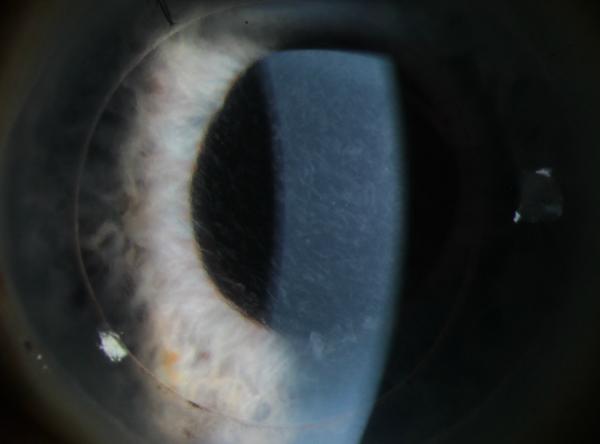
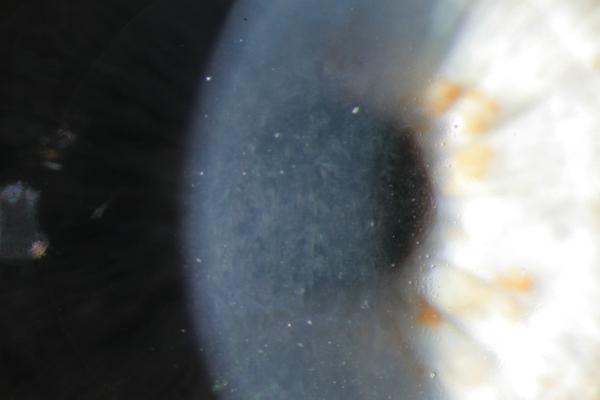
Abstract
Purpose
To examine the association between transient interface fluid (TIF) and textural interface opacity (TIO) following DSAEK surgery using intraoperative optical coherence tomography (iOCT) in the PIONEER study.
Methods
All consecutive eyes that underwent DSAEK between October 2011 and September 2013 from the PIONEER intraoperative and perioperative OCT study were included. iOCT images were captured after lenticule apposition with complete air fill and after air-fluid exchange. Postoperative day (POD) 1 OCT images were obtained. Outcome variables included the presence of TIO at the graft-host junction and the presence of intraoperative and postoperative interface fluid on OCT.
Results
Seventy-six eyes from 69 patients which underwent DSAEK with iOCT were included. The mean age was 71 years (range 31-90). The two most common indications for surgery were Fuchs’ dystrophy (63%) and pseudophakic bullous keratopathy (24%). In 18/76 (23.7%) eyes, TIF was visible on iOCT post air-fluid exchange. Of these eyes, 14 developed TIO. TIO was observed in 18/76 (23.7%) eyes. TIF on iOCT was associated with a significantly higher rate of postoperative TIO (OR=47.25; p<0.0001). Sixteen of the 18 eyes that had TIF on iOCT had had resolution on the POD 1 OCT. There was no significant difference in mean graft thickness between eyes with TIF on iOCT and those without (p=0.58).
Conclusions
Eyes with TIF on iOCT are more likely to develop TIO in the postoperative period. It is believed that the process of gap closure results in TIO, possibly secondary to precipitated solutes, retained viscoelastic, or lamellar irregularities caused by delayed adhesion or uneven matching of lamellar fibrils.
Keywords: Descemet's stripping endothelial keratoplasty, textural interface opacity, haze, transient interface fluid, intraoperative optical coherence tomography
INTRODUCTION
Over the past decade, Descemet's stripping automated endothelial keratoplasty (DSAEK) has become the most widely performed procedure for the management of corneal endothelial dysfunction1. The advantages of DSAEK over traditional penetrating keratoplasty have been well-described2. There are, however, post-operative challenges more specific to endothelial keratoplasty surgery including graft dislocation, “air bubble” management issues, and interface complications.
