Structured Abstract
Background
Ostomy surgery is common and has traditionally been associated with high rates of morbidity and mortality, suggesting an important target for quality improvement.
Objective
To evaluate the variation in outcomes after ostomy creation surgery within Michigan in order to identify targets for quality improvement.
Design
Retrospective cohort study.
Setting
The 34-hospital Michigan Surgical Quality Collaborative (MSQC).
Patients
Patients undergoing ostomy creation surgery between 2006-2011.
Main outcome measures
We evaluated hospitals' morbidity and mortality rates after risk-adjustment (age, comorbidities, emergency v. elective, procedure type).
Results
4,250 patients underwent ostomy creation surgery; 3,866 (91.0%) procedures were open and 384 (9.0%) were laparoscopic. Unadjusted morbidity and mortality rates were 43.9% and 10.7%, respectively. Unadjusted morbidity rates for specific procedures ranged from 32.7% for ostomy-creation-only procedures to 47.8% for Hartmann's procedures. Risk-adjusted morbidity rates varied significantly between hospitals, ranging from 31.2% (95%CI 18.4-43.9) to 60.8% (95%CI 48.9-72.6). There were five statistically-significant high-outlier hospitals and three statistically-significant low-outlier hospitals for risk-adjusted morbidity. The pattern of complication types was similar between high- and low-outlier hospitals. Case volume, operative duration, and use of laparoscopic surgery did not explain the variation in morbidity rates across hospitals.
Conclusions
Morbidity and mortality rates for modern ostomy surgery are high. While this type of surgery has received little attention in healthcare policy, these data reveal that it is both common and uncommonly morbid. Variation in hospital performance provides an opportunity to identify quality improvement practices that could be disseminated among hospitals.
Keywords: stoma care, ostomy surgery, ostomy complications, surgical collaborative
Introduction
Approximately 100,000 people in the United States undergo operations that result in a colostomy or ileostomy each year.1 The high incidence of ostomy surgeries in the United States is due in part to the increasing prevalence of colorectal cancer and diverticular disease.2, 3 Despite these being common operations, some reports indicate that up to 70% of patients experience postoperative complications.4 If accurate, these rates would exceed what is observed following established high-risk procedures, such as major cancer surgery in the elderly.5, 6 Complications result in high costs for the healthcare system, due to longer hospitalizations and higher readmission rates.7, 8 Given this, ostomy creation procedures may represent a significant financial burden to hospitals and source of suffering for patients who experience these complications, making these procedures a potential target for surgical quality improvement efforts.9
However, the published literature lacks accurate data regarding the true morbidity and mortality ascribed to modern ostomy surgery. Single institution studies and those based on administrative data lack generalizability and/or sensitivity for detecting complications, especially those diagnosed after hospital discharge.10, 11 Furthermore, it is unknown whether hospitals vary in their success with avoiding ostomy surgery complications. Other types of high risk surgery have demonstrated significant variation in outcomes, leading to opportunities for improvement through the replication of practices from the best-performing centers.12
We used statewide data from a validated clinical registry in Michigan to study rates of morbidity and mortality for ostomy surgery, and whether results vary across hospitals. We adjusted analyses for case-mix and patient factors. We then focused on potential mechanisms for hospital variation by studying differences between high- and lowperforming centers. Understanding hospital variation in perioperative morbidity has the potential to identify targets for collaborative quality improvement in our region.
Methods
Data Source and Study Population
Data was obtained from the Michigan Surgical Quality Collaborative (MSQC) prospective clinical registry between 2006 through 2011. The MSQC is a provider-led quality improvement organization funded by Blue Cross and Blue Shield of Michigan. Data from 34 participating hospitals were employed for this analysis. This project followed standard data definitions and collection protocols for the Michigan Surgical Quality Collaborative platform as previously described.13 Data collection occurs at the hospital level by designated MSQC data-collection nurses. Accuracy of data collection and maintenance is ensured by rigorous training of collection staff and data audits performed at participating sites. Variables collected include patient demographics, preoperative risk factors, laboratory values, perioperative factors and events, and 30-day postoperative morbidity and mortality. Additional hospital-level data was obtained from the American Hospital Association (AHA) Hospital and Health System Data Resources. Specific variables included total bed size, inpatient medical/surgical bed size, ICU bed size, teaching hospital status, and the presence of dedicated inpatient wound care teams.
