Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the presence of pathogenic IgG anti-nuclear antibodies. Pathogenic IgG autoantibody production requires B-cell activation, leading to the production of activation-induced deaminase (AID) and class switching of IgM genes to IgG. To understand how and when B cells are activated to produce these IgG autoantibodies, we studied cells from 564Igi, a mouse model of SLE. 564Igi mice develop a disease profile closely resembling that found in human SLE patients, including the presence of IgG anti-nucleic acid antibodies. We have generated 564Igi mice that conditionally express an activation-induced cytidine deaminase transgene (Aicdatg), either in all B cells or only in mature B cells. Here we show that class-switched pathogenic IgG autoantibodies were produced only in 564Igi mice in which AID was functional in early developing B cells, resulting in loss of tolerance. Furthermore, we show that the absence of AID in early developing B cells also results in increased production of self-reactive IgM, indicating that AID, through somatic hypermutation (SHM), contributes to tolerance. Our results suggest that the pathophysiology of clinical SLE might also be dependent on AID expression in early developing B cells.
Keywords: Systemic lupus erythemathosus, pathogenic autoantibodies generated from early B cells, dual role of AID in early B cell tolerance, conditional expression of AID, AID in receptor editing
Introduction
Patients with systemic lupus erythematosus (SLE) have circulating, pathogenic anti-nucleic acid antibodies (Abs) [1] that combine with self-antigen to form immune complexes (ICs) that are deposited in tissues causing inflammation and damage [2, 3]. In healthy individuals, self-reactive B cells are eliminated through several mechanisms, including deletion, anergy, and receptor editing [4–6]. In SLE, mechanisms of tolerance are somehow disturbed or avoided, allowing for the production and secretion of pathogenic autoantibodies. As anti-nuclear IgM is not pathogenic, SLE is dependent on the class-switching of autoantibody genes from IgM to IgG [7]. IgG autoantibody production requires the activation of B cells, leading to the expression of activation-induced cytidine deaminase (Aicda) [8, 9], which is necessary for class switch recombination (CSR) of IgM genes to other isotypes [9]. Activation-induced deaminase (AID) is also required for somatic hypermutation (SHM) [8], and in birds for somatic gene conversion [10] [11]. [11–13]CSR, SHM and gene conversion diversify the antibody repertoire and are crucial for affinity maturation, which leads to an enhanced, highly specific, and normal adaptive immune response [14, 15]. However, under what conditions and at which point during B-cell development CSR is necessary for the generation of pathogenic autoantibodies has not been established.
AID was originally shown to be expressed in germinal centers [9]. Thus, it is largely believed that the class-switching required for the generation of IgG, including pathogenic IgG autoantibodies, is a process that occurs only in mature B cells within peripheral germinal centers [16] [17). However, we and others have found that Aicda is also expressed early during B- cell development (pre-B cells and immature B cells) [18–23] Furthermore the activation of immature B cells requires BCR/TLR engagement and is T cell-independent [20].This raises the possibility that Aicda expression in pre-B and immature B cells may play a necessary role in the genesis of class-switched, self-reactive and pathogenic antibodies. We investigated this possibility using the 564Igi mouse model of SLE
564Igi mice have rearranged immunoglobulin (Ig) heavy (H) and light (L) chain genes derived from an autoreactive pathogenic hybridoma (fused from an autoimmune SWR X NZB F1 mouse) introduced into the IgH and IgL loci of a C57BL/6 mouse (Supporting Information Fig. 1)[24]. The 564 antibody has a characteristic idiotype (Id), and B cells carrying the corresponding B-cell antigen receptor (BCR) are Id+. In anti-nuclear antibody (ANA) assays, serum antibodies from 564Igi mice bind to nucleoli of HEp-2 cells [24] (Supporting Information Fig. 1), suggesting that the recognized self-antigens are RNA or RNA-associated nuclear antigens. The rearranged 564 IgH gene was introduced into the endogenous joining (JH) region, allowing 564 Cμ to switch to any isotype. Thus, even on the non-autoimmune C57BL/6 background, class-switched, pathogenic, Id+, anti-RNA Abs are produced and lead to glomerulonephritis, as is characteristic of human lupus. Strikingly, this autoantibody production is T cell-independent but dependent on TLR7 and TLR8 [24] [25].
We fail to detect any non-anergic Id+ B cells in the periphery of 564Igi mice [24]. Nonetheless, pathogenic IgG Id+ Abs are produced. A key question is what cells are responsible for production of these antibodies. It is possible that anergic mature B cells are activated in vivo by TLR/BCR mediated signaling and differentiate into antibody secreting cells (ASC). Alternatively, some immature Id+ B cells may be able to class-switch, differentiate into ASC and evade anergy [26]. In order to determine whether production of pathogenic IgG antibodies in 564Igi mice is the consequence of Aicda expression early during B-cell development, we generated 564Igi mice that conditionally express an activation-induced cytidine deaminase transgene (Aicdatg) [27] either in all B cells or only in mature B cells. Here we show that class switched pathogenic IgG autoantibodies were produced in mice in which functional AID was present in early developing B cells, and not in mice that only had functional AID in mature B cells. Furthermore, we show that the absence of AID in early developing B cells results in increased self-reactive IgM production, indicating that AID-mediated SHM, paradoxically, contributes to tolerance.
Results
Similar numbers of mononuclear cells in the bone marrow and spleen of 564Igi and 564Igi-cre mice
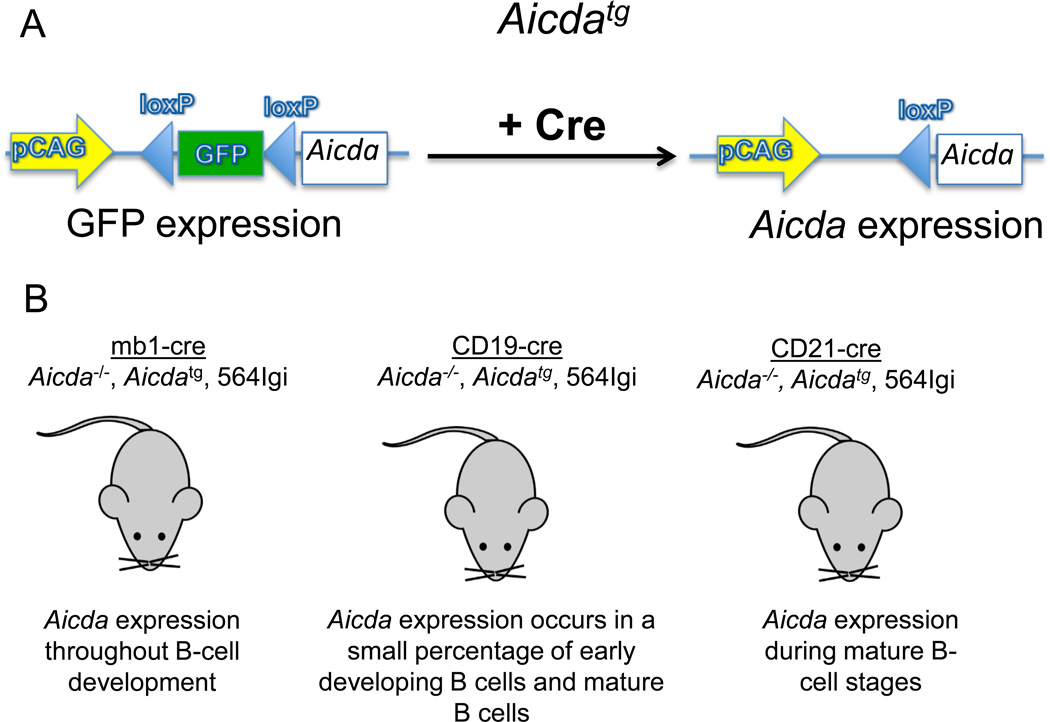
To learn how Aicda expression at different stages of B-cell development might affect autoantibody production, we introduced the transgene Aicdatg [27] into 564Igi Aicda−/− mice (Fig.1A). This transgene carries a floxed GFP gene in front of the Aicda coding sequence. Cre-mediated deletion of the floxed GFP gene results in loss of the GFP marker and expression of Aicda driven by the strong pCAG actin promoter (Fig.1A). To achieve stage specific expression of Aicda, 564Igi AicdatgAicda−/− mice were crossed with each of three Cre knock-in mouse strains that express Cre under the control of a different and endogenous promoter: the mb1 promoter [28], which is active throughout B-cell development, the Cd19 promoter [29], which is variably active during B-cell development, and the Cd21 promoter [30], which is active exclusively in mature B cells (Fig. 1B and Supporting Information Fig. 2).
Figure 1. Schematic of the 564Igi-cre mouse models.
(A) The Aicda transgene (Aicdatg) construct. Upon Cre-mediated recombination, GFP is excised, allowing the expression of Aicdatg. (B) On an Aicda−/−background, Aicdatg and 564IgH and IgL chain genes are bred to CD19-cre, mb1-cre and CD21-cre mice. These mice express Aicda at different stages of B-cell development.
Conditional expression of Aicdatg in the three 564Igi-cre lines had no significant effect on the absolute number of viable B cells (B220+) in the BM (Supporting Information Fig. 3A and B) nor on the absolute number of viable immature B cells in the BM (AA4.1+), (Supporting Information Fig. 3B) compared with 564Igi mice. Likewise, expression of Aicdatg did not affect total viable B-cell numbers in the spleen. In the spleens of 564Igi CD21-cre mice, there was a modest increase in the total number of mononuclear cells (p<0.05) (Supporting Information Fig. 4A).
In sum, these results indicate that the expression of Aicdatg does not alter the development of B lineage lymphocytes in 564Igi mice, consistent with a previous report for C57BL/6 mice. [27].