Interface complications are of particular interest because of their myriad etiologies. Although the mechanism for many interface complications, such as infection, epithelial downgrowth, and interface hemorrhage, is usually apparent, some mystery has remained about the phenomenon of “interface haze.” Interface haze refers to the typically early presence of a diffuse, central, often undulating, architectural pattern of grayish opacities at the level of the graft-host junction. This type of haze has been variably described as “interface wavelike deposits”3, “reticular haze”4,5, “ground glass interface haze6, and most recently “textural interface opacity”7. In keeping with the largest study attempting to classify the condition, we have elected to refer to this phenomenon as textural interface opacity (TIO).
Several etiologies for TIO have been proposed, most commonly mechanical irregularity induced by the microkeratome blade or the presence of persistent interface fluid or viscoelastic7. Although multiple authors have reported TIO developing in the setting of persistent interface separation3-5,7, confounding the latter theory is the observation that TIO occurs frequently in eyes in which there is no identifiable postoperative interface fluid.
We hypothesize that the presence of transient, occult intraoperative interface fluid (TIF) may account for such cases in which TIO develops in the absence of identifiable postoperative interface fluid. Using the results of intraoperative optical coherence tomography (iOCT) we present new information on the relationship between interface fluid and postoperative TIO.
METHODS
The PIONEER study is a prospective intraoperative and perioperative OCT study initiated at the Cleveland Clinic in November of 2011. All patients were consented and the study was approved by the institutional review board at the Cleveland Clinic and is adherent to the principles that were established in the Declaration of Helsinki. For this report, all eyes from the PIONEER study that underwent DSAEK by a single surgeon (JMG) between November 2011 and October 2013. There were no exclusion criteria for the use of iOCT.
The same surgical technique was used for all patients8. In brief, a temporal, 5 mm scleral tunnel incision was used and Descemet's membrane was stripped under sodium hyaluronate (Healon, Abbott Medical Optics). The peripheral host stroma was roughened with a Terry scraper (Storz). Viscoelastic was removed with single port irrigation and aspiration. All donor tissue was pre-cut by the surgeon's eye bank. Prior to folding, a thin bead of sodium hyaluronate was injected onto the endothelial surface of all donor tissue. Non-coapting forceps were used for donor insertion. Corneal vent incisions were not created except in rare circumstances (see Results).
A portable spectral domain optical coherence tomography (SD-OCT) system (Envisu C2200, Bioptigen, Research Triangle Park, NC) with customized microscope mount was utilized for intraoperative imaging. A standard imaging protocol employing a 12 x 12 mm cube was utilized in all cases. iOCT images were routinely performed after the donor tissue was thought to be completely apposed with a full anterior chamber air fill. Based on the OCT appearance, if the surgeon felt that additional external manipulations were indicated the OCT was repeated at the conclusion of these maneuvers and before starting the ten minute “wait period.” After performing a complete air-fluid exchange and prior to patching the eye closed, iOCT was repeated. One day postoperative OCT (RTVue version 6.8.0.27, Optovue, Inc., Fremont, CA) images were routinely obtained.
Patient demographics including patient age, ocular comorbidities and previous ocular surgeries were recorded. Donor tissue parameters including preoperative graft thickness and endothelial cell density were logged. Clinical outcomes data collected included any clinician documentation of “interface haze,” defined as a diffuse, central, undulating, architectural pattern of grayish opacities at the level of the graft-host junction. The postoperative interval at which the haze was first noted and the occurrence of any postoperative complications were also recorded. iOCT and postoperative OCT images were evaluated for the presence of interface fluid. Slit-lamp photographs were taken for demonstrative cases but not routinely performed. The Student t-test was used to compare continuous variables and the χ2 test was used to compare categorical variables. Odds ratios were also calculated (GraphPad InStat Version 3.10; GraphPad Software, Inc., San Diego, CA).