Patients were identified for study if they underwent ostomy creation surgery within the study period and were over 18 years of age. These procedures were identified by Current Procedural Terminology (CPT) codes and included the following: ostomy creation only (44187, 44188, 44310, 44320), repair of perforation with ostomy (44605), resection with ostomy (44141, 44144, 44146, 44155, 44208, 44212), abdominoperineal resection (APR) (45110, 45395), and Hartmann's procedure (44143, 44206). (Table 1)
Table 1. Definition of Procedure Codes.
| Ostomy Creation | |
| 44187 | Laparoscopic ileo/jejunostomy |
| 44188 | Laparoscopic colostomy |
| 44310 | Ileostomy/jejunostomy |
| 44320 | Colostomy |
| Repair of Perforation | |
| 44605 | Repair of bowel lesion with ostomy |
| Resection with Ostomy | |
| 44141 | Hemicolectomy with ostomy |
| 44144 | Hemicolectomy with ostomy |
| 44146 | Hemicolectomy with ostomy |
| 44155 | Colectomy with ileostomy |
| 44208 | Laparoscopic colectomy with coloproctostomy |
| 44212 | Laparoscopic total proctocolectomy |
| Abdominoperineal Resection | |
| 45110 | Rectal resection with ostomy |
| 45395 | Laparoscopic rectal resection with ostomy |
| Hartmann's Procedure | |
| 44143 | Partial colectomy with creation of Hartmann's pouch |
| 44206 | Laparoscopic partial colectomy with creation of Hartmann's pouch |
Outcomes
The primary outcome for this study was 30-day morbidity. Secondary outcomes of interest included 30-day mortality and specific postoperative complications such as surgical site infection (superficial, deep, and organ space defined separately), deep venous thrombosis, urinary tract infection, acute renal failure, postoperative bleeding requiring transfusion, stroke, unplanned intubation, fascial dehiscence, prolonged mechanical ventilation over 48 hours, myocardial infarction, pneumonia, pulmonary embolism, sepsis, and renal insufficiency. Major complications excluded superficial surgical site infection, renal insufficiency, urinary tract infection, and deep venous thrombosis in the absence of pulmonary embolism. Surgical complications were defined as postoperative bleeding requiring transfusion, fascial dehiscence, and surgical site infection. Medical complications included all others listed above.
Statistical Analysis
In order to rank hospitals based on performance, we applied risk-adjusted 30-day morbidity as the primary outcome. Risk-adjustment models were developed using backward stepwise logistic regression (α= 0.05 significance threshold) that included the following variables- patient age, sex, race, BMI, diabetes, smoking status, alcohol use, dyspnea, do-not-resuscitate (DNR) status, preoperative functional status, chronic obstructive pulmonary disease (COPD), pneumonia, ascites, congestive heart failure, need for dialysis, hemiplegia, history of transient ischemic attack (TIA), disseminated cancer, steroid use, bleeding disorders, chemotherapy, radiotherapy, sepsis, esophageal varices, prior myocardial infarction, angina, hypertension requiring medication, peripheral vascular disease, prior operations, American Society of Anesthesiologists (ASA) class, operative duration, surgeon specialty, admission prior to date of operation, emergency procedure status, and preoperative diagnosis.
The final model included 13 variables: diabetes (none, non-insulin dependent, insulin dependent), admission prior to date of operation (>24 hours inpatient stay immediately prior to operation), preoperative steroid usage, congestive heart failure, emergency status, DNR status, preoperative functional status (independent, partially dependent, totally dependent), dyspnea (none, with exertion, at rest), ascites, open versus laparoscopic operation, case complexity (ostomy creation only, repair of perforation with ostomy, resection with ostomy, abdominoperineal resection, Hartmann's procedure), prior operation, and ASA class (I-II, III, IV-V). The C-statistic for the model was 0.701 and the Hosmer-Lemeshow p-value was 0.359. High-and low outlying hospitals were identified if the confidence interval of their risk-adjusted complication rate did not encompass the overall mean rate across hospitals for that outcome. To identify potential mechanisms underlying differences between outcomes between low- and high-outlier hospitals, we assessed complication profile (major, surgical, and medical), emergent procedures, procedure-mix, and the proportion of cases performed after more than one day of hospitalization (which is a surrogate for operations on inpatient consults or severely ill patients) between high-and low outlying hospitals. Differences between hospitals were compared using t-test for continuous variables, while Chi-squared and Fisher's exact tests were employed to assess differences in categorical variables.
