ABSTRACT
SET is an endogenous protein phosphatase 2A (PP2A) inhibitor and is associated with a poor prognosis in human leukemia. Previously, we reported increased SET protein levels in canine lymphoma cell lines and the potential therapeutic application of SET antagonists in canine lymphoma. Here, we found that canine cells express several isoforms of the SET protein. We cloned 4 isoforms of SET, named SETα, β, γ and δ. Genomic BLAST showed that the SET genes are located on chromosomes X, 7, 1 and 8, respectively. An immunofluorescent study showed nuclear localization of SETα and β, and nuclear and cytosolic localization of SETγ and δ. We confirmed that SETα and β possess the ability to associate with PP2A. Our data reveal the existence of unique SET isoforms that should be taken into account in SET-targeting drug development studies in dogs.
Keywords: cancer, canine, phosphorylation, protein phosphatase 2A, SET/I2PP2A
The human SET gene was discovered as a component of the SET-CAN fusion gene, which was produced by a somatic translocation in a case of acute undifferentiated leukemia [17]. It has been reported that SET positively regulates multiple oncogenic pathways, and elevated SET protein levels are observed in various human tumors [4,5,6, 8, 12, 18, 19]. SET, also known as I2PP2A, is a potent physiological inhibitor of protein phosphatase 2A (PP2A). PP2A is a major cellular protein serine/threonine phosphatase and regulates a wide range of biological processes, including cell proliferation, apoptosis, development and motility [3]. Loss or inhibition of PP2A has revealed that it possesses a critical tumor suppressor function [2] and the SET protein directly binds with PP2A through both its N-terminus and C-terminus regions and subsequently inhibits PP2A phosphatase activity [1].
There are 2 isoforms of human SET, the 290 amino acid SETα and the 277 amino acid SETβ, both of which exhibit PP2A inhibitory activity [15]. However, it is unknown whether canine cells express SET isoforms. We have previously reported that OP449, a peptide antagonist of SET, recovered PP2A activity and exerted anti-tumor effects on canine lymphoma cells [10]. In canine lymphoma cells, the effects of OP449 were dependent on SET protein levels in the cells. Therefore, it is important to identify the isoforms of canine SET and clarify whether they are functional PP2A inhibitors.
MATERIALS AND METHODS
Cell culture: Canine melanoma cell lines (CMeC1, CMeC2, KMeC and LMeC) were kindly provided by Dr. Takuya Nakagawa. The canine lymphoma cell line Ema has previously been described [10]. CMeC1, CMeC2 and Ema were grown in RPMI1640 that contained 10% fetal bovine serum (FBS) and 1× anti-biotic/anti-mycotic (Life Technologies, Carlsbad, CA, U.S.A.). HeLa, HEK293T and canine renal tubule epithelial cell line MDCK were grown in Dulbecco’s Modified Eagle Medium (DMEM) that contained 10% FBS and 1× anti-biotic/anti-mycotic. PBMCs were isolated from Beagle and cultured in RPMI1640 that contained 10% FBS.
Immunoblotting: Immunoblotting was performed as previously described [13]. Briefly, cells were lysed in a buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM ethylenediaminetetraacetic acid (EDTA) (pH 8.0), 5 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton X100, 1 mM Na3VO4, 20 mM sodium pyrophosphate and Roche’s complete protease inhibitor cocktail. The proteins were separated by SDS-PAGE and transferred onto PVDF membrane (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Membranes were blocked with 0.5% skim milk and treated with the following primary antibodies: anti-human SETα (Abcam #ab1183, Cambridge, UK), anti-human SETα/β (Bioss #BS-5943R, Woburn, MA, U.S.A.), anti-PP2A C subunit (Millipore #07-324, Billerica, MA, U.S.A.) and anti-VCP/p97 (GeneTex, Hsinchu, Taiwan). Bands were detected using ECL Western Blotting Detection System (GE Healthcare, Freiburg, Germany) and visualized using a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan).