RESULTS
Seventy-six eyes from 69 patients were included in this study. Our patient population included 28 men (41%) and 41 women (59%). The mean age at the time of surgery was 71 years, ranging from 31 to 90. The two most common indications for surgery were Fuchs’ dystrophy (n=48; 63%) and pseudophakic bullous keratopathy (PBK) (n=18; 24%). Six patients (8%) had a previous failed penetrating keratoplasty. Other etiologies for endothelial failure included toxic anterior segment syndrome (n=1), posterior polymorphous corneal dystrophy (n=1), aphakic bullous keratopathy (n=1), and herpetic endotheliitis (n=1). Forty-nine (64%) eyes were pseudophakic at the time of surgery, 27 (36%) were phakic, and one eye was aphakic. Four eyes had previous glaucoma surgery. Seven eyes were functionally unicameral (prior vitrectomy with open posterior capsule). All patients had a minimum of one month follow-up.
All corneal grafts were pre-cut by the surgeon's eye bank. Graft size varied from 7.5 mm to 8.0 mm. The mean preoperative graft thickness for all donor tissue was 129±34 μm as reported by the eye bank. There was no significant difference in graft thickness in eyes with TIF (133 μm) and those without TIF (128 μm) (p = 0.58). There was also no significant difference in graft thickness in those eyes with TIO (136 μm) and without postoperative TIO (127 μm) (p = 0.71). There was also no significant difference in donor endothelial cell counts between eyes with TIF and those without (p=0.74).
Forty-nine (64.5%) eyes underwent DSAEK alone, 24 (31.6%) underwent combined DSAEK and cataract extraction, one underwent DSAEK with scleral-sutured intraocular lens (IOL), and one had DSAEK with an iris-sutured IOL. Three eyes underwent phakic DSAEK surgery. Corneal vent incisions were performed in two cases (2.6%). In both of these cases, venting incisions were performed based on the identification of significant central interface fluid after routine corneal sweeping. In both cases, the vent was manually gaped with a Sinskey hook while the cornea was externally swept toward the incision. In both cases, the interface gap did not resolve and no future venting incisions were attempted, regardless of interface appearance on iOCT.
Table 1 summarizes all cases of patients with TIF or TIO. In 58 (76.3%) eyes, no interface fluid was visible on iOCT at the end of surgery following air-fluid exchange. In 18 (23.7%) eyes, TIF was visible on iOCT. TIO was documented in 18 (23.7%) eyes in the postoperative period. Of the 18 patients with TIO, 14 (77.8%) had TIF on iOCT at the conclusion of the surgery. Figure 1 summarizes the distribution of cases with TIO and TIF. TIF on iOCT was associated with a higher rate of postoperative TIO (OR=47.25; p<0.0001).
| Cases | TIF on iOCT | TIO | POD when TIO first documented | Indication for Surgery | Surgical Comorbidities | Concurrent Procedure |
|---|---|---|---|---|---|---|
| 1 | Yes | No | - | Fuchs | None | None |
| 2 | Yes | No* | - | Fuchs | None | None |
| 3 | Yes | No* | - | Fuchs | None | None |
| 4 | Yes | No | - | Failed DSAEK graft | Trabeculectomy, PPV, scleral sutured IOL | Partial anterior vitrectomy |
| 5 | Yes | Yes | 13 | PBK | Tube shunt, ACIOL, iris defect | None |
| 6 | Yes | Yes | 34 | Fuchs | None | None |
| 7 | Yes | Yes | 1 | PBK | PPV, scleral buckle, ACIOL | ACIOL explant, iris sutured IOL |
| 8 | Yes | Yes | 28 | Fuchs | None | Cataract |
| 9 | Yes | Yes | 29 | Fuchs | None | Cataract |
| 10 | Yes | Yes | 11 | Fuchs | None | Cataract |
| 11 | Yes | Yes | 7 | Fuchs | None | None |
| 12 | Yes | Yes | 29 | Fuchs | None | Cataract |
| 13 | Yes | Yes | 21 | Fuchs | None | Cataract |
| 14 | Yes | Yes | 7 | Fuchs | None | None |
| 15 | Yes | Yes | 30 | Fuchs | None | Cataract |
| 16 | Yes | Yes | 7 | Fuchs | None | Cataract |
| 17 | Yes | Yes | 7 | PBK | None | None |
| 18 | Yes | Yes | 9 | Fuchs | None | Cataract |
| 19 | No | Yes† | 53 | Fuchs | None | None |
| 20 | No | Yes | 30 | Failed PK | Failed PK | None |
| 21 | No | Yes‡ | 43 | Fuchs | None | Cataract |
| 22 | No | Yes§ | 306 | Fuchs | None | Cataract |
Graft detachment was noted on POD 1
The patient had a peripheral postoperative fluid cleft which resolved and resulted in peripheral interface haze in the same location
“Trace haze” was documented in the setting of unexplained vision loss. The patient was later diagnosed with nonarteritic anterior ischemic optic neuropathy
“Trace haze” was documented in the far postoperative period, unlikely to represent the architectural haze in question
Figure 1.