All statistical analyses were performed using Stata statistical software version 12.1 (College Station, Texas). This study was approved by the University of Michigan Institutional Review Board.
Results
Study population
We identified 4,250 patients who underwent ostomy creation surgery in Michigan between 2006-2011. Patient and operative characteristics are shown in Table 2 and Figure 1. The median age of the study population was 65.1 years and 50.2% of patients were male. The median hospital length of stay was 10 days (IQR= 10 days). Emergency surgical procedures comprised 37.2% of the study population. There were 3,866 (91.0%) procedures performed open and 384 (9.0%) were laparoscopic. Differences in case mix are detailed in Figure 1. The most common preoperative diagnoses were diverticular disease/inflammation (n=1,615; 38.0%) and colorectal cancer (n=1,003; 23.6%). (Table 2) The unadjusted morbidity rate following emergent procedures (55.4%) was significantly higher than elective cases (37.0%, p<0.01). (Figure 2) Mortality was higher for emergent when compared to elective cases (18.8% vs. 5.9%, p<0.01). Morbidity and mortality rates were highest for resection and Hartmann's procedures. (Figure 3)
Table 2.
Patient population.
| Patient Characteristic | Statistic | ||
|---|---|---|---|
| Descriptives | |||
|
| |||
| Median age (years) | 65.1 | ||
| Male sex (%) | 50.2 | ||
| Non-white race (%) | 24.7 | ||
| Mean BMI (Kg/m2) | 27.8 | ||
|
| |||
| Clinical | n (%) | ||
|
| |||
| Independent functional status | 3235 (76.2) | ||
| Hypertension requiring medication | 2451 (57.7) | ||
| ASA Class ≥4 | 1185 (27.9) | ||
| Albumin <3.5 g/dl | 1127 (26.5) | ||
| Smoking in past year | 1096 (25.8) | ||
| Diabetes mellitus | 803 (18.9) | ||
| Sepsis | 692 (16.3) | ||
| Chronic obstructive pulmonary disease | 507 (11.9) | ||
| Steroid use | 388 (9.1) | ||
| Disseminated cancer | 353 (8.3) | ||
| Dialysis | 160 (3.8) | ||
| DNR | 121 (2.9) | ||
|
| |||
| Operative Diagnosis | n (%) | ||
|
| |||
| Diverticular disease/inflammation | 1615 (38.0) | ||
| Colorectal cancer | 1003 (23.6) | ||
| Other | 610 (14.4) | ||
| Obstruction/hernia/volvulus | 430 (10.1) | ||
| Other neoplasm | 173 (4.0) | ||
| Inflammatory bowel disease | 157 (3.7) | ||
| Vascular infufficiency | 151 (3.6) | ||
| Prolapse/ostomy complications | 71 (1.7) | ||
| Bleeding | 40 (0.9) | ||
Figure 1. Distribution of ostomy surgery cases for elective (n= 2,667; 62.8%) and emergent procedures (n= 1,583; 37.2%).

Figure 2.