Cloning of canine SET isoforms and transfection: Thymus cDNA from the Beagle was used as a template for PCR. The 37-mer oligonucleotide 5′-TATTTCCGGTGAATTCCTTTCGTGGTTTCCTCACAGC-3′ and the 36-mer oligonucleotide 5′-CTTTGTAGTCGGATCCTGGAATCCATCAGTGTTCCA-3′ were designed as the 5′ primer and the 3′ primer, respectively, to sequence canine SET variants. PfuUltra II (Life Technologies) was used for the PCR amplification, which was conducted with 40 cycles of 20 sec at 95°C, 20 sec at 60°C and 60 sec at 72°C. The PCR products were cloned into pLVSIN-EF1α-IRES-ZsGreen Vector (Takara, Tokyo, Japan) by InFusion (Takara), according to the manufacturer’s instructions. Plasmids were sequenced using BigDye Terminator v3.1 cycle sequencing kit and ABI3130 sequencer (Life technologies). Plasmids were transfected into cells using Polyethylenimine Max (Polysciences, Taipei, Taiwan), according to the manufacturer’s instructions.
Immunoprecipitation: HEK293T cells transiently expressing FLAG-SET variants were lysed in 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 1% Triton X100, 1 mM Na3VO4, 20 mM sodium pyrophosphate and Roche’s complete protease inhibitor cocktail. The supernatants were incubated with FLAG-M2 affinity gel (Sigma, St. Louis, MO, U.S.A.). The beads were washed in a washing buffer that contained 50 mM MOPS-NaOH (pH 7.4), 150 mM NaCl, 5 mM EGTA and 1% NP-40.
Immunofluorescent staining: Cells were grown on glass coverslips and subsequently fixed with 4% paraformaldehyde for 20 min at room temperature and blocked with 3% skim milk in phosphate buffered saline-Tween (PBS-T) (137 mM NaCl, 2.7 mM KCl, 1.76 mM KH2PO4, 10 mM Na2HPO4 and 0.05% Tween 20). After incubation with anti-SET antibodies or an anti-FLAG antibody (SIGMA) overnight at 4°C, Alexa 488 or 594-conjugated secondary antibodies (Life Technologies), Phalloidin Far-Red Fluorescent Acti-stainTM 670 (Cytoskeleton, Denver, CO, U.S.A.) and Hoechst 33342 (Dojindo, Tokyo, Japan) were added before the cells were incubated for 1 hr at room temperature. Fluorescence images were captured using a confocal laser-scanning microscope (LSM710, Zeiss, Tokyo, Japan).