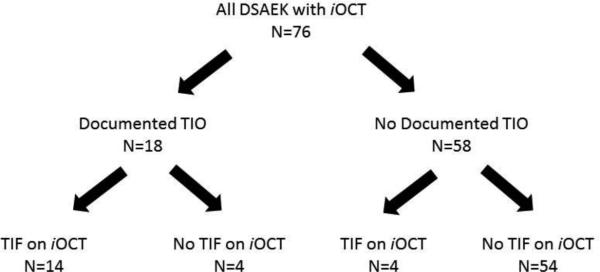
Representation of all eyes included in analysis.
Legend: iOCT: intraoperative OCT; TIO: textural interface opacity; TIF: transient interface fluid
Of the 18 eyes with TIF, 14 developed TIO. In the 4 eyes that did not develop TIO, two experienced graft dislocation on the first postoperative day and were successfully re-bubbled. In both of these cases, there was no TIF visible on iOCT when the graft was re-attached. Only 2 of the 18 eyes (11.1%) that had TIF on iOCT had persistence of the fluid on the first postoperative day OCT. One of these eyes had resolution of the interface gap by the seventh postoperative day, while the other continued to have slight persistence of the interface gap three months after surgery. The overall graft dislocation rate in all eyes was 7.9%.
DISCUSSION
The use of iOCT to visualize the DSAEK graft-host interface was first described in 2010 by Knecht, et al9. These authors reported two cases in which occult central donor-host interface separation was identifiable on iOCT. Interestingly, both of these grafts were completely attached on the first postoperative day, indicating that complete intraoperative donor-host graft apposition was not required for subsequent graft adherence. Some authors have reported the resolution of intraoperative gap with interventions such as the creation of venting incisions9-10 or additional aspiration11. In other patients, the effect of venting incisions on intraoperative gap was more limited9. Anshu et al reported a case of persistent interface fluid on the one-month postoperative visit in which a drainage incision was created with a diamond blade, but the interface gap persisted4.
In this large prospective consecutive case series, we demonstrate a strong correlation between the presence of central TIF and the appearance of postoperative TIO. Eyes with TIF had a statistically significant increased risk of developing TIO. Of the four patients in whom TIF was present but TIO did not develop, two had graft dislocations on POD 1 and therefore did not have “resolution” of the TIF. It is not clear why TIO was not visible in the other two cases, but it is possible that TIO was present but not documented.
Also worth discussing are the four cases in which “haze” was documented despite the absence of initial TIF. In three of these cases, the interface haze was noted later in the postoperative period than the typical 30 days seen with TIO and may have represented other forms of interface haze. In one case, “peripheral haze” was documented in the same location as a resolved fluid cleft. Two of the patients in this group had “trace haze” documented in the setting of unexplained decreased vision. This may reflect observer bias related to the decreased VA. The last case was also documented as “trace haze” and only noted approximately ten months after the surgery. We believe these four cases are likely false positives, but chose to include them to be as rigorous as possible with our data analysis.