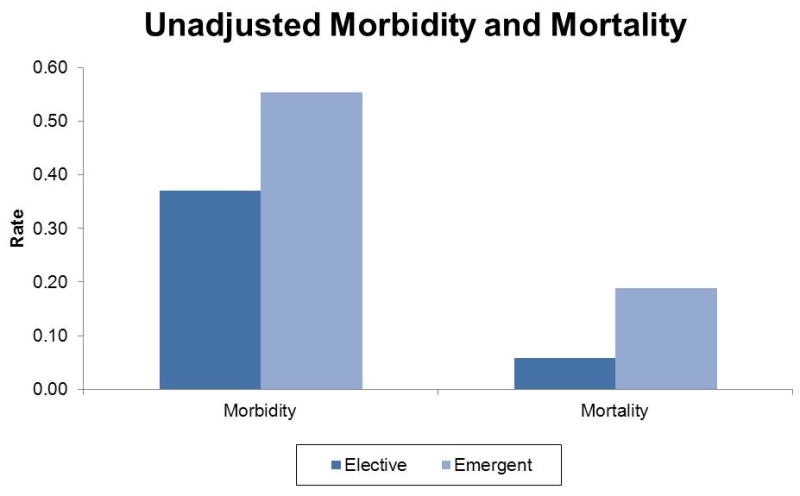
Unadjusted morbidity and mortality displayed by emergent status.
Figure 3.
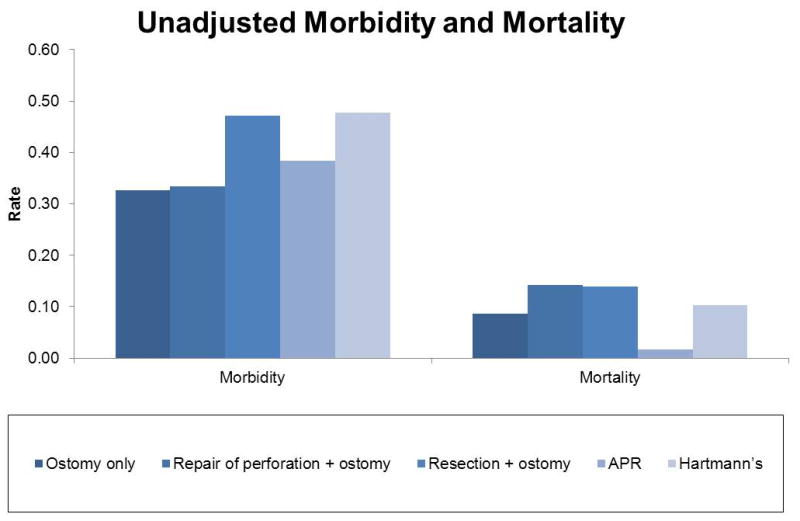
Unadjusted morbidity and mortality displayed by procedure type.
Variation in morbidity after ostomy creation procedures
Risk-adjusted morbidity rates across all 34 centers were high, with a mean of 43.9% (range= 30.1%-58.5%). The most common complications were need for postoperative ventilation (13.7%), postoperative transfusion (8.9%), sepsis (7.9%), and pneumonia (7.1%). (Table 3.) Hospitals were ranked by risk-adjusted 30-day morbidity rates. Plot of risk-adjusted morbidity rates including 95% confidence intervals for each center was generated in order to identify hospitals whose performance is significantly different from the overall mean. (Figure 4) As a result, three low-outlier (low complication rate) and five high-outlier hospitals were identified. No outliers with respect to risk-adjusted mortality were identified (range= 6.1%-10.8%).
Table 3. List of Common and Selected Complication Rates.
| Complication | Unadjusted Rate |
|---|---|
| Prolonged ventillation | 13.7% |
| Sepsis | 7.9% |
| Pneumonia | 7.1% |
| Superficial SSI | 6.8% |
| Organ space SSI | 4.2% |
| Wound dehiscence | 2.4% |
| Deep SSI | 2.3% |
| Acute renal failure | 2.3% |
| Myocardial infarction | 1.3% |
Figure 4.