RESULTS
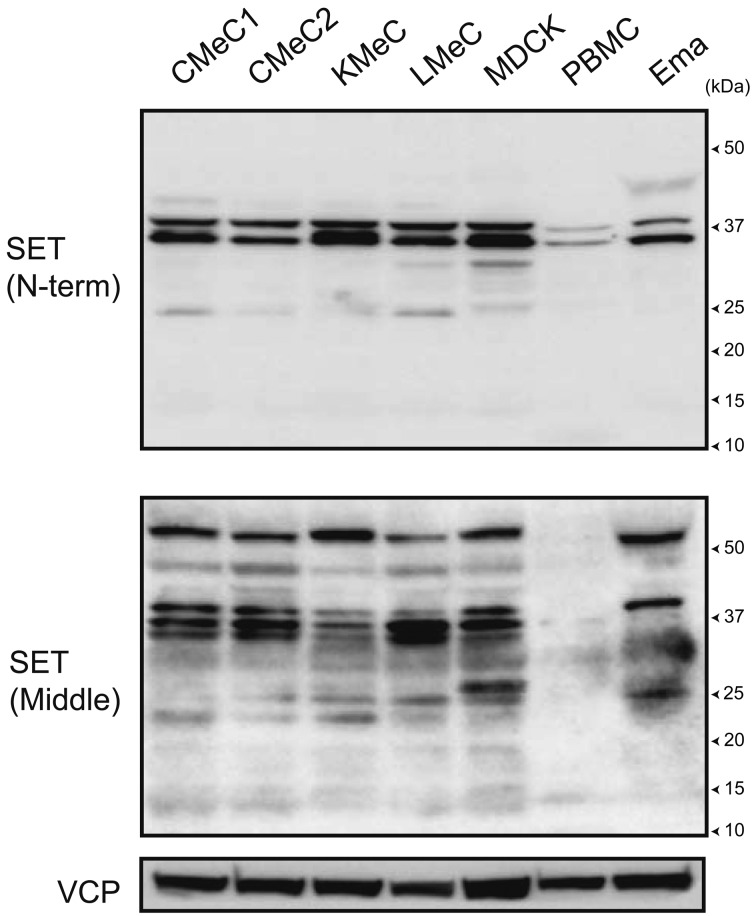
To examine the protein expression of SET isoforms in canine cells, normal peripheral blood mononuclear cells (PBMC), melanoma cell lines CMeC1, CMeC2, KMeC and LMeC, lymphoma cell line Ema and the renal tubule epithelial cell line MDCK were lysed for immunoblotting. The major bands were observed around 37 kDa by 2 types of anti-SET antibodies against 3–18 aa of human SETα (N-term) and 175–215 aa of human SETα (Middle). The minor bands were commonly observed around 25 kDa by the 2 antibodies. Only anti-SETα (Middle) detected the major band around 50 kDa and week bands around 15 kDa. These data indicate that canine cells express more than one SET isoform (Fig. 1).
Fig. 1.
SET protein expression in canine cells. SET protein expression in canine cells was analyzed by immunoblotting. Two types of anti-SET antibodies against 3–18 aa of human SETα (N-term) and 175–215 aa of human SETα (Middle) were used. VCP was used as a loading control.
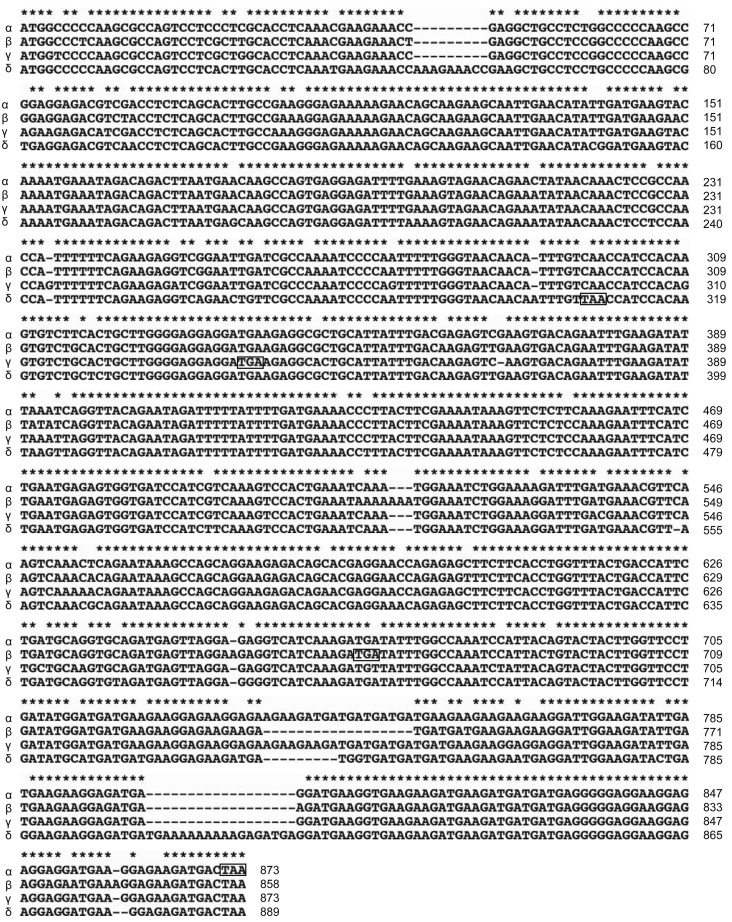
To determine the sequence of canine SET isoforms, we cloned the protein cDNA from the Beagle thymus, as described in the Materials and Methods. Interestingly, the same primer isolated 4 types of cDNA sequences. cDNA sequences for 4 isoforms, named α, β, γ and δ, were aligned using Clustal X2 (Fig. 2). Genomic BLAST (Basic Local Alignment Search Tool) revealed that isoform α, β, γ and δ genes are 99% homologous to CanFem3.1 whole shotgun sequences NW_003726126.1, NW_003726067.1, NW_003726047.1 and NW_003726071.1 and are located on chromosomes X, 7, 1 and 8, respectively. Isoform α expresses 290 aa protein, which is the same size as human SETα. Alignment of the protein sequences of human SETα and canine SETα revealed that approximately 94% were homologous between the 2 species (Fig. 3A). In contrast, isoforms β, γ and δ have a single base insertion and express 223 aa, 112 aa and 102 aa C-terminus truncated SET proteins, respectively (Figs. 2 and 3B).