Efficient stage-specific Cre-mediated recombination in 564Igi-cre mice
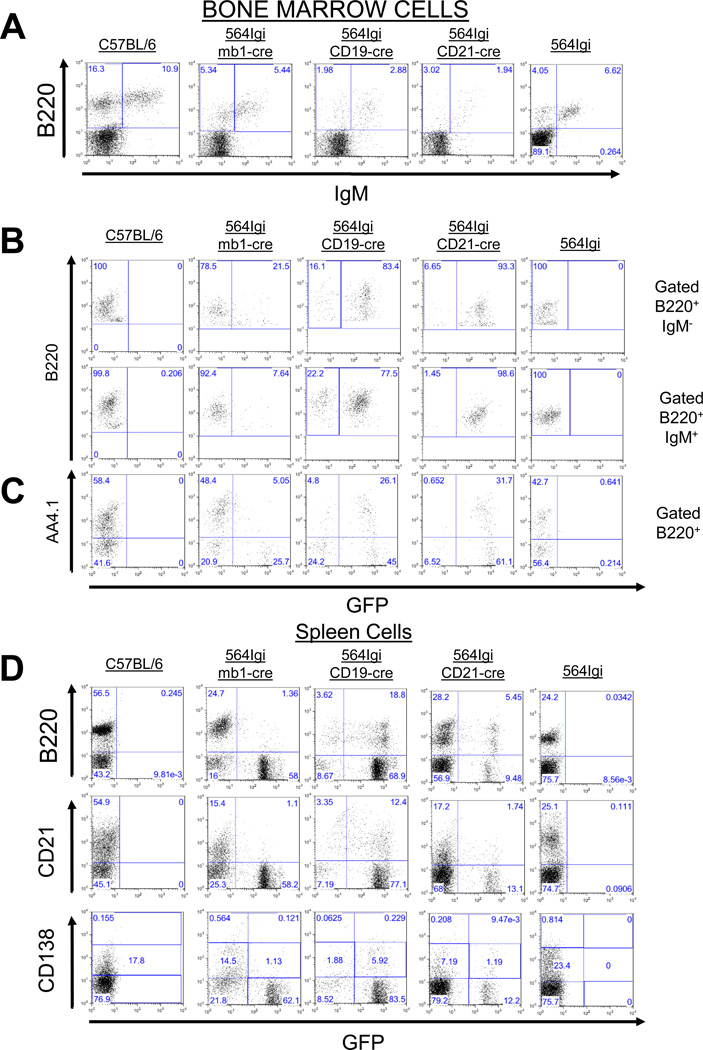
Each of the three Cre knock-in mouse strains that we used in our study would be expected to express Aicdatg at a characteristic stage of B-cell development and with a characteristic efficiency, depending on the specific promoter. To determine if Cre-mediated Aicdatg expression occurred as expected in our 564Igi-cre mice, we used the loss of GFP expression as a marker of Cre-mediated Aicdatg expression. Using flow cytometry, we examined GFP expression in BM B220+IgM− (pro- and pre-B) cells, as well as B220+IgM+ (immature and re-circulating mature B) cells and B220+AA4.1+ (pre-B and immature B) cells (Fig. 2A–C). In splenocytes, GFP expression was measured in the total B-cell pool (B220+ cells), as well as in mature (CD21+) B cells (Fig. 2D). We also examined GFP expression in splenic antibody-secreting plasma cell precursors (CD138lo) and terminally differentiated plasma cells (CD138hi) (Fig. 2D). As expected, in 564Igi-mb1-cre mice, the vast majority of B lineage cells in the BM expressed Aicdatg, with close to 80% of pro- and pre-B cells (B220+IgM−) and 92% of immature or re-circulating mature B cells (B220+ IgM+) failing to express GFP (Figure 2B, 2C). Similarly, 90% of pre-B and immature B cells (B220+ AA4.1+) were GFP− (Fig. 2C). Consistent with early expression of the Aicdatg locus, over 90% of splenic B cells, plasma cell precursors, and plasma cells were GFP− (Fig. 2D).
Figure 2. Mb1, CD19 and CD21 promoters allow regulated and efficient expression of Cre in immature and mature B cells.
(A) Bone marrow (BM) cells of C57BL/6, 564Igi mb1-cre/Aicdatg, 564Igi CD19-cre/Aicdatg, 564Igi CD21-cre/Aicdatg and 564Igi mice were stained with anti-B220 and anti-IgM antibodies and analyzed by flow cytometry. (B) Upon cre recombination in 564Igi-cre mice GFP is excised allowing for the expression of AID. Therefore we were able to assess the efficiency of Cre-mediated recombination through GFP expression. GFP expression was assessed by flow cytometry in B220+ BM cells gated on B220+ and IgM+/−. (C) GFP expression in AA4.1+ (immature B cells [66]) cells from various mice was assessed by flow cytometry, as in B. (D) GFP expression in B220+/−, CD21+/− and CD138+/− cells from the spleens from various mice were determined by flow cytometry, as in B. All flow cytometry plots are representative of at least 4 experiments.
564Igi-CD19-cre and 564Igi-CD21-cre mice also behaved as expected. In 564Igi CD19-cre mice, there was inefficient Aicdatg expression, with a majority of B lineage cells in the BM and spleen expressing GFP (Fig. 2B–D). In 564Igi CD21-cre mice, most of the BM B220+ cells (92.7%) were GFP+ because there are very few CD21+ cells in the BM. Aicdatg expression was evident primarily in splenic B cells of 564Igi CD21-cre mice, indicating that little Cre-mediated recombination occurred in the BM (Fig. 2B–D).
The Aicdatg is expressed at high levels and is functional in B220+ GFP− B cells of 564Igi-cre mice
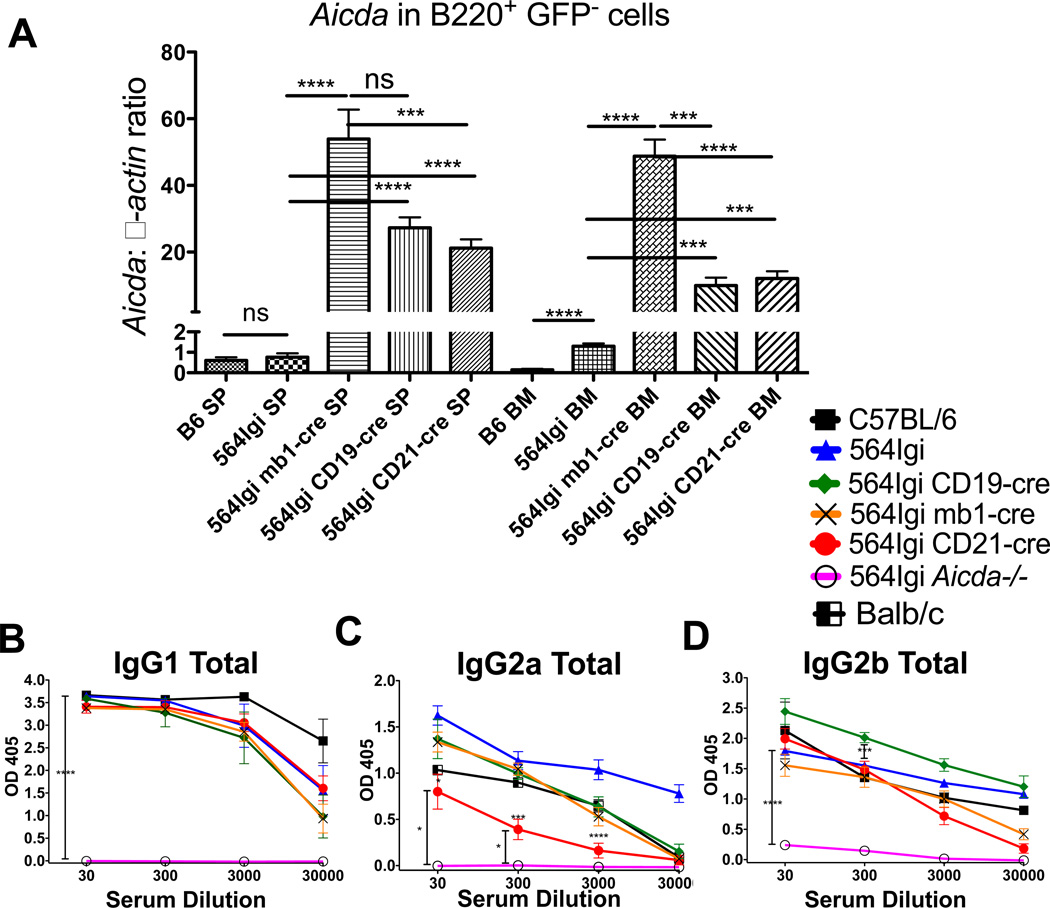
To confirm that Aicdatg is expressed in B220+GFP− B cells of 564Igi-cre mice, we sorted GFP−B220+ cells from the spleen and BM and measured Aicda expression using qRT-PCR (Fig. 3A). Aicdatg is expressed at very high levels compared with endogenous Aicda expression in non-transgenic B6 or 564Igi mice. Of note, endogenous Aicda expression is significantly higher in BM B cells of 564Igi mice compared with BM B cells from C57BL/6 mice. Aicdatg-encoded AID appears to be functional in GFP− B cells, as 564 IgH genes from all 564Igi-cre mice undergo CSR leading to production of IgG1, IgG2a and IgG2b antibodies (Fig. 3B–D). Even though there is up to 30–50× more Aicda mRNA expressed in 564Igi-cre mice compared with non-transgenic 564Igi mice, total serum IgG1 antibody titers in 564Igi-cre mice appear comparable to those of 564Igi and C57BL/6 mice. There appears to be lower antibody titers of IgG2a antibodies in 564Igi CD21-cre mice and slightly higher titers of IgG2b in 564Igi CD19-cre mice compared with 564Igi mice (Fig. 3C and D). These results suggest that Aicda expression in mature, CD21+ B cells is sufficient for efficient CSR, since Aicda is not expressed in early developing B cells in 564Igi CD21-cre mice (Fig. 2C).
Figure 3. Aicda is upregulated in B220+ B cells from the bone marrow and spleen of 564Igi-cre mice.
(A) mRNA transcript levels of Aicda relative to β-actin in BM B220+GFP cells were measured by real-time PCR (qPCR) from C57BL/6, 564Igi, 564Igi mb1-cre, 564Igi CD19-cre, and 564Igi CD21-cre mice. Three mice were tested for each strain. Three dilutions of cDNA were tested from each mouse, and each dilution was tested in triplicate. Data are shown as the mean ± SEM of n = 3 samples and are pooled from 3 independent experiments. Mice were tested at 3 to 4 months of age. (B–D) Total antibody titers, based on isotype-specific ELISAs of (B) IgG1, (C) IgG2a, and (D) IgG2b, in the sera of the indicated mouse strains. C57BL/6, n = 4; 564Igi, n = 7; 564Igi mb1-cre, n = 9; 564Igi CD19-cre, n = 7; 564Igi CD21-cre, n = 25; 564Igi Aicda−/−, n = 8. (A–D) Statistical analysis is based on a two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0 .001 and ****p < 0 .0001.
