (green) (C) or analyzed in the rhodamine channel (red) for GBCA-HDL uptake (D). The merged image (E) demonstrates uptake of the rhodamine B-labeled HDL formulations by intraplaque macrophages. The autofluorescent elastic lamina is observed as multiple wave-like structures and is disrupted in an atherosclerotic lesion. (F) Hematoxylin and eosin (H&E) staining of the matched histopathological aorta section. Abbreviations: apo, apolipoprotein; Gd, Gd-based contrast agent; high density lipoproteins, HDL; KO, knockout.
Together, these findings strongly support the hypothesis that a naturally occurring oxidative modification of apo A-I in synthetic HDL may serve as a natural way to deliver the incorporated GBCA to macrophages of atherosclerotic plaques, eliminating the need for macrophage-targeting moieties. The data also suggest that oxidized synthetic 22-mer peptides that correspond to amphipathic apo A-I helixes 4 and 6 are able to functionally replace native apo A-I in GBCA-HDL, thereby significantly simplifying the nanoparticle preparation and encouraging the further development of these contrast agents for atherosclerotic plaque detection.
3. DISCUSSION
During recent years, there has been a dramatic increase in academic and industrial research efforts in the field of lipoprotein-like formulations, primarily with the aim of developing enhanced delivery vehicles for therapeutics and diagnostics (21–22,39–44). While the use of native HDL as a delivery vehicle is impractical, synthetic HDL reconstituted from lipids and apolipoproteins in vitro (20) have several competitive advantages as compared with other delivery platforms including plaque-targeting MRI contrast agents (45–46): 1) apo A-I, the major HDL protein, is an endogenous protein and does not trigger immunoreactions, 2) the small size (8–12 nm) allows HDL to enter and accumulate in the plaque, and 3) a variety of drugs and imaging agents can be incorporated into this platform (40,42,44,47).
In the human body, HDL transport cholesterol from the peripheral tissues to the liver for catabolism into bile acids (19). Thus, native HDL that contain unmodified lipids and apolipoproteins are not recognized by macrophage scavenger receptors (48). Similar to native HDL, synthetic HDL particles also possess the intrinsic property to target the liver in vivo and are not normally uptaken by macrophages. As such, synthetic HDL particles have been recently suggested as a delivery vehicle for targeted MRI of the liver (21). Thus, with respect to macrophage-targeted delivery of drugs and imaging agents, there is the need to target HDL to macrophages using targeting moieties, an example of such is the apo E-derived lipopeptide (22).
The results of the present study demonstrate for the first time that a naturally occurring modification of apo A-I converts HDL into a substrate for macrophages. Importantly, this represents a natural way to target the incorporated drugs and/or imaging agents to macrophages in vivo. Synthetic HDL particles containing either oxidized or unmodified apo A-I were successfully loaded with Gd- and rhodamine B-labeled lipids and subsequently purified and characterized using a variety of biophysical procedures. The purified particles were comparatively studied for in vitro macrophage uptake and for in vivo detection of macrophage-rich atherosclerotic plaques in apo E KO mice fed on a Western diet for at least 5 months. In the in vitro studies, after incubating the J774 macrophages with the contrast agents for 4 h, GBCA-HDL with oxidized apo A-I showed substantially higher macrophage uptake relative to its unmodified counterpart as detected by rhodamine B fluorescence intensities of cell lysates and the T1 values estimated from the T1-weighted images of cell pellets. In this study, we used high-purity apo A-I preparations isolated from human serum and characterized these preparations using a variety of techniques including analytical reversed-phase high performance liquid chromatography (RP-HPLC), mass spectrometry (MS), and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described previously (34,38,49). While we observed some macrophage uptake of HDL particles with unmodified protein in vitro, this may be explained by the activity of myeloperoxidase (MPO), which is secreted by macrophages and neutrophils and may cause oxidative damage to apo A-I (50–51). As hypothesized, MRI images of the diseased aortic wall in apo E KO mice taken 24 h after administration reveal an increased enhancement in the abdominal aorta (NER = 90%) for particles with oxidized apo A-I as compared to its unmodified counterpart, which shows some but not significant enhancement of the aortic wall (NER = 35%). As suggested by the confocal microscopy and the in vitro macrophage uptake results, the enhanced MR signal of macrophage-rich atherosclerotic plaques observed in apo E KO mice can result from the increased uptake of these particles by intraplaque macrophages. Consistent with the in vitro studies, some enhancement of the diseased aorta was observed for GBCA-HDL with unmodified apo A-I, which may be explained by the partial oxidation of apo A-I by MPO expressed in macrophage-rich atherosclerotic lesions (51–52).