Fig. 2.
Nucleotide sequence alignment of canine SET isoforms. cDNA sequences for canine SET isoforms were aligned using Clustal X2. “*”, “:” and “.” indicate “full,” “strong,” and “weak” conserved residues, respectively. The square indicates the stop codon.
Fig. 3.
Protein sequence alignment of canine SET isoforms. (A) Protein sequences for canine SETα and human SETα were aligned using Clustal X2. “*”, “:” and “.” indicate “full,” “strong,” and “weak” conserved residues, respectively. The solid and dotted lines indicate the immunogen sequence of anti-SET (N-term) and anti-SET (Middle), respectively. (B) Protein sequences for the canine SET isoforms were aligned using Clustal X2. “*”, “:” and “.” indicate “full,” “strong,” and “weak” conserved residues, respectively. The square indicates the residues corresponding to around Val105 of human SETα, which is essential for PP2A association.
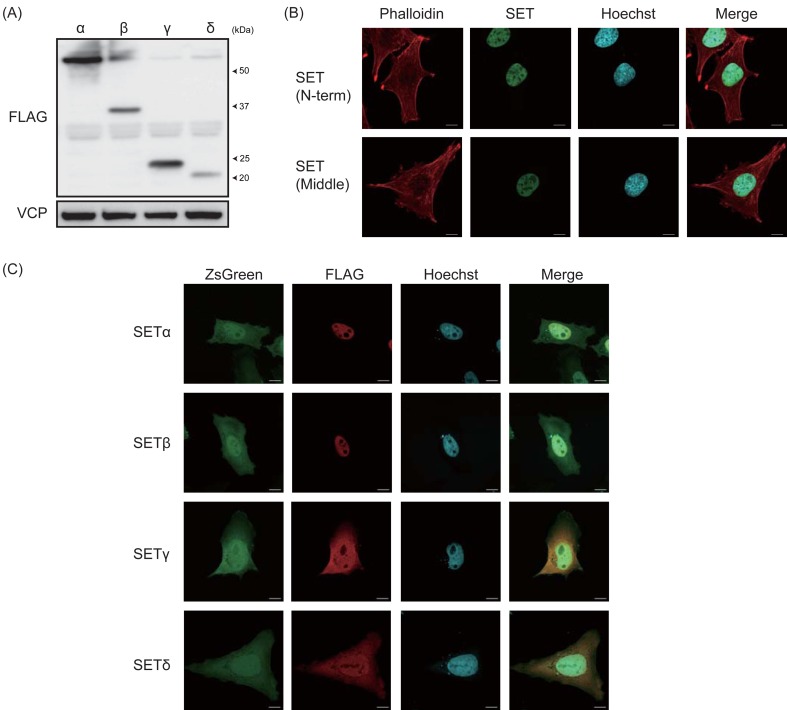
To examine whether canine SET isoforms are conformationally stable, HEK293T cells were transfected to express the canine SET isoforms, and protein levels were determined by immunoblotting (Fig. 4A). The anti-FLAG antibody detected the bands around 52 kDa for FLAGx3-SETα, around 37 kDa for FLAGx3-SETβ, around 24 kDa for FLAGx3-SETγ and around 20 kDa for FLAGx3-SETδ. Although these bands are much higher than the predicted size of canine SETα, β, γ and δ, which are 33.5 kDa, 26.1 kDa, 12.6 kDa and 11.9 kDa, respectively, a similar upper shift was also observed for human SETα with FLAGx3 tag (Data not shown). Next, we examined the localization of canine SET isoforms. Both anti-SET (N-term) and anti-SET (Middle) antibodies exhibited nuclear localization of the endogenous canine SET in CMeC1 cells (Fig. 4B). To determine the localization of individual isoforms, HEK293T cells were transfected to express canine SET isoforms. pLVSIN-EF1α-IRES-ZsGreen canine SET isoforms vector express both SET isoforms and ZsGreen fluorescent protein independently, and ZsGreen localizes both in cytosol and nucleus. As shown in Fig. 4C, SETα and β mainly localize in the nucleus, but SETγ and δ localize in both the nucleus and the cytosol.