CSR in early developing B cells leads to the loss of tolerance in 564Igi mice
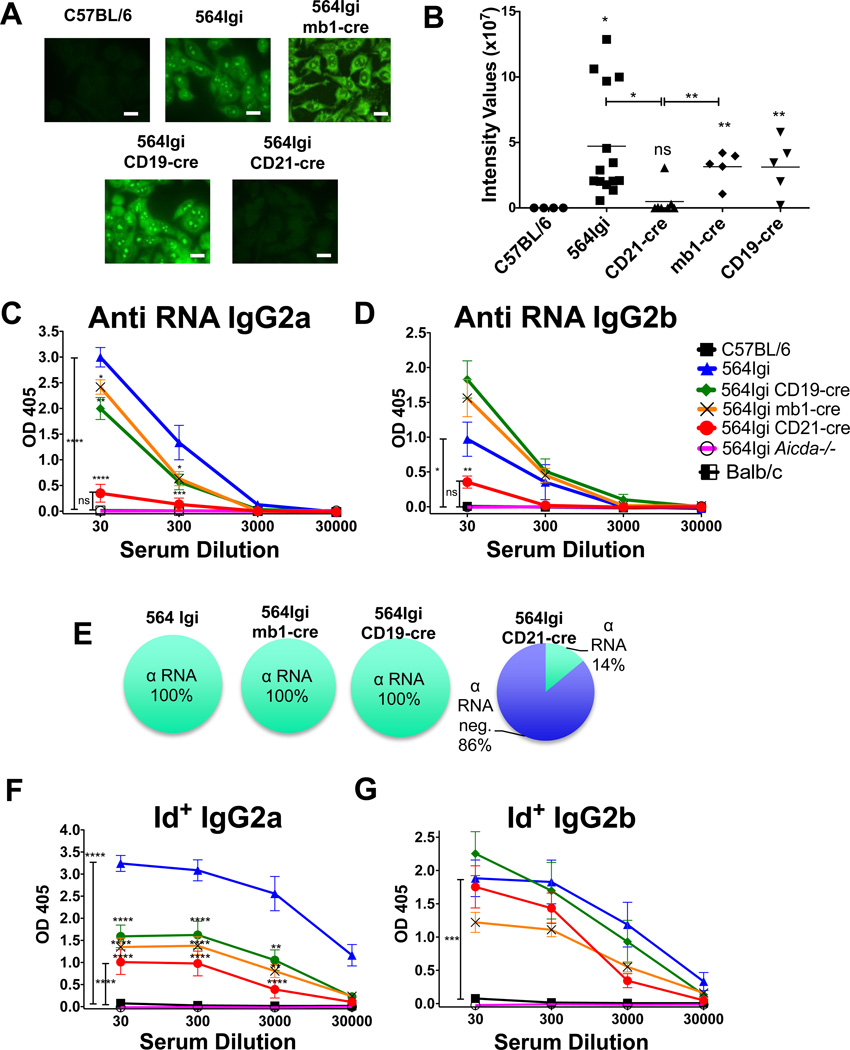
In ANA assays, 564Igi serum stains the nucleoli and cytoplasm of HEp-2 cells in a pattern indicative of the presence of autoantibodies reactive towards RNA or RNA-associated molecules (Fig. 4A and B). Consistent with this, 564Igi mice, unlike C57BL/6 mice, produce circulating Id+ anti-RNA IgG2a and IgG2b antibodies as measured by ELISA (Fig. 4C–G).
Figure 4. Generation of IgG anti-RNA antibodies requires Aicda expression at the immature B-cell stage.
(A) Presence of IgG anti-nuclear antibody (ANA) was assessed by fluorescence microscopy, using Hep-2 cells and treated with sera, and then stained with fluorescent anti-IgG. Scale bars are 20μm. Images are representative of 4 independent experiments. (B) Quantification of the intensity values from ANA staining in (A). Asterisks above points indicate significant differences compared with C57BL/6. Each symbol represents an individual mouse, pooled from 4 independent experiments, and means are indicated. (C and D) Sera were tested for (C) anti-RNA IgG2a and (D) IgG2b antibodies by ELISA. C57BL/6, n = 8; 564Igi, n = 8; 564Igi mb1-cre, n = 10; 564Igi CD19-cre, n = 18; 564Igi CD21-cre, n = 25; 564Igi Aicda−/−, n = 8. Asterisks above points indicate significant differences compared with 564Igi. Data were pooled from 5 independent experiments. (E) Percentage of tested mice with any detectable anti-RNA IgG antibodies. (F) Serum 564 Idiotype positive (Id+) antibodies were detected by isotype-specific ELISA in the indicated strains of mice. Legend is the same as in C and D. (F) IgG2a, (G) IgG2b. Asterisks above points indicate significant differences compared with 564Igi. (B, C, D, F, G) Statistical analysis is based on a two-tailed Student’s t-test; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
We next determined the effect of Aicda expression levels and timing on this loss of B-cell tolerance in 564Igi mice by measuring the levels of class-switched autoantibodies produced by our three 564Igi-cre mouse strains (Fig. 4A–G). As assessed by ANA assays, it is apparent that autoantibodies are produced in 564Igi mb1-cre and 564Igi CD19-cre, where Aicda is expressed throughout B cell development (albeit with different efficiencies in the two strains), but not in 564Igi CD21-cre mice, where AID is only activated in mature B cells (Fig. 4A and B).
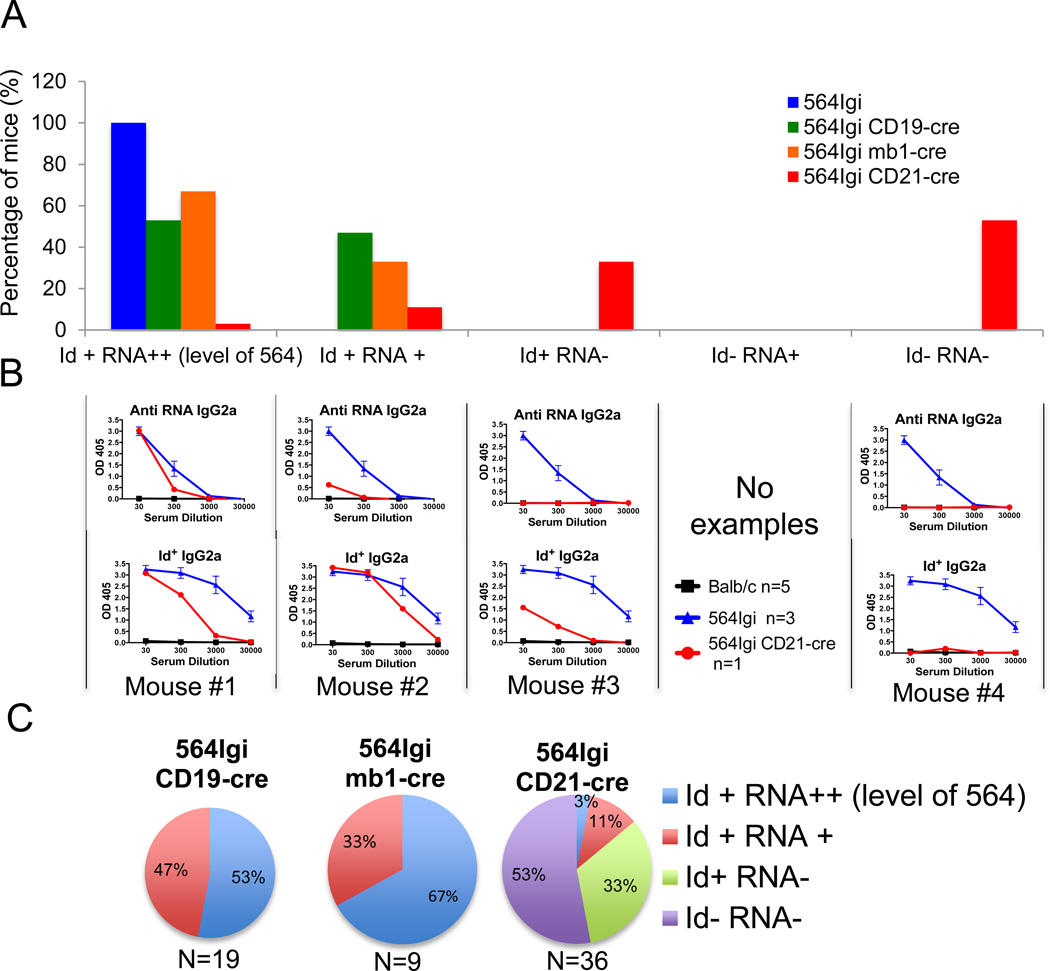
564Igi CD19-cre mice and 564Igi mb1-cre mice both secrete high levels of Id+, anti-RNA, anti-nucleolar IgG2a and IgG2b antibodies, similar to 564Igi (Fig. 4). Ten out of nineteen (53%) of 564Igi CD19-cre and six out of nine (67%) 564Igi mb1-cre mice had Id+ anti-RNA antibodies with titers similar to those found in 564Igi mice, while nine out of nineteen (47%) of 564Igi CD19-cre mice and three out of nine (33%) of 564Igi mb-cre mice had lower titers compared with 564Igi mice (Fig. 5A–C).
Figure 5. Few 564Igi CD21-cre mice have anti-RNA and Id+ antibodies.
(A) Percentage of mice from the indicated strain of mice that produce serum IgG2a Id+ and anti-RNA antibodies, as assessed by ELISA as in Figure 4. Mice that are Id+ RNA++ have high levels of anti-RNA antibodies, similar to 564Igi mice, and have Id+ antibodies. Mice that are Id+ RNA+ have lower levels of anti-RNA antibodies and are Id+. Mice that are Id+ RNA−–have no detectable anti-RNA IgG2a antibodies but have Id+ antibodies. Mice that are Id RNA−– have neither anti-RNA antibodies nor Id+ antibodies. (B) ELISA analysis of serum IgG2a antibodies from one individual example of 564Igi CD21-cre compared with sera from 564Igi as described in A. Data for 564Igi are shown as mean ± SEM of 3 samples representative of 5 independent experiments. (C) Percentage of mice from the indicated strain with specific antibody reactivity, as assessed by ELISA as in Figure 4.
Sera from 564Igi CD21-cre mice, on the other hand, did not bind nucleoli in ANA assays (Fig. 4A and B) and rarely had any detectable IgG2a or IgG2b anti-RNA autoantibodies (Fig. 4C–E). Only five out of thirty six (14%) of the 564Igi CD21-cre mice had serum antibodies with RNA reactivity, and only one mouse out of thirty six (3%) had IgG anti-RNA antibody titers similar to those found in the sera of 564Igi mice (Fig. 5A and B). Four mice out of thirty six (11%) 564Igi CD21-cre mice had Id+ IgG antibodies with very low RNA binding capabilities, while the majority (thirty one out of thirty six or 86%) had either Id+ IgG antibodies with no RNA binding specificity or antibodies that were neither Id+ nor anti-RNA (Fig 5A–C). The percentage of mice with varying reactivity is shown in Fig. 5C. These results suggest that 564Igi CD21-cre mice do not produce IgG autoantibodies, a stark contrast to the high levels of IgG autoantibodies found in 564Igi mb1-cre and 564Igi CD19-cre mice.
Most of the CD21+ B cells and all of the CD138+ cells in the spleen of 564Igi CD21-cre mice were GFP− (Fig. 2D), indicating that the Aicdatg was expressed. The AID protein was fully functional, as it was sufficient for CSR to the IgG1 isotype (Fig. 3B). We have previously shown that 564Igi mice do not express Id+ IgG1 antibodies but can produce non-autoreactive IgG1 antibodies [24]. These results indicate that B cells from 564Igi CD21-cre mice express a functional Aicdatg capable of mediating CSR but do not class-switch the 564 IgH and IgL genes to the IgG2a and IgG2b isotypes.
It is interesting to note that 564Igi CD19-cre and 564Igi mb1-cre mice expressed Aicdatg at the immature B-cell stage (AA4.1+ GFP−) in the BM (Fig. 2C) (4.8% and 48%, respectively) and were able to produce anti-RNA IgG autoantibodies (Fig. 4A–G). However, 564Igi CD21-cre mice did not express Aicdatg in immature BM cells (AA4.1+ GFP+) (Fig. 2C), and did not secrete any anti-RNA IgG autoantibodies (Fig.4). These results show a correlation between Aicdatg expression in BM immature AA4.1+ B cells and presence of IgG anti-RNA antibodies. Therefore, it seems that Aicda expression during B-cell development is necessary for the production of anti-RNA IgG autoantibodies.