The findings of the current study provide a molecular explanation to clarify the sometimes confusing and contradictory literature surrounding the question of whether GBCA-HDL containing no additional targeting moieties can be specifically targeted to macrophages and, consequently, whether there is the need for these moieties to target HDL to macrophages (22,26,53). In these in vivo MRI studies of apo E KO mice, the mean atherosclerotic wall/muscle NER values reported for seemingly similar GBCA-HDL were 35% (26), 53% (22), and 91% (53), respectively. Together with the data reported for non-specific Gd-containing micelles, where the mean NER value was 34% (54) and was not significantly different from the pre-contrast level, this raises a concern about macrophage specificity of GBCA-HDL containing unmodified lipid and protein components. In addition, the high variation in the NER of the diseased aortic wall observed for GBCA-HDL (22,26,53) suggests differences in the nature of the particles used or in the molecular mechanisms of macrophage uptake. The data of the current study suggest that macrophage specificity of the reported GBCA-HDL reconstituted using apo A-I purified from human serum (22,26,53) can be provided by oxidized apo A-I, the content of which in fresh plasma samples exhibits considerable inter-individual variability (33–34,55).
With respect to diagnostics, human apo A-I is a large protein, which is purified from human plasma. Thus, in addition to the immense monetary cost, the further development of apo A-I-containing imaging agents would require a number of safety precautions followed by a complicated translation into clinical practice. In this study, we evaluated whether synthetic 22-mer peptides that correspond to apo A-I helixes 4 and 6 containing Met-112 and Met-148, respectively, can be used in place of native apo A-I to assist in the self-assembly of the fully functional Gd-containing HDL particle, and when oxidized, target this particle to macrophages. The comparative studies demonstrated that a 1:1 mixture of these peptides successfully mimics native apo A-I with respect to their ability to build HDL particles. The in vitro macrophage uptake results combined with the in vivo MRI studies on the detection of atherosclerotic plaques in apo E KO mice as well as confocal microscopy of the diseased aorta sections convincingly show that when oxidized, these peptides effectively target paramagnetic and fluorescent HDL to macrophages.
4. CONCLUSIONS
This study clearly demonstrates that a naturally occurring oxidative modification of apo A-I in reconstituted HDL targets the incorporated Gd-based MRI contrast agent to macrophages in vitro and to macrophage-rich atherosclerotic plaques in apo E KO mice in vivo, eliminating the need for targeting moieties. Importantly, native apo A-I in these HDL-like particles can be functionally replaced by synthetic peptides that correspond to amphipathic apo A-I helixes 4 and 6, which contain methionine residues Met-112 and Met-148. Our results strongly encourage the further development of these macrophage-specific contrast agents with broad application in cardiology, oncology and other diseases, where macrophage imaging has important diagnostic and prognostic value. Examples include the detection of macrophage-rich vulnerable plaques in atherosclerosis and evaluation of tumor-associated macrophages in cancer.
5. EXPERIMENTAL PROCEDURES
5.1. Chemicals, Lipids and Cells
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DMPG), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (rhodamine-PE, Rho B-PE) and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (gadolinium salt) (14:0 PE-DTPA-Gd) were purchased from Avanti Polar Lipids (Alabaster, AL). Sodium cholate, hydrogen peroxide and other chemicals were purchased from Sigma Chemical Company (St. Louis, MO). The murine macrophage cell line J774A.1 was obtained from the American Type Culture Collection (ATCC, Manassas, VA).