Fig. 4.
Expression and localization of canine SET isoforms. (A) HEK293T cells were transfected to express FLAGx3-tagged canine SET isoforms, and protein expression was analyzed by immunoblotting. VCP was used as a loading control. (B) The localization of endogenous SET in CMeC1 cells was examined by immunofluorescent staining using anti-SET (N-term) and anti-SET (Middle) (Green). Phalloidin (Red) and Hoechst (Blue) were used to stain F-actin and the nucleus. (C) HeLa cells were transfected to express FLAGx3-tagged canine SET isoforms and ZsGreen, and the localization of SET isoforms was determined by immunofluorescent staining using an anti-FLAG antibody (Red). Hoechst (Blue) was used to stain the nucleus.
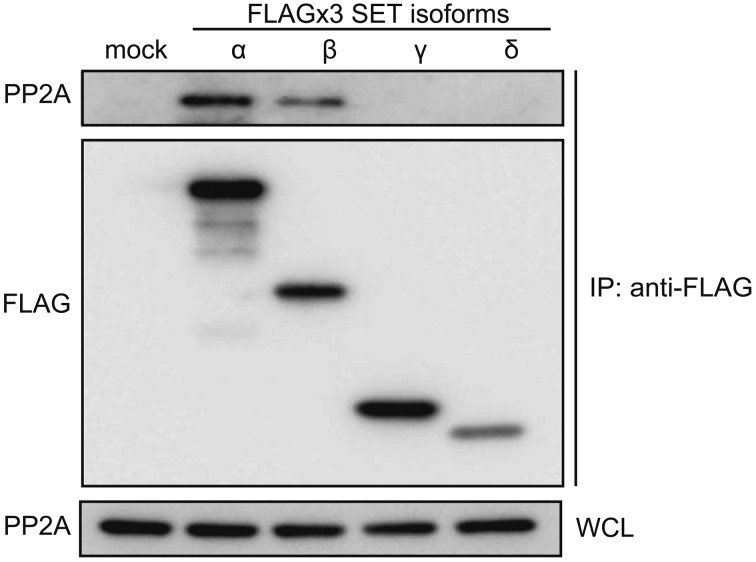
Because the SET protein directly associates with PP2A to inhibit its activity, we examined whether canine SET isoforms possess the ability to associate with PP2A, using immunoprecipitation (Fig. 5). HEK293T cells were transfected to express canine SET isoforms, and FLAGx3-SET isoforms were immunoprecipitated using anti-FLAG M2 beads and subjected to immunoblotting. Canine SETα and β, but not γ and δ, associated with PP2A, indicating that these 2 isoforms have the ability to suppress PP2A activity.
Fig. 5.
Association of canine SET isoforms with PP2A. HEK293T cells were transfected to express FLAGx3-tagged canine SET isoforms, and the association with PP2A was examined by immunoprecipitation using anti-FLAG M2 beads. WCL: whole cell lysates.