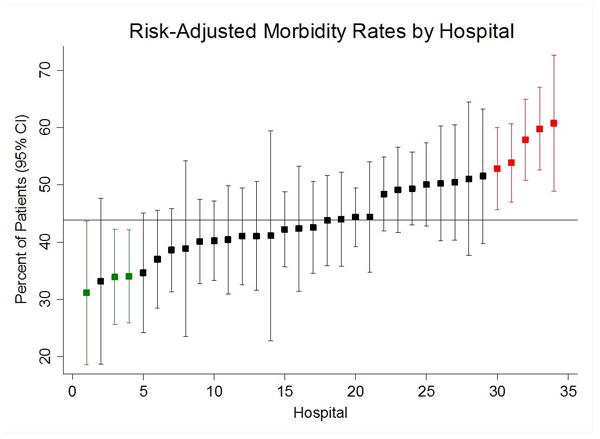
Risk-adjusted morbidity rates for all 34 hospitals including 95% confidence intervals. Average percentage (43.8%) is displayed as a horizontal line. Outlier hospitals are identified is their 95% confidence intervals do not intersect with the average morbidity line.
Understanding Hospital Variation
Potential mechanisms for observed differences in outcomes between low- and high- outlier hospitals was assessed through categorizing complications by nature (major, surgical, and medical). Predictably, high-outlier centers had significantly higher rates of major (42.4% vs. 25.1%, p<0.001), surgical (26.0% vs. 14.4%, p<0.001), and medical (39.7% vs. 24.0%, p<0.001) complications when compared to low-outlier centers. While rates of postoperative morbidity were clearly higher at high-outlier hospitals, there were no systematic differences in the profile of such complications. In other words, major, surgical, and medical complications all increased proportionally from low- to high-outlier centers. We also examined individual complications (e.g. pneumonia, myocardial infarction, superficial surgical site infection) and did not find patterns of differences in the distribution of complication types between high- and low-outlier hospitals (data not shown). These analyses refuted our hypothesis that best-performing hospitals might be avoiding particular types of complications
Operative and patient characteristics were also compared between low- and high-outlier hospitals. (Table 4) There were no significant differences in the proportion of cases performed emergently, laparoscopically, or after more than one day of hospitalization (a proposed surrogate measure for cases performed after urgent, inpatient consultation, or on patients with severe comorbidities). Additionally, there were no significant differences in patient age or the percent of patients receiving ostomy surgery for malignancy. There was a small, statistically-significant difference in median operative time between low (120 minutes) and high-outlier (135 minutes, p<0.001) hospitals. Lower median length of stay was observed at low-outlier hospitals when compared to high-outlier hospitals (8.5 days vs. 10 days, p=0.004). No differences in average bed size, ICU availability, or residency training status were observed (data not shown). Further, all high- and low- outlier centers had wound care teams available at their hospital.
Table 4.
Comparison of characteristics of High- and Low-outlier hospitals.
| Characteristic | Low-outliers | High-outliers | p-value |
|---|---|---|---|
|
| |||
| Statistic | (n=346) | (n=750) | |
| Median operative duration (minutes) | 120 | 135 | <0.001 |
| Cases performed after >1 day LOS (%) | 47.1% | 50.1% | 0.278 |
| Laparoscopic procedures (%) | 13.6% | 10.3% | 0.107 |
| Emergency procedures (%) | 39.3% | 40.3% | 0.763 |
| Median length of stay | 8.5 | 10 | 0.004 |
| Median patient age | 64.5 | 64.9 | 0.269 |
| Patients with colorectal malignancy (%) | 26.3% | 21.9% | 0.106 |
| Patients with inflammatory disease (%) | 41.0% | 39.7% | 0.682 |
| Average number of comorbid conditions | 3.6 | 3.6 | 0.735 |
| Hospital average number of medical/surgical beds | 223 | 317 | 0.532 |
| Hospital average number of ICU beds | 27 | 47 | 0.383 |
Discussion
Surgery in which an ostomy is created is common in general surgical practice; thus understanding the risks associated with these procedures is important for hospitals, surgeons, and for the purposes of resource planning and appropriate patient counseling. This study demonstrates that morbidity following ostomy creation procedures is high and varies significantly across Michigan hospitals. These observations are not explained by patient and procedural factors or baseline hospital characteristics. While certain patient comorbidities are associated with a higher risk of postoperative complications, this work suggests that hospital and surgeon practices may also contribute to significant differences in morbidity rates, because adjusting for these factors did not change the finding of significant variation.