5.2. Peptides and Proteins
Synthetic peptides were ordered from New England Peptide (Gardner, MA). Apolipoprotein (apo) A-I was purified from human serum as previously described (49).
Unmodified apo A-I protein was isolated from the initial preparation by preparative RP-HPLC as described (34). Protein composition of peaks in the HPLC profile was determined by SDS-PAGE (12.5% acrylamide) using the standard Laemmli system (56) as well as by analytical HPLC as previously described (34). Oxidized apo A-I containing sulfoxides at methionines 112 and 148 was prepared, purified and characterized as described previously (34). The same procedure was used to oxidize methionine residues in synthetic peptides H4 and H6 that correspond to apo A-I helixes 4 and 6, respectively.
5.3. Preparation and Characterization of Paramagnetic and Fluorescent Lipoproteins
The discoidal GBCA-HDL complexes were synthesized essentially as described (22,38,44) except that no dialysis was undertaken. The molar ratio was 1:65:25:38:2 for apo A-I:DMPC:DMPG: PE-DTPA-Gd:rhodamine B-PE. Briefly, 14:0 PE-DTPA-Gd, DMPC, and DMPG in organic solvents were mixed, dried in a stream of argon, and placed under vacuum for 8 h. Amount of 14:0 PE-DTPA-Gd was controllably varied in different preparations. To synthesize fluorescently labeled nanoparticles, rhodamine B-PE in chloroform was also added to a lipid mixture. Then, lipid films were dispersed in phosphate-buffered saline (PBS), pH 7.4 and sonicated for 5 min. After incubation for 30 min at 30°C, solution containing either oxidized or unmodified apo A-I in PBS, pH 7.4 was added and the mixture was incubated at 30°C for 3 h. The obtained GBCA-HDL particles were then purified on a calibrated Superdex 200 HR gel filtration column (GE Healthcare Biosciences, Pittsburgh, PA) using the BioCAD 700E Workstation (Applied Biosystems, Carlsbad, CA). The same procedure was used to prepare GBCA-HDL with a 1:1 mixture of peptides H4 and H6 in either oxidized or unmodified form.
The obtained GBCA-HDL particles were characterized by analytical RP-HPLC and non-denaturing gel electrophoresis as described previously (38). Protein concentrations in the GBCA-HDL particles were measured using the Lowry method as modified by Markwell et al. (57). Final protein compositions were determined in the prepared particles by analytical RP-HPLC as previously described (38). Gd concentrations were analyzed by ICP-MS. Total cholesterol was determined enzymatically using a Boehringer-Mannheim kit and the manufacturer’s suggested procedure. Phospholipids were determined by a phosphorus assay (58). Relaxation time of the synthesized GBCA-HDL complexes, T1, was determined at 3 T using a Philips Achieva 3.0T X-series Quasar wholebody MR system (Philips Medical System, Netherlands). The longitudinal relaxivity, r1, was calculated based on r1 (mM−1 s−1) ) (1/T1s - 1/T1c)/C, where T1s are relaxation times with contrast agent and T1c are relaxation times without contrast agent. C is the Gd concentration in mM.
The mean size of the particles was determined using electron microscopy (EM) essentially as described (38). Briefly, the GBCA-HDL complexes (at a concentration of about 0.3 mg of protein/ml) were extensively dialyzed against 5 mM ammonium bicarbonate, mixed with the same volume of 2% phosphotungstate, pH 7.4, and examined using a FEI Tecnai 12 Spirit BioTwin transmission electron microscope (FEI Company, Hillsboro, OR) at 80 KV accelerating voltage on carbon-coated Formvar grids. Microphotographs were photographed at an instrument magnification of 87000× and 92000×, and mean particle dimensions of 50 particles were determined from each negative.