DISCUSSION
In this study, we found that canine cells express at least 4 SET isoforms, 3 of which are C-terminally truncated. Among them, SETα and β can associate with PP2A, identifying them as endogenous inhibitors. The human SET protein directly binds with PP2A through both its N-terminus and C-terminus regions, and only one of them is sufficient for PP2A association [1]. The Valine 105 residue of human SETα is essential for the association of N-terminus SET. The sequences around this residue are highly conserved in canine SETα and β, but not γ and δ, and may determine the ability for association.
The predicted size of canine SETα, β, γ and δ is 33.5 kDa, 26.1 kDa, 12.6 kDa and 11.9 kDa, respectively. During immunoblotting for endogenous proteins, major bands were observed around 37 kDa in the canine cells. It is possible that these bands correspond to canine SETα, because human SETα, which predicted size is also 33.5 kDa, is detected around 37 kDa. It is also possible that the bands just above 25 kDa correspond to endogenous SETβ. It remains unclear whether SETγ and δ endogenously express, because anti-SET (N-term) did not detect bands corresponding to these isoforms. The bands around 15 kDa observed in the blots for anti-SET (Middle) antibodies are not SETγ and δ. The anti-SET (Middle) antibody cannot recognize SETγ and δ, because the immunogen sequence for this antibody is lost in these 2 isoforms. It may be possible that the ~15-kDa bands observed by the anti-SET (Middle) antibody correspond to N-terminus truncated SET isoforms, which can be expressed by a second open reading frame. We also detected ~50-kDa bands by the anti-SET (Middle) antibody, and it is not clear whether these bands correspond to another SET isoform or non-specific binding. Further observations are necessary to clarify this point.
Human SET isoforms are mainly localized in nucleus [14, 20]. Consistent with this, we observed nuclear localization of endogenous canine SET and FLAG-tagged canine SETα and β. It has been reported that human SET has nuclear localization signal in its N-terminus regions [20]. Because canine SET γ and δ localize both in cytosol and nucleus, it is possible that C-terminus region of SET is also important for nuclear localization.
Recently, several studies have reported that SET is an attractive drug target for various human tumors [9, 11, 16]. We have previously reported that COG/OP449 (Oncotide Pharmaceuticals, NC, U.S.A.), a peptide antagonist of SET, recovered PP2A activity and exerted anti-tumor effects on canine lymphoma cells [10]. The effects of OP449 were dependent on SET protein levels, which are now named SETα. However, the present study reveals that canine SETβ is also a potential PP2A inhibitor. OP449 peptides bind to the C-terminal region of SET, which corresponds to 190–290 of human SETα [7]. Because SETβ does not contain most of this region, it is unclear whether OP449 can antagonize canine SETβ functions. This point should be taken into account in future SET-targeting drug development studies in dogs.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant Number 25660227). The funding source had no role in the study design; collection, analysis or interpretation of data; in the writing of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
- 1.Arnaud L., Chen S., Liu F., Li B., Khatoon S., Grundke-Iqbal I., Iqbal K.2011. Mechanism of inhibition of PP2A activity and abnormal hyperphosphorylation of tau by I2(PP2A)/SET. FEBS Lett. 585: 2653–2659. doi: 10.1016/j.febslet.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold H. K., Sears R. C.2008. A tumor suppressor role for PP2A-B56alpha through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev. 27: 147–158. doi: 10.1007/s10555-008-9128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brautigan D. L.2013. Protein Ser/Thr phosphatases–the ugly ducklings of cell signalling. FEBS J. 280: 324–345. doi: 10.1111/j.1742-4658.2012.08609.x [DOI] [PubMed] [Google Scholar]