High serum IgM anti-RNA antibody titers in the absence of AID during B-cell development
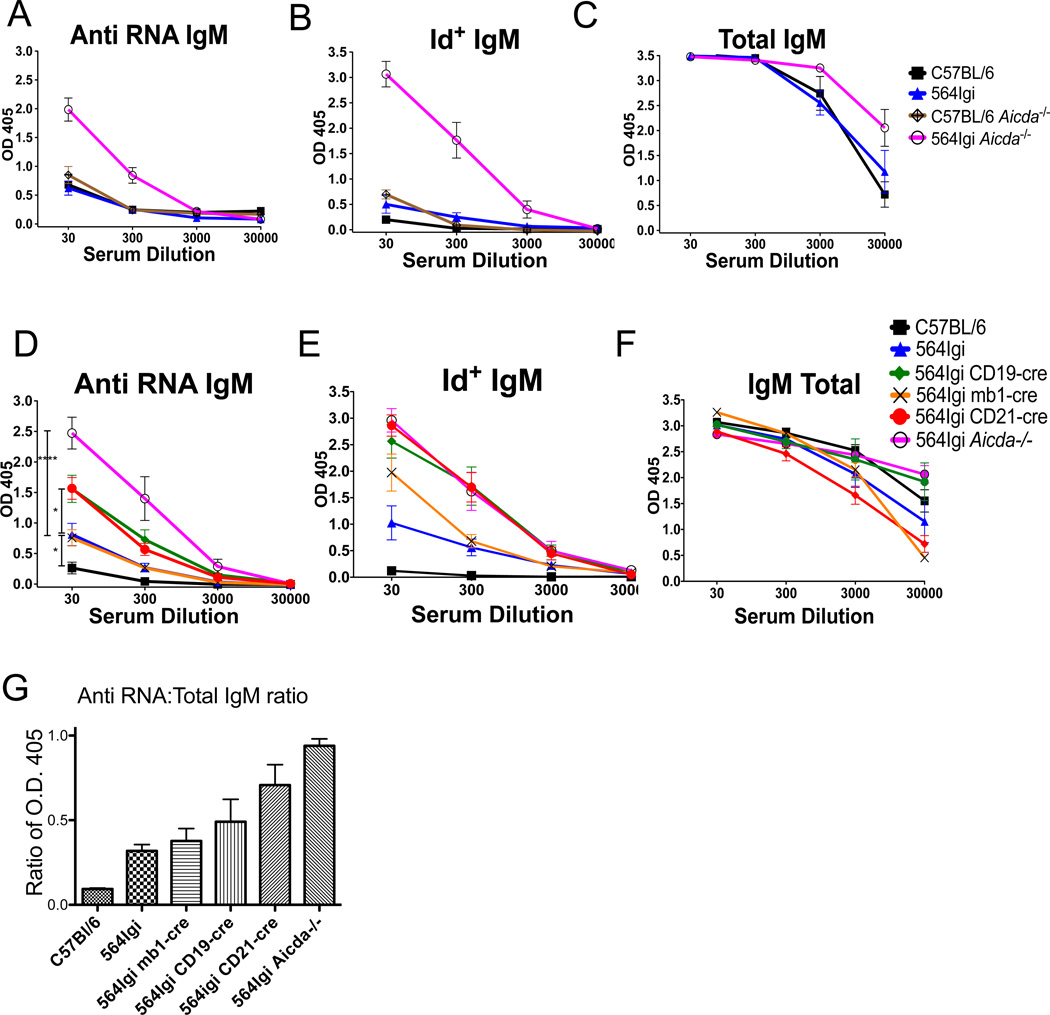
IgM autoantibody titers (Id+ anti-RNA) in 564Igi mice are low, but slightly higher than those in C57BL/6 mice (Fig. 6A and B). This is consistent with the idea that a majority of self-reactive IgM+ B cells are functionally deleted during B cell development in 564Igi mice. This finding in addition is also consistent with the fact that most of Id+ B cells in 564igi are anergic [24]. Interestingly, 564Igi Aicda−/− mice produce high levels of Id+ anti-RNA IgM Abs, while total IgM antibody titers remain normal (Fig. 6). C57BL/6 Aicda−/− mice have little serum anti-RNA and no serum Id+ IgM antibodies (Fig. 6A and B), demonstrating that the anti-RNA IgM antibodies in 564Igi Aicda−/− mice are the products of the 564 knock-in IgH and IgL genes. These results suggest that a central B-cell tolerance checkpoint is ineffective in the absence of AID.
Figure 6. Production of anti-RNA Id+ IgM is increased in mice lacking AID during B-cell development.
(A) Serum anti-RNA IgM antibody titers were measured by ELISA in the indicated strains of mice. C57BL/6, n = 4; 564Igi, n = 13; C57BL/6 Aicda−/−, n = 6; 564Igi Aicda−/−, n = 15. (B) Serum Id+ IgM antibody titers measured by ELISA in the indicated strains of mice. C57BL/6, n = 5; 564Igi, n = 10; C57BL/6 Aicda−/−, n = 10; 564Igi Aicda−/−, n = 10. (C) Total serum IgM antibody titers measured by ELISA in the indicated strains of mice. C57BL/6, n = 9; 564Igi, n = 10; C57BL/6 Aicda−/−, n = 5; 564Igi Aicda−/−, n = 5. (D) Serum anti-RNA IgM antibody titers in 564Igi-cre mice were measured by ELISA. Statistical analysis is based on a two-tailed Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 (E) Serum Id+ IgM antibody titers in 564Igi-cre mice were measured by ELISA. (F) Total serum IgM antibody titers in 564Igi-cre mice were measured by ELISA. (D–F) C57BL/6, n = 8; 564Igi, n = 15; 564Igi mb1-cre, n = 10; 564Igi CD19-cre, n = 21; 564Igi CD21-cre, n = 26, 564Igi Aicda−/−, n = 11. All data in (A–F) show mean ± SEM of the indicated numbers of samples, pooled from of 5 independent experiments. (G) Ratio of the average optical densities (O.D.) from IgM anti-RNA ELISA and total IgM ELISA for the indicated mouse strain as in Figure 4. Ratio of O.D. 405 nm for anti-RNA IgM ELISA at 1:30 serum dilution to the O.D. 405 nm from total IgM ELISA at 1:3000 serum dilution.
In 564Igi CD21-cre and 564Igi CD19-cre mice, there were significantly (p<0.05) elevated levels of serum anti-RNA Id+ IgM autoantibodies compared with 564Igi mice (Fig. 6D and E). In contrast 564Igi mb1-cre mice had low levels of anti-RNA IgM Abs in their sera, similar to 564Igi mice (Fig. 6D and E). All 564Igi-cre mouse strains have similar levels of total serum IgM antibodies compared with 564Igi and C57BL/6 (Fig. 6F). The ratio of values (OD405) from the anti-RNA IgM and total IgM assays (anti-RNA/total IgM) indicates that there are twice as many total IgM antibodies as there are anti-RNA antibodies in the serum of 564Igi and 564Igi mb1-cre mice (Fig. 6G). Strikingly, 564Igi CD21-cre mice show a ratio of 0.7, close to that of 564Igi Aicda−/−, and 564Igi CD19-cre mice show an intermediate ratio above 0.5 (Fig. 6G). These results suggest that B cells from 564Igi CD21-cre and some 564Igi CD19-cre mice lack functional AID at the appropriate developmental stages to prevent the secretion of self-reactive IgM antibodies.
In 564Igi Aicda−/− mice, RAG-mediated receptor editing and other mechanisms of tolerance are insufficient to suppress self-reactive IgM Abs from being produced, leading to high levels of Id+ anti-RNA IgM antibodies (Fig. 6A). This suggests that AID may be playing a role in limiting the production of self-reactive IgM. A possible mechanism is that SHM of 564Igi IgH and IgL genes at the immature B-cell stage prevents the secretion of self-reactive IgM.
SHM and secondary rearrangements are involved in B-cell tolerance
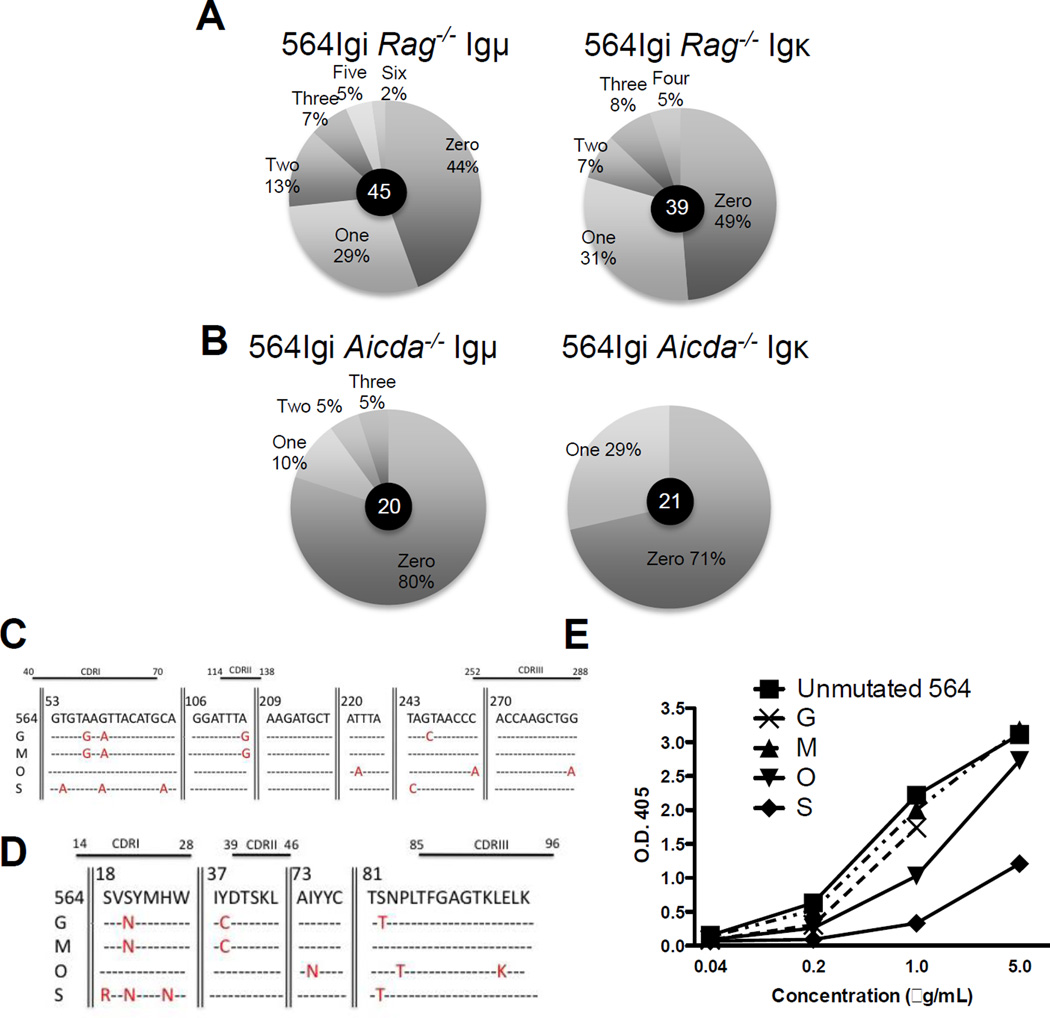
In order to determine whether 564Igi IgH and IgL genes are somatically mutated in 564Igi mice, we cloned and sequenced IgH and IgL genes from B220+Id− B cells from a 564Igi Rag−/− mice. This approach was taken in order to assure that any nucleotide exchanges were due to SHM and not due to secondary rearrangements of IgH and IgL genes. Our results show that 564 Igμ and Igκ genes from 564Igi Rag−/− splenic B cells have high mutation frequencies compared with 564Igi Aicda−/− mice (Fig. 7A–D). Sequence analysis of IgH and IgL genes from 564Igi Rag−/− mice indicates that B220+ Id− splenic B cells have both mutated and unmutated IgH and IgL genes (Supporting Information Fig. 5 and Supporting Information Fig.6, Fig. 6C and D). A high proportion of the unmutated IgH or IgL genes are likely expressed with a mutated partner IgL or IgH gene, respectively, to explain the lower titer of the 564 Id+ and RNA reactivity in 564Igi Rag−/− mice (Supporting Information Fig. 7A and B). The ratio of anti RNA IgM titer to the total IgM titer in the sera is lower in 564 Rag−/− than in 564Igi Aicda−/− (Supporting Information Fig. 7D). These results suggest that AID contributes to the editing of 564 IgH and IgL chain genes resulting in the loss of RNA reactivity of IgM antibody in 564Igi. In addition, 564 IgH and IgL genes from 564Igi Rag−/− mice have high replacement to silent (r:s) mutation ratios, especially in the CDR regions of IgL genes (Supporting Information Fig. 6). A high r:s ratio in the CDR region supports the notion that these non-RNA-reactive antibodies are products of antigen selected B cells with mutated Ig genes. Multiple examples of unmutated 564 IgH genes were co-transfected into HEK293T cells with mutated IgL chain genes (clones G, M, O, S) (Fig. 7C and D). The resulting recombinant antibodies were tested for RNA reactivity. Compared with the unmutated 564 IgH and IgL genes, some of these antibodies (i.e. clone O and S) are significantly less reactive towards RNA (Fig. 7E).