Risk-adjusted morbidity rates in this study were impressively high, ranging by hospital from 30.1 to 58.5 percent. These rates exceed what has been reported for emergent general surgical procedures, where the propensity for poor outcomes is well established.14 Furthermore, because our database does not currently include ostomy specific complications such as dehydration, skin brakkdown, and prolapse, these rates may underestimate the true burden of morbidity. Though our cohort included emergent and non-emergent cases, colorectal operations performed in the elective setting have similarly high morbidity rates when an ostomy is placed.15, 16 Lower morbidity and mortality rates have been reported in several large series where segmental colectomy with primary anastomosis is performed.17, 18 This suggests that an increase in overall risk for complications occurs when an ostomy is performed. While rates were high across all centers, we also observed a significant difference between hospitals— an observation not previously described in the literature. Despite this, we were unable to explain our findings based on the clinical data available. Further, hospital characteristics including overall bed size, number of ICU beds, teaching hospital status, and wound care teams were not significantly different across hospitals. True case volumes for individual hospitals or surgeons are not available given that the MSQC collects data on a case-sample basis, but the volume of cases in the registry was also not associated with outcomes variation.
Factors explaining hospital variation in postoperative morbidity are not well understood, particularly in the setting of ostomy creation. Recent work has shown that colorectal procedures represent the greatest share of adverse events of any procedure category within general surgical practices.19 Surgeons and hospitals have responded to this in several ways. Attention to preoperative optimization and surgical technique may significantly reduce infectious complications, as shown in several studies.20-22 Utilization of ostomy nurses for stoma marking and patient education may also improve outcomes.23 Differences in implementation and adherence to these practices is unknown and could inform targeted quality improvement within our collaborative.
In order to study this further, the MSQC plans to direct attention to those sites identified as high- and low-outliers with respect to complications following ostomy creation. This will include studying specific processes of care related to surgery with ostomy placement. Preoperatively, this will focus on patient presentation, triage, and management by medical and surgical teams. In discussing this topic with MSQC member surgeons, we have learned that the greatest variability in surgical practice may occur during this period where patient optimization may be important. There will be additional focus on technical aspects of these procedures. Finally, the MSQC is interested in how ostomy patients are managed postoperatively. This includes, but is not limited to, dedicated ostomy nurses, care by medical vs. surgical teams, and wound care.
This work has several limitations, first being its retrospective study design. Further, the clinical registry used attempts to collect all relevant clinical information. However, unmeasured variation in case severity limits the analysis. We are also unable to differentiate between colostomies and ileostomies, because we use CPT codes for identification of patients. At this time, we do not capture “ostomy specific” complications, although future data collection may be modified to include these, given the findings of the present study. Nonetheless, we believe this work identifies a group of procedures particularly high-risk of any complication. Patient presentation is also important as it has the ability to inform both patient preparation and operative planning. As explained above, future work will address these limitations by focusing on specific practice-level differences in patient care at high- and low- outlier centers within our collaborative in an effort to identify “best practices” that might be disseminated statewide. This approach has proven effective within regional collaboratives for the development and implementation of quality improvement initiatives.24
In conclusion, this study demonstrates that morbidity following ostomy surgery is high. Even though significant hospital variation exists, morbidity rates were high even at the best performing centers— suggesting that ostomy surgery represents an important target for quality improvement.
Footnotes
Disclosure: The authors have no conflicts to disclose.