5.4. Macrophage Uptake of Paramagnetic and Fluorescent Lipoproteins in vitro
In vitro experiments for quantifying macrophage uptake of the paramagnetic and fluorescent HDL complexes were performed essentially as reported (22,59). Briefly, the BALB/c murine macrophage J774A.1 cells (ATCC TIB-67) were cultured at 37°C with 5% CO2 in DMEM (Cellgro, Mediatech Inc, Manassas, VA) with 2mM glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 10% fetal bovine serum (FBS) (Cellgro, Mediatech Inc, Manassas, VA) and grown to approximately 90% of confluence in 6-well tissue culture plates (Corning, Tewksbury, MA). Cells were incubated for varied time periods from 4 to 24 h at 37°C with GBCA-HDL nanoparticles containing apo A-I or peptides H4 and H6 in either oxidized or unmodified form at a concentration of 0.01 or 0.05 mM Gd. After incubation, cells were washed twice with PBS and lysed using Promega passive lysis buffer (Promega, Madison, WI). The rhodamine B fluorescence was measured in the lysates with a 540 nm excitation and a 590 nm emission filters using the Gemini EM fluorescence microplate reader (Molecular Devices, Sunnyvale, CA). The protein concentration in the lysates was determined using the Bradford reagent (Bio-Rad, Richmond, CA) and the SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA).
MRI studies of cell pellets were performed essentially as reported (22). Briefly, after incubation with GBCA-HDL containing apo A-I or peptides H4 and H6 in either oxidized or unmodified form at 37°C for 4 h at concentration of 0.05 mM Gd, approximately 106 J774 macrophages were washed twice with PBS, collected in 15 ml BD Falcon tubes (BD Biosciences, San Jose, CA), and washed twice in PBS by centrifugation. Then, 4% paraformaldehyde solution was added to a final volume of 200 ul. The cells were dispersed, transferred into yellow 200 μl pipette tips with sealed ends and left overnight to form loosely packed cell pellets. The tips containing cell pellets were placed in a custom-made sample holder, capable of carrying five tips. Then, high resolution T1-weighted images were generated using a Philips Achieva 3.0T X-series Quasar system and a spin echo (SE) sequence (TR/TE = 600 ms/14 ms, FOV = 3.0 X 3.0 cm, matrix size 256 X 256, slice thickness = 0.5 mm, number of averages = 8).
The obtained images were analyzed using Image J software (National Institutes of Health, Bethesda, MD). The contrast-to-noise ratio (CNR) was defined as CNR = (Itreatment - Icontrol)/Inoise, where Itreatment is the signal intensity of the cell pellet after incubation with GBCA-HDL, Icontrol is the signal intensity of the cell pellet incubated with medium only and Inoise is the standard deviation outside the cell pellet. The normalized enhancement ratio (NER) was calculated as NER = [(Itreatment - Icontrol)/Icontrol] × 100%.
5.5. Animal MRI studies
Animal MRI studies were performed using male WT C57BL/6J mice and male apo E KO B6.129P2-Apoetm1Unc/J mice from the Jackson Laboratory (Bar Harbor, ME) essentially as published (22,59). The animal protocols and procedures were approved by the University of Massachusetts Medical School Animal Care and Use Committee. Four groups of WT mice (n = 2 per group) and four groups of the apo E KO animals (n = 4 per group) were fed either a normal chow diet (WT mice) or a Western diet (21% fat and 0.15% cholesterol; apo E KO mice), for a period of at least 5 months. Before and 24 h after tail vein administration of the equivalent of 0.05 mmol Gd/kg of GBCA-HDL, MRI of the abdominal aorta was performed using a Philips Achieva 3.0T X-series Quasar system. During MRI, mice were positioned in a custom-made solenoid transmit-receive (T/R) coil and anesthetized with a 4% isoflurane-O2 gas mixture (400 cm3/min initial dose) and maintained with a 1.5% isoflurane-O2 gas mixture (100 cm3/min maintenance dose) delivered through a nose cone. During the examination, the respiratory rate was monitored using a respiratory sensor connected to a monitoring and gating system (Model 1025, SA Instruments, Inc., Stony Brook, NY, USA) and placed on the abdomen. When high resolution T1-weighted multi-slice SE images (TR/TE=600 ms/14 ms, FOV = 3.0 X 3.0 cm, matrix size 256 X 256, 22 contiguous 0.5 mm-thick axial slices, number of averages = 8, total scan time = 41 min) were acquired, the slices were matched at each time point to the baseline pre-contrast scan.