Figure 7. 564Igi Rag−/− mice have mutated Ig genes.
(A,B) Antibody genes were cloned and sequenced from single B cells. The percentage of antibody gene sequences with a specific number (zero, one, two etc.) of mutations is displayed. (A) The number of mutations in 564 Igμ and 564 Igκ chain sequences in B220+Id B cells from the spleen of 564Igi Rag−/− mice and (B) B cells from the spleen on 564Igi Aicda−/− mice, which were used as control for the background mutation frequency. The number of genes sequenced is indicated in the center of individual pie charts (C) Mutated Igκ genes, assessed as in (A,B). Base pair locations are given relative to the first base in the sequence. Some portions of the sequence are not shown in this figure, indicated by vertical lines. (D) Protein sequences of genes displayed in (C). Amino acid locations are given relative to the first amino acid in the sequence. Missense mutations are indicated in red. Relative locations of complementarity determining regions (CDRs) are also given above the DNA and protein sequences. (E) Anti-RNA ELISA was performed to compare recombinant antibodies with specific mutations with antibodies that were unmutated recovered from transfected HEK293T cells. Antibody concentrations were determined by analysis of supernatants on anti-IgG antibody ELISA using purified human IgG antibody of known concentration.
SHM in 564 IgM antibody genes during early B-cell development leads to loss of self-reactivity
In order to determine whether the absence of IgM anti-RNA autoantibodies in 564Igi and 564Igi mb1-cre mice resulted from secondary rearrangement or SHM, we cloned and sequenced 564Igi antibody genes from single BM pre-B cells (B220lowκ−) and single immature B cells (B220lowκ+).
In most of the single pre-B and immature B cells from 564Igi mb1-cre mice that we analyzed, we found that Igκ genes were readily amplified with 564-Vκ primers. (Supporting Information Fig. 8C). On the other hand, neither Igμ nor Igγ genes could be amplified from these cells with 564VH-specific primers (Supporting Information Fig. 8A and B). This indicates that the H-chain knock-in genes in these pre-B and immature cells have been altered to the point that they cannot amplify with 564-VH specific primers. One possible explanation for such dramatic alterations is RAG-mediated secondary rearrangements of H-chain genes [31] that have been shown to play a role in the editing of anti-dsDNA antibody genes [32] [6]. In contrast to the extensive alterations of early B-cell Igμ genes in 564Igi mb1-cre mice, the early B cell Igμgenes in 564Igi CD21-cre mice could be amplified with 564 VH-specific primers (Supporting Information Fig. 8D). This surprising result suggests that RAG mediated receptor editing does not occur in the AID-deficient immature B cells that are present in 564Igi CD21-cre mice. The linkage of RAG-mediated receptor editing with AID expression in early B cells is not clear and needs further study. Previous reports have suggested a co-dependency between RAG and AID function [33] [34].
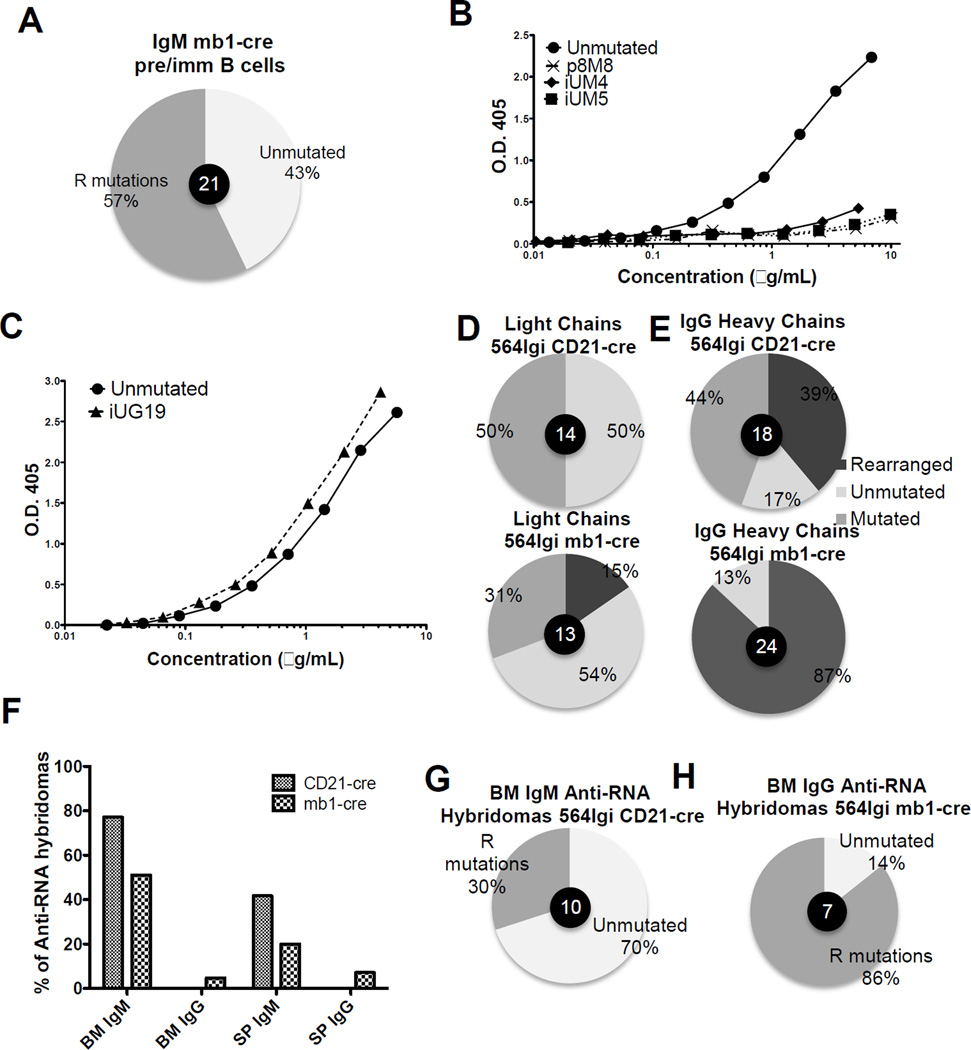
We next wanted to determine whether AID-mediated SHM of IgH and/or IgL genes could be an additional mechanism of tolerance in early developing B cells. Pre-B cells and immature B cells from 564Igi mb1-cre mice have high mutation frequencies with 57% of early developing B cells tested having replacement mutations in IgH or IgL genes (Fig. 8A and Supporting information Fig. 9 and Fig. 10). In order to test whether these mutations lead to a loss of RNA reactivity, Igμ and Igκ genes from 564Igi mb1-cre single cells were expressed in HEK293T cells and the resulting recombinant antibodies were tested for RNA reactivity. Recombinant antibodies genes from a single pre-B cell (p8M8) and from single immature B cells (iUM4, and iUM5) all had minimal RNA reactivity compared with the unmutated 564 antibody (Fig. 8B). The protein sequences are displayed in Supporting Fig. 11. These results are similar to those shown with mutated 564 IgH and IgL genes from 564Igi Rag−/− mice (Fig. 7). Thus, the combination of RAG-mediated receptor editing [31] [32] and SHM of 564 IgH and IgL genes early during B-cell development is a likely explanation for the low serum titers of IgM anti-RNA antibodies observed in 564Igi, 564Igi mb1-cre and 564Igi Rag−/− mice.
Figure 8. 564 Igμ and Igκ genes are mutated in 564Igi mb-1-cre mice, resulting in loss of RNA reactivity during B cell development.
(A) Percentage of Igμ mutated sequences from 564Igi mb1-cre BM pre-B and immature B cells was determined by analyzing antibody gene sequences. Genes were cloned and sequenced from single cells. (B and C) Antibody concentrations were determined by analysis of supernatants by ELISA using purified antibody of known concentration as standard. (B) Igμ and Igκ genes were amplified from developing B cells (C) Igγ and Igκ genes were amplified from an immature B cell and RNA-reactivity was compared with the unmutated 564 IgH and Igκ genes. Unknown concentrations were determined using a standard curve from an human IgG of known concentration. O.D. 405 was determined by anti-RNA ELISA at all concentrations. (D,E) cDNA was prepared from 250 000 CD138+ cells from the spleen of 564Igi CD21-cre and 564Igi mb1-cre mice. PCR was performed using 564 Igγ- and Igκ-specific primers. These “bulk” PCR products were cloned and sequenced. The percentage of clones that were unmutated, mutated and secondarily rearranged is displayed. The number of sequences analyzed are indicated in the center of pie charts (F) Bone marrow- and spleen-derived hybridomas that were positive for growth were screened for both anti-RNA IgM and IgG antibody production by ELISA. Shown is the percentage of RNA reactive hybridomas per total positive for growth. 96 IgM 564Igi CD21-cre spleen-derived, 96 IgM 564Igi CD21-cre BM-derived, 96 IgG 564Igi CD21-cre spleen-derived, 96 IgG 564Igi CD21-cre BM-derived, 169 IgM 564Igi CD21-cre spleen-derived, 94 IgM 564Igi CD21-cre BM-derived, 112 IgG 564Igi CD21-cre spleen-derived, 60 IgG 564Igi CD21-cre BM-derived. (G) Igμ and Igκ antibody genes were cloned and sequenced from selected anti-RNA IgM antibody-producing hybridomas from 564Igi CD21-cre BM. Shown is the percentage of hybridomas with replacement (R) mutations in either IgH or IgL genes. 10 hybridomas were analyzed. (H) Igγ and Igκ genes were cloned and sequenced from anti-RNA IgG antibody-producing hybridomas from 564Igi mb1-cre BM. Shown is the percentage of hybridomas with R mutations in either IgH or IgL genes. 7 hybridomas were analyzed.