The composition of TIF is likely a variable mixture of aqueous, balanced salt solution, and, in some cases, viscoelastic. Slowly resolving TIF, especially that which requires weeks or longer to resolve, may represent viscoelastic or a viscoelastic-aqueous mixture (Figures 2 and 3). Fast resolving TIF (not visible clinically or by OCT on the first postoperative exam) may represent aqueous or BSS fluid pockets (Figure 4). The fact that both slow and fast resolving TIF are associated with TIO indicates that retained viscoelastic may not be the sole etiology of TIO.
Figure 2.
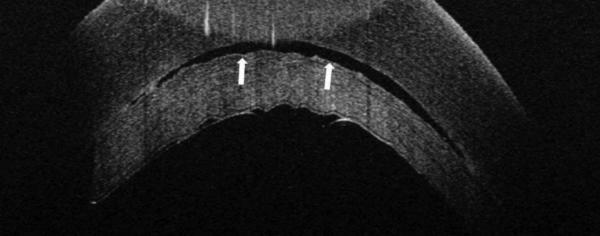
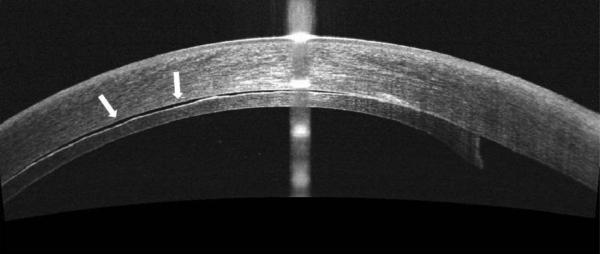
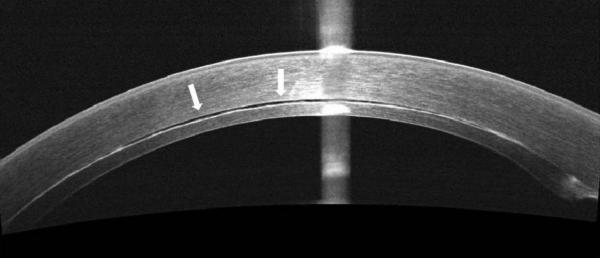
In some cases, TIF is seen on iOCT, and persists beyond POD 1, also resulting in TIO. This persistent TIF is thought to be secondary to retained viscoelastic.
A. iOCT with interface space. This space is seen in multiple cross-sectional images.
B. POD 1 OCT. Note the areas of hyper-reflectivity along the stromal surface of the donor lenticule.
C. POM 1 OCT showing a stable amount of persistent interface fluid D. POM 1 Photo showing wave-like TIO.
Figure 3.
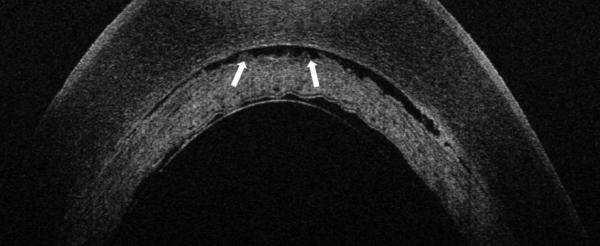

Another example of TIF seen on iOCT, with near-complete resolution of TIF on POD 1, resulting in TIO.
A. iOCT with interface space. Note the undulating contour of the stromal surface of the lenticule.
B. POD 1 OCT with near complete resolution of TIF. Arrows point to trace residual interface fluid. C. POM 1 Photo showing wave-like TIO.
Figure 4.

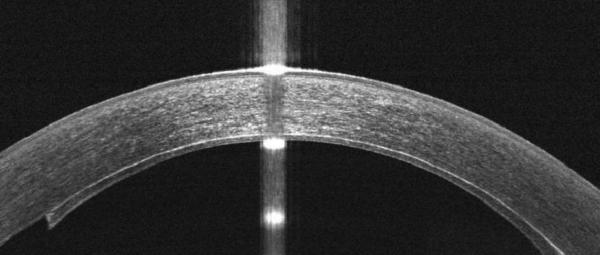
In other cases, TIF is seen on iOCT, resolves by POD 1, and results in TIO.