The obtained images were analyzed using Image J software (National Institutes of Health, Bethesda, MD). Regions of interest (ROI) containing tissue directly surrounding the vessel lumen were drawn in the aortic vessel wall, in surrounding muscle tissue and outside each mouse to calculate the corresponding average signal intensities and noise level (Iw, Im and Inoise, respectively). The contrast-to-noise ratio between the wall and adjacent muscle (CNR) was determined according to CNR = (Iw - Im)/Inoise. The mean normalized enhancement ratio of aortic wall (NER) relative to surrounding muscle was defined as NER = {[(Iw/Im)post/(Iw/Im)pre]/Inoise × 100%. The mean ± SD of NER for apo E KO mice (n = 4 per test group) and WT mice (n = 2 per control group) were calculated by analysis of aortic MR images of the matched (pre- and post-injection) slices for each mouse.
5.6. Histology and Microscopy
After MRI experiments, abdominal aortas matching the MR imaging area were removed, embedded in Tissue Tek (Miles Scientific, Naperville, IL), frozen and cut into 8 μm-thick serial sections perpendicular to the vessel direction. For immunohistochemistry, slides were fixed in ice-cold acetone for 10 min and air dried. Macrophages were detected by Alexa Fluor 647-labeled rat anti-mouse CD68 antibodies (AbD Serotec, Raleigh, NC). After immunostaining, sections were washed twice in PBS and once in distilled water, and mounted in Prolong Gold antifade mounting medium with 4′,6-diamino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA). Confocal imaging was performed using a Leica TCS SP5 II laser scanning confocal microscope (Leica Microsystems, Mannheim/Wetzlar, Germany) with Acousto-Optical Beam Splitter (AOBS) equipped with hybrid detectors (HyD), a detector for transmitted light including differential interference contrast (DIC) and 8 laser lines at 405, 458, 476, 488, 496, 514, 561, and 633 nm. Images were acquired with a 20X multi-immersion objective lens, HyD detectors, a 514 laser for Rhodamine B, a 633 nm laser for Alexa Fluor 647 and DIC, and a 405 nm laser for DAPI. Another set of sections was stained with hematoxylin and eosin and examined by Olympus BHTU microscope (Olympus, Hyde Park, NY).
5.7. Statistical Analysis
Data are presented as the means ± standard deviation. Statistical analysis was performed using GraphPad Prizm 4.0 ANOVA for computations within and between groups. Results were considered statistically significant at p < 0.05.
Acknowledgments
We are grateful to the Advanced Magnetic Resonance Imaging Center of the University of Massachusetts Medical School for imaging experiments. We also owe a debt of gratitude to Dr. Shaokuan Zheng, who did an excellent job conducting imaging studies, for his important expertise, experience, and skills and for numerous valuable discussions. Animal handling was performed at the Department of Animal Medicine of the University of Massachusetts Medical School. Transmission electron microscopy was performed at the Core Electron Microscopy Facility of the University of Massachusetts Medical School. Histological and confocal studies were performed at the DERC Morphology Core Facility and the Cell Biology Confocal Core Facility of the University of Massachusetts Medical School, respectively. We thank Drs. Gregory Hendricks, Jeffrey Nickerson, Jean Underwood, and Yu Liu for helpful discussions of results.
This study was supported in part by a grant R43HL110417 (Alexander B. Sigalov, principal investigator) from the National Heart, Lung, and Blood Institute/National Institutes of Health.