Antibody genes coding for anti-RNA antibodies can class switch to IgG in early developing B cells
Out of 40 immature B cells tested from the BM of 564Igi mb1-cre mice, only one cell had an Igγ gene that could be amplified with 564VH-specific primers (Supporting Information Fig. 8 and Fig. 9A). This clone, iUG19, had one replacement mutation in the heavy chain and no mutations in the light chain (Supporting Information Fig. 11). The mutation did not affect the RNA reactivity of the antibody (Fig. 8C). This is one example of an anti-RNA IgG antibody encoded by a early developing B cell.
To investigate whether secondary recombination played a role in the prevention of self-reactive IgG production in 564Igi mb1-cre and 564Igi CD21-cre mice, we cloned and sequenced Igγ genes from bulk CD138hi antibody-producing cells from the spleens of both mice. Secondary rearrangements were not often detected in Igκ genes (Fig. 8D). Igγ genes, on the other hand, were frequently rearranged in both 564Igi mb1-cre and 564Igi CD21-cre mice (87% and 39%, respectively) (Fig. 8E). The variable region of rearranged genes often consisted of a VH region similar, but not identical, to the 564 VH region and a dramatically different DJ region. This likely indicates rearrangements between 564 and endogenous variable regions.
To further validate the correlation between Aicda expression and anti-RNA antibody production, we created hybridomas from BM and SP cells from both 564Igi mb1-cre and 564Igi CD21-cre mice. The hybridoma supernatants were screened for anti-RNA IgM and IgG antibody production. 564Igi CD21-cre BM and SP generated a higher percentage of hybridomas that produced anti-RNA IgM antibodies compared 564Igi mb1-cre mice (Fig. 8F). However, neither the BM nor the SP of 564Igi CD21-cre mice generated hybridomas that produce anti-RNA IgG antibodies (Fig. 8F). Both BM and SP hybridomas from 564Igi mb1-cre mice, on the other hand, produced anti-RNA IgG antibodies; 4.73% (8/169) of BM and 7.14% (8/112) of SP (Fig. 8F).
We then selected anti-RNA producing hybridomas and sequenced their antibody genes using 564 IgH- and Igκ-specific primers. From 564Igi CD21-cre IgM BM hybridomas, 70% of the anti-RNA antibodies had no replacement mutations, while 30% had replacement mutations but still retained RNA reactivity (Fig. 8G and Supporting Information Fig. 12 and Fig. 14A and B). These results are in agreement with the findings that 564Igi CD21-cre mice have high titers of serum anti-RNA IgM antibodies (Fig. 6D–G). Since 564Igi CD21-cre mice do not express Aicda early in B-cell development, the 564 IgM antibody genes retain RNA binding specificity.
In 564Igi mb1-cre mice, the anti-RNA IgG-producing hybridomas from both SP and BM had many replacement mutations and deletions (Fig. 8H and Supporting Information Fig. 13 and Fig. 14A and C). Even though they were highly mutated, these IgG antibodies retained RNA reactivity. These results are in agreement with the serological analysis of 564Igi mb1-cre mice where there are high titers of IgG anti-RNA antibodies compared with 564Igi CD21-cre mice (Fig. 4).
564Igi CD19-cre mice are less effective in expressing Cre-recombinase at pro/pre and immature stages of B-cell development compared with 564Igi mb1-cre mice (Fig. 2B and C), resulting in lower expression of Aicda. Thus, in many early developing cells, the Igμ and Igκ genes would be unmutated and could explain the high IgM anti-RNA titers in these mice (Fig. 6D–G). However, expression of Aicda via Cre-recombinase activity in a low number of early B cells is sufficient to cause CSR of Igμ genes to Igγ, resulting in the high titers of IgG anti-RNA antibodies in the sera of 564Igi CD19-cre mice (Fig. 4D).
Because 564Igi CD21-cre mice express AID only in mature B cells whereas 564Igi mb1-cre mice express AID in both early and mature B cells, our results from these mouse strains provide strong support for our model that AID expression in early B cells is critical for the CSR that gives rise to pathogenic IgG antibodies in the 564Igi mouse model of SLE and, in addition, for our model that AID expression and SHM provides an important mechanism for receptor editing of antibodies having autoreactive specificities in early B cells.
Discussion
SLE is an autoimmune disease characterized by the presence of IgG1 in humans and IgG2a and IgG2b autoantibodies in mice, many of which are directed against nuclear antigens. Human patients and mouse models of SLE have autoantibodies reactive to a variety of self-antigens [35], and some, but not all of these antibodies are directly pathogenic.
In lupus prone MRL-lpr/lpr (MRL-lpr) mice, pathogenic IgG anti-nuclear antibodies have been suggested to be produced by T cell-dependent activation of B cells within germinal centers (GCs) [36] [36][37] [38] [39]. Previous studies have shown that both murine and human anti-double-stranded DNA (anti-dsDNA) antibody producing cells can develop from non-DNA-reactive B cells. These studies further suggest a crucial role for AID mediated SHM and CSR in the production of anti-dsDNA antibodies [40–42].
Recent data however, have questioned whether GCs are the only site of affinity-based selection and mutation of self-reactive B cells. Autoantigen-specific responses have been found extra-follicularly at the T zone-red pulp border in the spleen and in actively dividing B cells where SHM takes place in situ rather than in the GC [43]
Our results using the 564Igi mouse model of SLE suggest yet another site for the production of IgG autoantibodies in SLE. We have previously shown that in 564Igi mice, Ig genes from B cells producing self-reactive antibodies undergo CSR in a T cell-independent but TLR-dependent manner [24]. Similar findings were reported in another mouse model of SLE, MRL/lpr [44]. Autoantibody production by 564Igi mice is dependent on the expression of either TLR7 or TLR8 [25]. We propose that in SLE patients, T-independent and TLR-dependent activation of early developing B cells is yet another mechanism by which autoantibodies are produced, similar to what has been observed in mouse models.
We discovered that in the bone marrow (BM) of wild-type mice, immature B cells can be induced to upregulate activation-induced cytidine deaminase (Aicda) expression and undergo functional CSR [20]. Strikingly, we found that this CSR in immature B cells was T-independent and required both BCR signaling and the presence of MyD88 [20]. We have also found that Aicda expression in pre-B cells similarly requires MyD88-mediated signaling, but Bruton tyrosine kinase (Btk) is not required [20]. This indicates that, in pre-B cells, BCR-mediated signaling is not required for Aicda expression.
These results have important implications for tolerance of self-reactive, immature B cells. Certain self-antigens that can activate both self-reactive BCRs and Myd88 (such as RNA) may induce the expression of Aicda in immature B cells. Aicda would then have the dual role of mediating both SHM and CSR, each with different consequences. SHM would lead to loss of self-reactivity and would thus constitute a receptor editing mechanism. CSR, on the other hand, would result in the generation of self-reactive IgG B cells, which might be refractory to subsequent tolerance mechanisms and capable of differentiating into antibody secreting cells (ASCs) [45, 46]. Alternatively, B cells expressing mutated IgG autoantibody genes are antigen selected and differentiate into ASCs [47].
Here we determined the effect of Aicda expression on the loss of B cell tolerance during various stages of B-cell development by measuring the levels of class-switched autoantibodies produced by our three 564Igi-cre lines (Fig. 4A–G). IgG autoantibodies are produced in 564Igi mb1-cre and 564Igi CD19-cre, where Aicda is expressed throughout B-cell development (albeit with different efficiencies in the two strains). However, no IgG autoantibodies were produced in 564Igi CD21-cre mice, where Aicda is efficiently expressed only in mature B cells (Fig 4A and B). Sequence analysis of Ig genes from 564Igi CD21-cre mice revealed a high frequency of replacement mutations in Igγ and Igκ genes. Similarly, analysis of hybridomas from BM and spleen of 564Igi CD21-cre mice revealed that none of the hybridomas produced anti-RNA IgG antibodies. On the other hand, we were able to obtain several RNA-binding IgG hybridomas from the BM of 564Igi mb1-cre mice. In addition we found one anti-RNA IgG producing single cell from an immature BM B cell from 564Igi mb1-cre mice.
The AID protein was fully functional in 564Igi CD21-cre mice, as it was sufficient for CSR to the IgG1 isotype (Fig. 3B). We have previously shown that 564Igi mice do not express Id+ anti-RNA IgG1 antibodies but can produce non-autoreactive IgG1 antibodies [24]. These results indicate that B cells from 564Igi CD21-cre mice express a functional Aicdatg capable of mediating CSR but do not class-switch the 564 IgH and IgL genes to the IgG2a and IgG2b isotypes.
564Igi mb1-cre mice, like 564Igi mice, have low titers of serum IgM autoantibodies, while 564Igi CD21-cre mice have higher titers of serum IgM autoantibodies, similar to those found in 564Igi Aicda−/− mice. This strongly suggests that a central B cell tolerance checkpoint is ineffective in the absence of AID early in B-cell development in 564Igi CD21-cre mice. Sequence analysis of 564 Igμ and Ig κ genes from single pre-B and immature B cells of 564Igi mb1-cre mice indeed revealed multiple cells with nucleotide exchanges in Ig genes that eliminated RNA-reactivity of the encoded IgM antibodies (Fig. 8B). In contrast, nearly all IgM hybridomas from 564Igi CD21-cre mice were RNA reactive.
These results clearly indicate that class switched, pathogenic IgG autoantibodies were produced only in mice in which AID was functional in early developing B cells. Further, we show that the absence of AID in early developing B cells results in increased self-reactive IgM production, indicating that AID-mediated SHM, paradoxically, contributes to tolerance.
It has been shown that self-reactive mature B cells can be activated through dual BCR and endosomal TLR signaling [48] and that dual ligation of these receptors can induce CSR [49]. We propose that immature B cells in the bone marrow can potentially be activated by similar mechanisms. Self nucleic acid, which is abundant in the bone marrow microenvironment, [50] would have the potential to bind and stimulate the self-reactive BCRs frequently found on the surface of immature B cells [51]. Once recognized, the self-antigen could be endocytosed and delivered to the endosome [52]. In the endosome, TLR7 and TLR8, which recognize nucleic acids, could potentially be stimulated by this internalized self-antigen [52]. Significantly, early developing and transitional B cells can be induced to express Aicda through combined TLR and BCR, leading to CSR of antibody genes to IgG and other isotypes [20].