Author contributions: All authors contributed to the conception of study and study design. All authors contributed to critical analysis of data and drafting of the initial and final manuscript. Dr. Hendren is supported by the National Cancer Institute through grant 1K07CA163665-01A1, and by the American Society of Colon and Rectal Surgeons Research Foundation
References
- 1.Goldberg M, Aukett LK, Carmel J, et al. Management of the patient with a fecal ostomy: best practice guideline for clinicians. J Wound Ostomy Continence Nurs. 2010;37:596–598. doi: 10.1097/WON.0b013e3181f97e37. [DOI] [PubMed] [Google Scholar]
- 2.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 3.U S Cancer Statistics Working Group-United States Cancer Statistics: 1999-2009 Incidence and Mortality Data. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2012. Volume Available at http://www.cdc.gov/uscs. [Google Scholar]
- 4.Robertson I, Leung E, Hughes D, et al. Prospective analysis of stoma-related complications. Colorectal Dis. 2005;7:279–285. doi: 10.1111/j.1463-1318.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Duron JJ, Duron E, Dugue T, et al. Risk factors for mortality in major digestive surgery in the elderly: a multicenter prospective study. Ann Surg. 2011;254:375–382. doi: 10.1097/SLA.0b013e318226a959. [DOI] [PubMed] [Google Scholar]
- 6.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 7.Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55:175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 8.Mala T, Nesbakken A. Morbidity related to the use of a protective stoma in anterior resection for rectal cancer. Colorectal Dis. 2008;10:785–788. doi: 10.1111/j.1463-1318.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Arendonk KJ, Tymitz KM, Gearhart SL, Stem M, Lidor AO. Outcomes and costs of elective surgery for diverticular disease: a comparison with other diseases requiring colectomy. Arch Surg. 2012:1–6. doi: 10.1001/jamasurg.2013.1010. [DOI] [PubMed] [Google Scholar]
- 10.Parmar KL, Zammit M, Smith A, Kenyon D, Lees NP. A prospective audit of early stoma complications in colorectal cancer treatment throughout the Greater Manchester and Cheshire colorectal cancer network. Colorectal Dis. 2011;13:935–938. doi: 10.1111/j.1463-1318.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 11.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256:973–981. doi: 10.1097/SLA.0b013e31826b4c4f. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor GT, Plume SK, Olmstead EM, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. JAMA. 1996;275:841–846. [PubMed] [Google Scholar]
- 13.Campbell DA, Jr, Englesbe MJ, Kubus JJ, et al. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145:985–991. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
- 14.Becher RD, Hoth JJ, Miller PR, Mowery NT, Chang MC, Meredith JW. A critical assessment of outcomes in emergency versus nonemergency general surgery using the American College of Surgeons National Surgical Quality Improvement Program database. Am Surg. 2011;77:951–959. [PubMed] [Google Scholar]
- 15.Bennis M, Parc Y, Lefevre JH, Chafai N, Attal E, Tiret E. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg. 2012;255:504–510. doi: 10.1097/SLA.0b013e31824485c4. [DOI] [PubMed] [Google Scholar]
- 16.Ciga MA, Oteiza F, Fernandez L, de Miguel M, Ortiz H. Comparative study of one-stage colectomy of the descending colon in emergency and elective surgery without mechanical preparation. Dis Colon Rectum. 2010;53:1524–1529. doi: 10.1007/DCR.0b013e3181f05654. [DOI] [PubMed] [Google Scholar]
- 17.Schilling PL, Dimick JB, Birkmeyer JD. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698–704. doi: 10.1016/j.jamcollsurg.2008.06.138. [DOI] [PubMed] [Google Scholar]
- 18.Englesbe MJ, Brooks L, Kubus J, et al. A statewide assessment of surgical site infection following colectomy: the role of oral antibiotics. Ann Surg. 2010;252:514–9. doi: 10.1097/SLA.0b013e3181f244f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilling PL. Prioritizing quality improvement in general surgery. J Am Coll Surg. 2008;207:698–704. doi: 10.1016/j.jamcollsurg.2008.06.138. [DOI] [PubMed] [Google Scholar]
- 20.Bass EM, Del Pino A, Tan A, Pearl RK, Orsay CP, Abcarian H. Does preoperative stoma marking and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40:440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 21.Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. J Am Coll Surg. 2010;211:232–238. doi: 10.1016/j.jamcollsurg.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes care. 2011;34:256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Likosky DS, Nugent WC, Ross CS. Improving outcomes of cardiac surgery through cooperative efforts: the northern new England experience. Semin Cardiothorac Vasc Anesth. 2005;9:119–121. doi: 10.1177/108925320500900203. [DOI] [PubMed] [Google Scholar]
- 24.Likosky DS. Improving outcomes of cardiac surgery through cooperative efforts: the northern new England experience. Semin Cardiothorac Vascl Anesth. 2005;9:119–21. doi: 10.1177/108925320500900203. [DOI] [PubMed] [Google Scholar]