References
- 1.Michaud CM, Murray CJ, Bloom BR. Burden of disease--implications for future research. Jama. 2001;285(5):535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Shah PK. Pathophysiology of coronary thrombosis: role of plaque rupture and plaque erosion. Prog Cardiovasc Dis. 2002;44(5):357–368. doi: 10.1053/pcad.2002.123473. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia V, Bhatia R, Dhindsa S, Virk A. Vulnerable plaques, inflammation and newer imaging modalities. J Postgrad Med. 2003;49(4):361–368. [PubMed] [Google Scholar]
- 4.Sharif F, Murphy RT. Current status of vulnerable plaque detection. Catheter Cardiovasc Interv. 2010;75(1):135–144. doi: 10.1002/ccd.22164. [DOI] [PubMed] [Google Scholar]
- 5.Schaar JA, Mastik F, Regar E, den Uil CA, Gijsen FJ, Wentzel JJ, Serruys PW, van der Stehen AF. Current diagnostic modalities for vulnerable plaque detection. Curr Pharm Des. 2007;13(10):995–1001. doi: 10.2174/138161207780487511. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2(6):309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- 7.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 9.Wakhloo AK, Lieber BB, Seong J, Sadasivan C, Gounis MJ, Miskolczi L, Sandhu JS. Hemodynamics of carotid artery atherosclerotic occlusive disease. J Vasc Interv Radiol. 2004;15(1 Pt 2):S111–121. doi: 10.1097/01.rvi.0000109204.16955.84. [DOI] [PubMed] [Google Scholar]
- 10.MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, Shishkov M, Kauffman CR, Houser SL, Aretz HT, DeJoseph D, Halpern EF, Tearney GJ. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004;44(5):972–979. doi: 10.1016/j.jacc.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 11.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture Circulation. 1994;90(2):775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 12.Davies JR, Rudd JH, Weissberg PL. Molecular and metabolic imaging of atherosclerosis. J Nucl Med. 2004;45(11):1898–1907. [PubMed] [Google Scholar]
- 13.Rudd JH, Hyafil F, Fayad ZA. Inflammation imaging in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(7):1009–1016. doi: 10.1161/ATVBAHA.108.165563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 15.Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009;29(7):983–991. doi: 10.1161/ATVBAHA.108.165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutz AM, Seemayer C, Corot C, Gay RE, Goepfert K, Michel BA, Marincek B, Gay S, Weishaupt D. Detection of synovial macrophages in an experimental rabbit model of antigen-induced arthritis: ultrasmall superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2004;233(1):149–157. doi: 10.1148/radiol.2331031402. [DOI] [PubMed] [Google Scholar]
- 17.Collins DJ, Padhani AR. Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges. IEEE Eng Med Biol Mag. 2004;23(5):65–83. doi: 10.1109/memb.2004.1360410. [DOI] [PubMed] [Google Scholar]
- 18.Veldhuis WB, Floris S, van der Meide PH, Vos IM, de Vries HE, Dijkstra CD, Bar PR, Nicolay K. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. J Cereb Blood Flow Metab. 2003;23(9):1060–1069. doi: 10.1097/01.WCB.0000080701.47016.24. [DOI] [PubMed] [Google Scholar]
- 19.Rothblat GH, Mahlberg FH, Johnson WJ, Phillips MC. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J Lipid Res. 1992;33(8):1091–1097. [PubMed] [Google Scholar]
- 20.Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 21.Rui M, Guo W, Ding Q, Wei X, Xu J, Xu Y. Recombinant high-density lipoprotein nanoparticles containing gadolinium-labeled cholesterol for morphologic and functional magnetic resonance imaging of the liver. Int J Nanomedicine. 2012;7:3751–3768. doi: 10.2147/IJN.S33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Vucic E, Leupold E, Mulder WJ, Cormode DP, Briley-Saebo KC, Barazza A, Fisher EA, Dathe M, Fayad ZA. Incorporation of an apoE-derived lipopeptide in high-density lipoprotein MRI contrast agents for enhanced imaging of macrophages in atherosclerosis. Contrast Media Mol Imaging. 2008;3(6):233–242. doi: 10.1002/cmmi.257. [DOI] [PubMed] [Google Scholar]