In this manuscript we found that pre-B cells expressing Aicdatg have somatically mutated 564 IgH genes (i.e. recombinant p8M8) (Fig. 8B). Since pre-B cells do not express a fully functional BCR on their surface there must be a mechanism for the Aicda expression independent of the BCR-antigen interaction.
One possible mechanism is that the pre-BCR does in fact have some potential to signal and activate a pre-B cell. Supporting this possibility is the observation that a self-reactive Igμ pre-BCR can drive the proliferation of pre-B cells [53] [54], [55] [56]. It has also been shown that pre-B cells can express Aicda in the absence of Bruton’s tyrosine kinase (Btk), therefore, without signaling through a surface pre-BCR [20]. Aicda expression in pre-B cells, however, is dependent on Myd88 signaling [20]. This supports a TLR-dependent but pre-BCR-independent mechanism for Aicda expression in pre-B cells. One possible explanation is that transcribed endogenous retroviral elements activate early developing B cells through TLR7 and TLR8 [47] [57] [58]
Further suggestive evidence for CSR in early developing B cells is provided by studies in μMT mice. μMT mice do not have fully developed B cells. However, in μMT mice IgG antibody production occurs in the absence of IgM+ B cells [59] [60] [61]).
We propose that 564Igi CD21-cre mice do not produce self-reactive IgG antibodies because fully mature B cells that reach the periphery have already undergone receptor editing and are no longer self-reactive. In 564Igi CD21-cre mice, self–reactive early developing IgM+ B cells differentiate into terminally differentiated plasma cells or undergo functional deletion in the BM. The only IgM+ B cells that achieve maturity have lost self-reactivity through RAG-mediated receptor editing. Indeed when cDNA was amplified and cloned from RNA of “bulk” CD138+ cells almost all of the IgG clones had secondarily rearranged IgVH genes (Fig.8). Conventional antigens in the periphery, with T cell help, will activate mature B cells to class-switch in 564Igi CD21-cre, but none of these B cells will produce self-reactive IgG antibodies.
Another interesting aspect of class-switched autoantibody production is that relatively few B cells are needed to produce high serum titers of anti-RNA IgG antibodies. Two observations support this idea: first, 564Igi CD19-cre mice have high levels of IgG anti-RNA antibodies in their sera; however, very few cells have undergone Cre-mediated recombination and, therefore, very few cells express Aicda. Secondly, the percentage of hybridomas that produce anti-RNA IgG antibodies is very low in 564Igi mb1-cre mice, despite high serum titers of anti-RNA IgG antibodies. Since few B cells are needed to produce high levels of IgG autoantibodies, SLE treatment approaches that target B cells may not be effective.
In summary we have established a dual role for AID in early developing B cells: first, we demonstrate that AID is necessary in early developing B cells for genes coding for self-reactive antibodies to class switch to IgG. Second, AID serves as a mechanism for the IgM-mediated central tolerance process, shown previously in humans and mice [62], that prevents self-reactive IgM production.
Our finding of AID expression in pre-B and immature B cells within the bone marrow [20] raised a question regarding the function of the AID protein in these early B cells. Our current results indicate that AID-mediated CSR in these early B cells can lead to autoimmunity; this function would seem unlikely to be selected by evolution. However, AID expression early in B cell development could help to avoid autoimmunity by mutating self-reactive antibody genes. An estimated 75% of developing B cells are self-reactive [51], which may explain why there appear to be many mechanisms of tolerance that prevent these self-reactive developing B cells from becoming autoantibody producing cells. Our results suggest that SHM in developing B cells may be one mechanism of tolerance induction in early developing B cells. This could be the evolutionarily selected SHM function of early B cell Aicda expression, which leads to a co-selection of CSR-driven autoimmunity that is coupled within the same protein.
MATERIALS AND METHODS
Mice
All experiments with mice were performed in accordance with the regulations and with the approval of Tufts/NEMC IACUC. The creation of 564Igi mice was previously described [24] [63]. Aicda−/− and Aicdatg mice were obtained from Dr. T. Honjo (Kyoto University). C57BL/6, BALB/c, CD21-cre, CD19-cre and Rag2−/− mice were purchased from Jackson laboratories. Mb1-cre mice were obtained from Dr. M. Reth. 564Igi was bred to Aicda−/− and to Rag2−/− mice. 564Igi Aicda−/− mice were bred to CD21-cre, CD19-cre and mb1-cre mice on Aicda−/− background. The males of these strains were bred to females 564Igi Aicdatg on Aicda−/− background. This tactic was used to prevent Cre-mediated recombination early during embryogenesis [64]. The offspring were selected by genotyping for Cre, Aicdatg, 564 IgH and IgL genes and the mice are designated 564Igi CD21-cre, 564Igi CD19-cre and 564Igi mb1-cre. Experiments were performed with female and male offspring.
ELISA
ELISAs were run to determine the levels of total antibodies, 564 Idiotype+ (Id+) antibodies and anti-RNA antibodies in the sera of various mouse strains. Id+ assays were performed using monoclonal anti-564 idiotypic antibodies (B6-256). B6-256 anti-id antibodies recognizes the combination of 564Ig H and L chains only[T4] [65]. B6-256 anti-id were coated on ELISA plates (Immulon 1B) at 5 µg/ml in borate buffer as previously described [24]. Plates were blocked with 1% BSA in borate buffer and serum samples were added in serial dilutions in 1% BSA blocking buffer. Bound serum antibodies were detected using alkaline phosphatase (AP)-coupled goat-anti-mouse-IgG2a, -IgG2b and -IgM antibodies (Southern Biotech) diluted to 1µg/mL in 1% BSA blocking buffer. The plates were developed with the AP substrate 4-nitrophenyl phosphate disodium salt hexadrydrate (pNPP) at 1mg/mL in 0.1M glycine/1mM ZnCl2/1mM MgCl2 ELISA buffer. For measurement of total serum antibodies of different isotypes, a similar protocol was followed. Wells were coated with isotype-specific goat-anti-mouse-IgG2a, -IgG2b and -IgM antibodies at 1 µg/ml in borate buffer and bound serum antibodies were detected with AP-conjugated isotype-specific goat-anti-mouse antibodies (Southern Biotech) in 1% BSA blocking buffer. Anti-RNA ELISAs were performed according to Dr. Keith Elkon (University of Washington, Seattle, Washington). Nunc MaxiSorb flat bottom (eBiosciences) ELISA plates were coated with 100ml of poly-L-Lysine (Sigma Aldrich #P8920), incubated 4hrs at room temperature, and then coated with 50µl of 10µg/ml of yeast RNA (Ambion/Invitrogen) in borate buffer and incubated overnight at 4°C. ELISA plates were blocked in 1× borate buffer/ 0.05% tween 20/ 5% goat serum/0.1% NaN3 and washed 3 times with 1× borate buffer/ 0.05% tween 20. Serum samples were added in serial dilutions in goat serum blocking buffer and developed as for 564 idiotype ELISA. The data was acquired using a Spectra Max 340 ELISA plate reader (Molecular Devices) at the optical density of 405 nm (OD405).
Immunofluorescence Staining of HEp-2 Cells
Fixed human HEp-2 cells (Antibodies Inc.) were coated with mouse serum according to the manufacturer’s instructions, and anti-nuclear antibodies were detected using an Alexa 488® goat-anti-mouse IgG secondary antibody (Invitrogen). Slides were mounted with ProLong Gold anti-fade reagent (Invitrogen) and digitally photographed with a Nikon E400 fluorescence microscope. Pixel Fluorescent intensity was measured by MetaXpress® software.
Flow Cytometry
Cells were stained for flow cytometry according to standard procedures. Tissue samples were homogenized to generate single cell suspensions and diluted to 1×106 cells/mL in 1% heat-inactivated rabbit serum / 0.1% NaN3 / 1× DPBS with Ca2+ and Mg2+ FACS staining buffer. Cells were centrifuged and re-suspended in 50µL of fluorescent anti-IgM, anti-B220, anti-CD138, anti-CD21, or anti-AA4.1 antibodies (BD Biosciences/Southern Biotech) at 1µg/mL in FACS staining buffer. Each sample was washed in 2mL FACS staining buffer and re-suspended in 500µL FACS staining buffer for analysis. Propidium iodide (PI) was added to a final concentration of 10ng/mL just prior to analysis on a FACScalibur flow cytometer (BD Biosciences) to assess cell viability.
Isolation of B lymphocytes
Fluorescently labeled B220+ idiotype− B cells were sorted from the spleen of a Rag−/−564Igi mouse by flow cytometry using the MoFlo sorter. Cells were stained for flow cytometry according to standard procedures. B cells were stained with B6-256 anti-Id, as described [24], which was coupled to the Alexa-647® fluorophore according to the manufacturer’s instructions (Invitrogen Molecular Probes). B cells were also stained with an anti-CD45 (B220) antibody labeled with R-Phycoerythrin (PE) from Southern Biotech as a marker for B cells. Fluorescent antibodies were generally used at 1 µg/ml. B220+ GFP− from 564Igi, 564Igi mb1-cre, 564Igi CD19cre and 564Igi CD21-cre BM and spleen cells were sorted and RNA prepared as described below. B cells from the spleen of an Aicda−/−564Igi mouse were purified using the EasySep Mouse B-Cell Enrichment kit (StemCell 19754), which isolates B cells by magnetic negative selection.
Cloning and Sequencing
Total RNA was isolated from the cells using TRIZOL reagent (Invitrogen 15596-018) and converted to cDNA using the iScript cDNA synthesis kit (BioRad 170-8890). The μ heavy chain and the κ light chain genes were PCR amplified using primers specific for the 564 μ heavy chain and κ light chain genes with the following sequences: forward 564 VH 5’-CTG-CAA-CCG-GTG-TCC-ACT-CCC-AGG-TC-3’ reverse Cμ 5’-AGG-GGG-CTC-TCG-CAG-GAG-ACG-3’, forward 564 Vκ 5’-CTG-CAA-CCG-GTG-TAC-ATT-CCC-AAA-TT-3’ and reverse Cκ 5’-GCC-ACC-GTA-CGT-TTC-AGC-TCC-3’. The amplified genes were then transfected into competent E. coli cells using the TA cloning kit (Invitrogen K2020-20). Positive transformants were selected based on ampicillin resistance and blue-white colony screening with the β-galactosidase gene, lacZ. The plasmids were isolated from the bacterial cultures using the QIAprep Spin Miniprep Kit (Qiagen 27106) and the presence of the insert was verified by restriction mapping. The inserts were sequenced using the standard M13 forward (5'-TGT-AAA-ACG-ACG-GCC-AGT-3') and M13 reverse (5'-CAG-GAA-ACA-GCT-ATG-AC-3') primers on the ABI 3130XL Automated DNA sequencer.