A. iOCT with interface space. Arrows point to the space which is again seen in multiple cross-sectional images.
B. POD 1 OCT showing resolution of TIF and total attachment.
C. POW 1 Photo showing wave-like TIO.
The presence of TIF did not seem to impact the likelihood of postoperative donor adherence. Most grafts with an intraoperative interface gap had complete resolution of the interface fluid and were fully attached on the first postoperative day (77.8%). Although a higher percentage of these eyes had graft dislocations than eyes without TIF (11.1% vs. 6.9%), this finding was not statistically significant. It is interesting to note that removing small amounts of TIF with external measures proved to be extremely difficult. We observed that if initial corneal sweeps were performed with a high-pressure air-fill in the anterior chamber, further interventions such as repeated sweeping, increased time with air-fill, or placement of corneal venting incisions did not result in improvement or closure of the gap.
It is also interesting that some eyes develop TIF and others do not. Based on the parameters that we studied, we could not identify any preoperative risk factors to explain why TIF occurs. One hypothesis has suggested that thinner graft tissue would result in smaller or no TIF9. However, in our sample, graft thickness was not significantly different in eyes with interface gap compared to those without. Mean graft size and donor endothelial cell counts were also not significantly different in the two groups. Graft insertion technique has also been identified as a possible risk factor. We employed only one graft insertion technique in our study, but previous authors have reported cases of TIO with a variety of insertion styles and insertion devices7. Other parameters, such as post-cut donor-host curvature mismatch, may play a role but evaluating this theory is technically difficult.
Based on the results of this study, we suggest the classic “wave-like” interface haze, or TIO, occurs preferentially in eyes in which a central, loculated pocket of interface fluid forms during surgery. The mechanism by which of this pocket of fluid forms is not clear. It has been hypothesized that the separation between donor and host corneas may be caused by microkeratome-induced fibrillar irregularities extending from the donor surface. Newman et al, showed using electron microscopy that an eye with TIO had irregular collagen fibrils of varying lengths at the stromal surface of the donor tissue12. In this study, the height of sequestered fluid often was in great excess of the OCT observable irregularities in the donor tissue, however it is possible that very fine fibrils may not be visible on iOCT. Although irregularities in donor tissue may play a role in the actual pattern of TIO, it seems unlikely that it is solely responsible for TIF formation. Alternatively, curvature mismatch between the donor lenticule and host stroma may preclude immediate, complete central apposition at the time of surgery. As the sequestered fluid traverses the donor tissue, interface opacity may result from residual solutes or decomposition products are concentrated or precipitated on the donor stromal surface.
Hyperreflectivity on donor surface prior is visible prior to complete resolution of the interface gap (Figure 2). Whether this hyperreflectivity represents the simple apposition of two tissue planes, inherent irregularities of the donor surface, abnormalities in tissue hydration, or early deposition of concentrated solutes is unclear. We favor the latter explanation based on the observation that the prominent hyperreflectivity seen postoperatively was not visualized on iOCT at the time of graft insertion. The resulting wavy or reticular pattern of deposits may be related to the undulating surface contour of the donor lenticules (Figure 3). Perhaps the most important observation of our study is that that some degree of TIO developed even in cases in which the interface gap completely resolved by the first postoperative day. This finding was of particular importance because it may explain the majority of “idiopathic” TIO cases reported before the availability of iOCT.