- 23.Cormode DP, Briley-Saebo KC, Mulder WJ, Aguinaldo JG, Barazza A, Ma Y, Fisher EA, Fayad ZA. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4(9):1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 24.Frias JC, Lipinski MJ, Lipinski SE, Albelda MT. Modified lipoproteins as contrast agents for imaging of atherosclerosis. Contrast Media Mol Imaging. 2007;2(1):16–23. doi: 10.1002/cmmi.124. [DOI] [PubMed] [Google Scholar]
- 25.Frias JC, Ma Y, Williams KJ, Fayad ZA, Fisher EA. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano Lett. 2006;6(10):2220–2224. doi: 10.1021/nl061498r. [DOI] [PubMed] [Google Scholar]
- 26.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126(50):16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 27.Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JG, Massey D, Fuster V, Vetrovec GW, Fayad ZA. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2(5):637–647. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulder WJ, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JG, Vucic E, Amirbekian V, Tang C, Chin PT, Nicolay K, Fayad ZA. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58(6):1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 29.Sauer I, Nikolenko H, Keller S, Abu Ajaj K, Bienert M, Dathe M. Dipalmitoylation of a cellular uptake-mediating apolipoprotein E-derived peptide as a promising modification for stable anchorage in liposomal drug carriers. Biochim Biophys Acta. 2006;1758(4):552–561. doi: 10.1016/j.bbamem.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 30.Wang XS, Gruenstein E. Rapid elevation of neuronal cytoplasmic calcium by apolipoprotein E peptide. J Cell Physiol. 1997;173(1):73–83. doi: 10.1002/(SICI)1097-4652(199710)173:1<73::AID-JCP9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Tolar M, Keller JN, Chan S, Mattson MP, Marques MA, Crutcher KA. Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. J Neurosci. 1999;19(16):7100–7110. doi: 10.1523/JNEUROSCI.19-16-07100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anantharamaiah GM, Hughes TA, Iqbal M, Gawish A, Neame PJ, Medley MF, Segrest JP. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J Lipid Res. 1988;29(3):309–318. [PubMed] [Google Scholar]
- 33.von Eckardstein A, Walter M, Holz H, Benninghoven A, Assmann G. Site-specific methionine sulfoxide formation is the structural basis of chromatographic heterogeneity of apolipoproteins A-I, C-II, and C-III. J Lipid Res. 1991;32(9):1465–1476. [PubMed] [Google Scholar]
- 34.Sigalov AB, Stern LJ. Enzymatic repair of oxidative damage to human apolipoprotein A-I. FEBS Lett. 1998;433(3):196–200. doi: 10.1016/s0014-5793(98)00908-9. [DOI] [PubMed] [Google Scholar]
- 35.Sigalov AB, Petrichenko IE, Kolpakova GV. The ratio of non-oxidized/oxidized forms of apolipoprotein A-I can affect cholesterol efflux from human skin fibroblasts mediated by high density lipoprotein. Eur J Clin Chem Clin Biochem. 1997;35(5):395–396. [PubMed] [Google Scholar]
- 36.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105(34):12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pankhurst G, Wang XL, Wilcken DE, Baernthaler G, Panzenbock U, Raftery M, Stocker R. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J Lipid Res. 2003;44(2):349–355. doi: 10.1194/jlr.M200256-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Sigalov AB, Stern LJ. Oxidation of methionine residues affects the structure and stability of apolipoprotein A-I in reconstituted high density lipoprotein particles. Chem Phys Lipids. 2001;113(1–2):133–146. doi: 10.1016/s0009-3084(01)00186-4. [DOI] [PubMed] [Google Scholar]
- 39.Zhang WL, Gu X, Bai H, Yang RH, Dong CD, Liu JP. Nanostructured lipid carriers constituted from high-density lipoprotein components for delivery of a lipophilic cardiovascular drug. Int J Pharm. 2010;391(1–2):313–321. doi: 10.1016/j.ijpharm.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 40.Skajaa T, Cormode DP, Falk E, Mulder WJ, Fisher EA, Fayad ZA. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(2):169–176. doi: 10.1161/ATVBAHA.108.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glickson JD, Lund-Katz S, Zhou R, Choi H, Chen IW, Li H, Corbin I, Popov AV, Cao W, Song L, Qi C, Marotta D, Nelson DS, Chen J, Chance B, Zheng G. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Mol Imaging. 2008;7(2):101–110. [PubMed] [Google Scholar]