Antibody production
Cloning strategy, expression vectors, antibody expression in HEK293 cells, and antibody purification were as described [51]
Real-time PCR
All qPCR experiments were as follows: first-strand cDNA synthesis was performed on four-fold serial dilutions of purified RNA. Triplicates were amplified with commercially available mouse Aicda or Actb (endogenous control)-specific TaqMan primer/probe sets (Applied Biosystems) in an iQ5 real-time PCR system (Bio-Rad). Quantification was determined using standard curves for genes of interest and the Actb control.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Allen Parmelee, Stephen Kwok and Nicholas Cabal and Augustin Vanier for technical help. The authors are grateful to Dr. Tasuko Honjo for generously providing the Aicdatg mice, to Dr. Klaus Rajewsky for providing the mb1-cre mice established by Dr. Michael Reth; Dr. Janet Stavnezer for providing the Aicda−/− mice with the permission of Dr. Tasuko Honjo’s lab. We would also like to thank Dr. Hedda Wardemann for providing human Ig expression vectors and Dr. Sean Tracy for helpful suggestions on cloning and expressing mutated genes. We would like to thank Dr. Jin-Hwan Han for important suggestions and Dr. Robert Berland and Dr. Erik Selsing for critically reading the manuscript. This work was supported by National Institutes of Health grants (R01AI45104 and R01AI076409A for T.I.-K. and T32 AI007077-26A2 training grant, by Lupus Research Institute (T.I.-K.) R25 GM066567 (C.O.Medina). We are also grateful for generous support by the Eshe Fund and to the Keck Foundation.
Abbreviations
- abs
antibodies
- AID
activation induced deaminase
- Aicda
activation induced cytidine deaminase
- Aicdatg
activation-induced cytidine deaminase transgene
- BCR
B-cell receptor
- BM
Bone Marrow
- C
constant region of Ig
- CRE
causes recombination
- CSR
class switch recombination
- ELISA
enzyme linked immunosorbent assay
- GFP
green fluorescent protein
- HEp-2
human epithelium cell 2
- ICs
immune complexes
- Id
idiotype
- Ig
immunoglobulin
- H
heavy
- JH
heavy chain joining region
- L
light
- pCAG
chicken beta actin promoter
- qRT-PCR
quantitative polymerase chain reaction
- RAG
recombination activating gene
- SHM
somatic hypermutation
- SLE
systemic lupus erythematosus
- SP
spleen
- TLR
toll like receptor
- T
thymus derived-lymphocyte
- ASC
antibody secreting cell
- ANA
anti-nuclear antibody assay
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
References
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. The New England journal of medicine. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Winchester RJ, Kunkel HG, Agnello V. Occurrence of -globulin complexes in serum and joint fluid of rheumatoid arthritis patients: use of monoclonal rheumatoid factors as reagents for their demonstration. J Exp Med. 1971;134:286s–295s. [PubMed] [Google Scholar]
- 3.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 5.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 6.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci U S A. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 9.Muramatsu M, Sankaranand VS, Anant S, Sugai M, Kinoshita K, Davidson NO, Honjo T. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 10.Reynaud CA, Dahan A, Anquez V, Weill JC. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell. 1989;59:171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 12.Reynaud CA, Aoufouchi S, Faili A, Weill JC. What role for AID: mutator, or assembler of the immunoglobulin mutasome? Nat Immunol. 2003;4:631–638. doi: 10.1038/ni0703-631. [DOI] [PubMed] [Google Scholar]
- 13.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell. 2003;12:501–508. doi: 10.1016/s1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 14.Neuberger MS, Milstein C. Somatic hypermutation. Curr Opin Immunol. 1995;7:248–254. doi: 10.1016/0952-7915(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 15.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 16.Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Ann N Y Acad Sci. 1995;764:384–396. doi: 10.1111/j.1749-6632.1995.tb55853.x. [DOI] [PubMed] [Google Scholar]
- 17.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 18.Weller S, Faili A, Garcia C, Braun MC, Le Deist FF, de Saint Basile GG, Hermine O, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 20.Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesemann DR, Magee JM, Boboila C, Calado DP, Gallagher MP, Portuguese AJ, Manis JP, Zhou X, Recher M, Rajewsky K, Notarangelo LD, Alt FW. Immature B cells preferentially switch to IgE with increased direct Smu to Sepsilon recombination. J Exp Med. 2011;208:2733–2746. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Wuerffel R, Achour I, Lajoie B, Sen R, Dekker J, Feeney AJ, Kenter AL. Flexible ordering of antibody class switch and V(D)J joining during B-cell ontogeny. Genes Dev. 2013;27:2439–2444. doi: 10.1101/gad.227165.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Umiker BR, Andersson S, Fernandez L, Korgaokar P, Larbi A, Pilichowska M, Weinkauf CC, Wortis HH, Kearney JF, Imanishi-Kari T. Dosage of X-linked toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur J Immunol. 2014 doi: 10.1002/eji.201344283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giltiay NV, Chappell CP, Sun X, Kolhatkar N, Teal TH, Wiedeman AE, Kim J, Tanaka L, Buechler MB, Hamerman JA, Imanishi-Kari T, Clark EA, Elkon KB. Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. J Exp Med. 2013;210:2773–2789. doi: 10.1084/jem.20122798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muto T, Okazaki IM, Yamada S, Tanaka Y, Kinoshita K, Muramatsu M, Nagaoka H, Honjo T. Negative regulation of activation-induced cytidine deaminase in B cells. Proc Natl Acad Sci U S A. 2006;103:2752–2757. doi: 10.1073/pnas.0510970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci U S A. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Zemlin M, Wang YH, Munfus D, Huye LE, Findley HW, Bridges SL, Roth DB, Burrows PD, Cooper MD. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Nagy Z, Prak EL, Weigert M. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–755. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AG, Lu H, Raghavan SC, Muschen M, Hsieh CL, Lieber MR. Human chromosomal translocations at CpG sites and a theoretical basis for their lineage and stage specificity. Cell. 2008;135:1130–1142. doi: 10.1016/j.cell.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui X, Lu Z, Kurosawa A, Klemm L, Bagshaw AT, Tsai AG, Gemmell N, Muschen M, Adachi N, Hsieh CL, Lieber MR. Both CpG methylation and activation-induced deaminase are required for the fragility of the human bcl-2 major breakpoint region: implications for the timing of the breaks in the t(14;18) translocation. Mol Cell Biol. 2013;33:947–957. doi: 10.1128/MCB.01436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology (Oxford) 2007;46:1052–1056. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 36.Connolly K, Roubinian JR, Wofsy D. Development of murine lupus in CD4-depleted NZB/NZW mice. Sustained inhibition of residual CD4+ T cells is required to suppress autoimmunity. J Immunol. 1992;149:3083–3088. [PubMed] [Google Scholar]
- 37.Peng SL, Cappadona J, McNiff JM, Madaio MP, Owen MJ, Hayday AC, Craft J. Pathogenesis of autoimmunity in alphabeta T cell-deficient lupus-prone mice. Clin Exp Immunol. 1998;111:107–116. doi: 10.1046/j.1365-2249.1998.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J. Murine lupus in the absence of alpha beta T cells. J Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 39.Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B, Kelsoe G, Dianda L, Owen MJ, Hayday AC. Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non alpha/beta” T cells. J Exp Med. 1996;183:2271–2282. doi: 10.1084/jem.183.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambrianides A, Giles I, Ioannou Y, Mason L, Latchman DS, Manson JJ, Isenberg DA, Rahman A. Arginine mutation alters binding of a human monoclonal antibody to antigens linked to systemic lupus erythematosus and the antiphospholipid syndrome. Arthritis Rheum. 2007;56:2392–2401. doi: 10.1002/art.22743. [DOI] [PubMed] [Google Scholar]
- 41.Rahman A, Haley J, Radway-Bright E, Nagl S, Low DG, Latchman DS, Isenberg DA. The importance of somatic mutations in the V(lambda) gene 2a2 in human monoclonal anti-DNA antibodies. J Mol Biol. 2001;307:149–160. doi: 10.1006/jmbi.2000.4491. [DOI] [PubMed] [Google Scholar]
- 42.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 44.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waisman A, Kraus M, Seagal J, Ghosh S, Melamed D, Song J, Sasaki Y, Classen S, Lutz C, Brombacher F, Nitschke L, Rajewsky K. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Ig alpha/beta. J Exp Med. 2007;204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eilat D, Wabl M. B cell tolerance and positive selection in lupus. J Immunol. 2012;189:503–509. doi: 10.4049/jimmunol.1200848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. The Journal of experimental medicine. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z, Casali P. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kappaB pathway. Nat Commun. 2012;3:767. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hepburn AL, Lampert IA, Boyle JJ, Horncastle D, Ng WF, Layton M, Vyse TJ, Botto M, Mason JC. In vivo evidence for apoptosis in the bone marrow in systemic lupus erythematosus. Ann Rheum Dis. 2007;66:1106–1109. doi: 10.1136/ard.2006.065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 52.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Meffre E, Schaefer A, Wardemann H, Wilson P, Davis E, Nussenzweig MC. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J Exp Med. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ubelhart R, Bach MP, Eschbach C, Wossning T, Reth M, Jumaa H. N-linked glycosylation selectively regulates autonomous precursor BCR function. Nat Immunol. 2010;11:759–765. doi: 10.1038/ni.1903. [DOI] [PubMed] [Google Scholar]
- 55.Eschbach C, Bach MP, Fidler I, Pelanda R, Kohler F, Rajewsky K, Jumaa H. Efficient generation of B lymphocytes by recognition of self-antigens. Eur J Immunol. 2011;41:2397–2403. doi: 10.1002/eji.201041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herzog S, Jumaa H. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol. 2012;24:166–172. doi: 10.1016/j.coi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Stetson DB. Connections between antiviral defense and autoimmunity. Curr Opin Immunol. 2009;21:244–250. doi: 10.1016/j.coi.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck-Engeser GB, Eilat D, Wabl M. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology. 2011;8:91. doi: 10.1186/1742-4690-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 60.Macpherson AJ, Lamarre A, McCoy K, Harriman GR, Odermatt B, Dougan G, Hengartner H, Zinkernagel RM. IgA production without mu or delta chain expression in developing B cells. Nat Immunol. 2001;2:625–631. doi: 10.1038/89775. [DOI] [PubMed] [Google Scholar]
- 61.Hasan M, Polic B, Bralic M, Jonjic S, Rajewsky K. Incomplete block of B cell development and immunoglobulin production in mice carrying the muMT mutation on the BALB/c background. Eur J Immunol. 2002;32:3463–3471. doi: 10.1002/1521-4141(200212)32:12<3463::AID-IMMU3463>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 62.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, Conley ME, Cunningham-Rundles C, Durandy A, Meffre E. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J, Fernandez L, O’Connor KC, Imanishi-Kari T, Stollar BD. The rearranged V(H) domain of a physiologically selected anti-single-stranded DNA antibody as a precursor for formation of IgM and IgG antibodies to diverse antigens. J Immunol. 2001;167:3746–3755. doi: 10.4049/jimmunol.167.7.3746. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez L. B cell tolerance to nucleosomes in nomarmal mice and its breakdown in autoimmune-prone mice. PhD. Thesis in Immunology at Tufts University Sackler School of Biomedical Sciences. 2001 [Google Scholar]
- 66.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.