Although this study reflects results from a prospective iOCT study, assessment of TIO was not evaluated in a prospective fashion. This limits our ability to compare the severity of TIO and its relationship to the amount of TIF in an objective fashion. Given the retrospective analysis, we did not systemically grade TIO severity and acknowledge that there may have been differences in sensitivity of the observer in documenting interface haze. Also not addressed by our study is the visual impact of interface haze as it relates to best corrected visual acuity postoperatively. For this report, we have focused on the anatomic abnormality of TIO and the factors associated with its formation. For future studies, we expect to be able to provide longer follow-up and the long-term implications for visual acuity.
In summary, this report strongly supports the role of TIF in the development of postoperative TIO. This represents one of the first applications of iOCT to improve understanding of the pathogenesis of a postoperative abnormality in DSAEK surgery. We are currently in the process of developing a blinded, prospective trial to confirm or refute this hypothesis.
Acknowledgments
Support detail: NIH/NEI K23EY022947-01A1 (JPE)
Footnotes
Conficts of Interest and Source of Funding
Dr. Justis P. Ehlers and Sunil K. Srivastava have licensed intellectual property related to intraoperative OCT to Bioptigen, Inc.
REFERENCES
- 1.Eye Bank Association of America 2012 Eye Banking Statistical Report. 2013 Available at: http://www.restoresight.org/wp-content/uploads/2013/04/2012_Statistical_Report_FINAL-reduced-size-4-10.pdf. Accessed December 20, 2013.
- 2.Lee WB, Jacobs Ds, Musch DC, et al. Descemet's stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Kymionis GD, Ide T, Yoo SH. Interface wavelike deposits after descemet stripping automated endothelial keratoplasty. Arch Ophthamol. 2009;127:1289–90. doi: 10.1001/archophthalmol.2009.249. [DOI] [PubMed] [Google Scholar]
- 4.Anshu A, Planchard B, Price MO, et al. A cause of reticular interface haze and its management after descemet stripping endothelial keratoplasty. Cornea. 2012;31:1265–8. doi: 10.1097/ICO.0b013e31823d027d. [DOI] [PubMed] [Google Scholar]
- 5.Hurmeric V, Wang J, Kymionis GD, et al. Persistent lamellar interface fluid with clear cornea after descemet stripping automated endothelial keratoplasty. Cornea. 2011;30:1485–7. doi: 10.1097/ICO.0b013e3182068974. [DOI] [PubMed] [Google Scholar]
- 6.Tarnawska D, Wylegala E. Management of pseudophakic bullous keratopathy by combined Descemet-stripping endothelial keratoplasty and intraocular lens exchange. J Cataract Refract Surg. 2008;34:1708–14. doi: 10.1016/j.jcrs.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Vira S, Shih CY, Ragusa N, et al. Textural interface opacity after descemet stripping automated endothelial keratoplasty: a report of 30 cases and possible etiology. Cornea. 2013;32:e54–9. doi: 10.1097/ICO.0b013e31826429d5. [DOI] [PubMed] [Google Scholar]
- 8.Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty: a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology. 2008;115:1179–86. doi: 10.1016/j.ophtha.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Knecht PB, Kaufmann C, Menke MN, et al. Use of intraoperative fourier-domain anterior segment optical coherence tomography during descemet stripping endothelial keratoplasty. Am J Ophthalmol. 2010;150:360–365. e2. doi: 10.1016/j.ajo.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Wylegala E, Nowinska AK, Wroblewska-Czajka E, et al. Donor disc attachment with intraoperative spectral optical coherence tomography during descemet stripping automated endothelial keratoplasty. Indian J Ophthalmol. 2013;61:511–3. doi: 10.4103/0301-4738.119440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ide T, Wang J, Tao A, et al. Intraoperative use of three-dimensional spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2010;41:250–4. doi: 10.3928/15428877-20100303-15. [DOI] [PubMed] [Google Scholar]
- 12.Newman LR, Rosenwasser GO, Dubovy SR, Matthews JL. Clinicopathologic correlation of textural interface opacities in descemet stripping automated endothelial keratoplasty: a case study. Cornea. 2014;33:306–9. doi: 10.1097/ICO.0000000000000057. [DOI] [PubMed] [Google Scholar]