- 42.Lacko AG, Nair M, Prokai L, McConathy WJ. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin Drug Deliv. 2007;4(6):665–675. doi: 10.1517/17425247.4.6.665. [DOI] [PubMed] [Google Scholar]
- 43.Shahzad MM, Mangala LS, Han HD, Lu C, Bottsford-Miller J, Nishimura M, Mora EM, Lee JW, Stone RL, Pecot CV, Thanapprapasr D, Roh JW, Gaur P, Nair MP, Park YY, Sabnis N, Deavers MT, Lee JS, Ellis LM, Lopez-Berestein G, McConathy WJ, Prokai L, Lacko AG, Sood AK. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oda MN, Hargreaves PL, Beckstead JA, Redmond KA, van Antwerpen R, Ryan RO. Reconstituted high density lipoprotein enriched with the polyene antibiotic amphotericin B. J Lipid Res. 2006;47(2):260–267. doi: 10.1194/jlr.D500033-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005;36(12):2764–2772. doi: 10.1161/01.STR.0000190895.51934.43. [DOI] [PubMed] [Google Scholar]
- 46.Sinusas AJ, Bengel F, Nahrendorf M, Epstein FH, Wu JC, Villanueva FS, Zahi A, Fayad ZA, Robert J, Gropler RJ. Multimodality Cardiovascular Molecular Imaging, Part I. Circulation: Cardiovascular Imaging. 2008;1(3):244–256. doi: 10.1161/CIRCIMAGING.108.824359. [DOI] [PubMed] [Google Scholar]
- 47.Pan D, Caruthers SD, Chen J, Winter PM, Senpan A, Schmieder AH, Wickline SA, Lanza GM. Nanomedicine strategies for molecular targets with MRI and optical imaging. Future Med Chem. 2010;2(3):471–490. doi: 10.4155/fmc.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional?- The mouse’s tale. J Clin Invest. 2001;108(5):649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigalov A, Alexandrovich O, Strizevskaya E. Large-scale isolation and purification of human apolipoproteins A-I and A-II. J Chromatogr. 1991;537(1–2):464–468. doi: 10.1016/s0021-9673(01)88920-2. [DOI] [PubMed] [Google Scholar]
- 50.Bergt C, Marsche G, Panzenboeck U, Heinecke JW, Malle E, Sattler W. Human neutrophils employ the myeloperoxidase/hydrogen peroxide/chloride system to oxidatively damage apolipoprotein A-I. Eur J Biochem. 2001;268(12):3523–3531. doi: 10.1046/j.1432-1327.2001.02253.x. [DOI] [PubMed] [Google Scholar]
- 51.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol. 2010;23(3):447–454. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94(1):437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W, Cormode DP, Vengrenyuk Y, Herranz B, Felg JE, Klink A, Mulder WJ, Fisher EA, Fayad ZA. Collagen-Specific Peptide Conjugated HDL Nanoparticles as MRI Contrast Agent to Evaluate Compositional Changes in Atherosclerotic Plaque Regression JACC: Cardiovascular Imaging. 2013;6(3):373–384. doi: 10.1016/j.jcmg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, Fisher EA, Fayad ZA. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104(3):961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakano T, Nagata A. Immunochemical detection of circulating oxidized high-density lipoprotein with antioxidized apolipoprotein A-I monoclonal antibody. J Lab Clin Med. 2003;141(6):378–384. doi: 10.1016/S0022-2143(03)00026-X. [DOI] [PubMed] [Google Scholar]
- 56.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 57.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 58.Van Veldhoven PP, Mannaerts GP. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987;161(1):45–48. doi: 10.1016/0003-2697(87)90649-x. [DOI] [PubMed] [Google Scholar]
- 59.Chen W, Jarzyna PA, van Tilborg GA, Nguyen VA, Cormode DP, Klink A, Griffioen AW, Randolph GJ, Fisher EA, Mulder WJ, Fayad ZA. RGD peptide functionalized and reconstituted high-density lipoprotein nanoparticles as a versatile and multimodal tumor targeting molecular imaging probe. Faseb J. 2010;24(6):1689–1699. doi: 10.1096/fj.09-139865. [DOI] [PMC free article] [PubMed] [Google Scholar]