- 4.Carlson S. G., Eng E., Kim E. G., Perlman E. J., Copeland T. D., Ballermann B. J.1998. Expression of SET, an inhibitor of protein phosphatase 2A, in renal development and Wilms’ tumor. J. Am. Soc. Nephrol. 9: 1873–1880 [DOI] [PubMed] [Google Scholar]
- 5.Chao A., Tsai C. L., Wei P. C., Hsueh S., Chao A. S., Wang C. J., Tsai C. N., Lee Y. S., Wang T. H., Lai C. H.2010. Decreased expression of microRNA-199b increases protein levels of SET (protein phosphatase 2A inhibitor) in human choriocarcinoma. Cancer Lett. 291: 99–107. doi: 10.1016/j.canlet.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Christensen D. J., Chen Y., Oddo J., Matta K. M., Neil J., Davis E. D., Volkheimer A. D., Lanasa M. C., Friedman D. R., Goodman B. K., Gockerman J. P., Diehl L. F., de Castro C. M., Moore J. O., Vitek M. P., Weinberg J. B.2011. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood 118: 4150–4158. doi: 10.1182/blood-2011-04-351072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen D. J., Ohkubo N., Oddo J., Van Kanegan M. J., Neil J., Li F., Colton C. A., Vitek M. P.2011. Apolipoprotein E and peptide mimetics modulate inflammation by binding the SET protein and activating protein phosphatase 2A. J. Immunol. 186: 2535–2542. doi: 10.4049/jimmunol.1002847 [DOI] [PubMed] [Google Scholar]
- 8.Cristóbal I., Garcia-Orti L., Cirauqui C., Cortes-Lavaud X., García-Sánchez M. A., Calasanz M. J., Odero M. D.2012. Overexpression of SET is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematologica 97: 543–550. doi: 10.3324/haematol.2011.050542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell A. S., Allen-Petersen B., Daniel C. J., Wang X., Wang Z., Rodriguez S., Impey S., Oddo J., Vitek M. P., Lopez C., Christensen D. J., Sheppard B., Sears R. C.2014. Targeting Inhibitors of the Tumor Suppressor PP2A for the Treatment of Pancreatic Cancer. Mol. Cancer Res.; Epub ahead of print. doi: 10.1158/1541-7786.MCR-13-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara N., Kawasaki H., Yabe R., Christensen D. J., Vitek M. P., Mizuno T., Sato K., Ohama T.2013. A potential therapeutic application of SET/I2PP2A inhibitor OP449 for canine T-cell lymphoma. J. Vet. Med. Sci. 75: 349–354. doi: 10.1292/jvms.12-0366 [DOI] [PubMed] [Google Scholar]
- 11.Kubota D., Yoshida A., Kawai A., Kondo T.2014. Proteomics Identified Overexpression of SET Oncogene Product and Possible Therapeutic Utility of Protein Phosphatase 2A in Alveolar Soft Part Sarcoma. J. Proteome Res. 13: 2250–2261. doi: 10.1021/pr400929h [DOI] [PubMed] [Google Scholar]
- 12.Neviani P., Santhanam R., Trotta R., Notari M., Blaser B. W., Liu S., Mao H., Chang J. S., Galietta A., Uttam A., Roy D. C., Valtieri M., Bruner-Klisovic R., Caligiuri M. A., Bloomfield C. D., Marcucci G., Perrotti D.2005. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8: 355–368. doi: 10.1016/j.ccr.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 13.Ohama T., Brautigan D. L.2010. Endotoxin conditioning induces VCP/p97-mediated and inducible nitric-oxide synthase-dependent Tyr284 nitration in protein phosphatase 2A. J. Biol. Chem. 285: 8711–8718. doi: 10.1074/jbc.M109.099788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saddoughi S. A., Gencer S., Peterson Y. K., Ward K. E., Mukhopadhyay A., Oaks J., Bielawski J., Szulc Z. M., Thomas R. J., Selvam S. P., Senkal C. E., Garrett-Mayer E., De Palma R. M., Fedarovich D., Liu A., Habib A. A., Stahelin R. V., Perrotti D., Ogretmen B.2013. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol. Med. 5: 105–121. doi: 10.1002/emmm.201201283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito S., Miyaji-Yamaguchi M., Shimoyama T., Nagata K.1999. Functional domains of template-activating factor-I as a protein phosphatase 2A inhibitor. Biochem. Biophys. Res. Commun. 259: 471–475. doi: 10.1006/bbrc.1999.0790 [DOI] [PubMed] [Google Scholar]
- 16.Sobral L. M., Sousa L. O., Coletta R. D., Cabral H., Greene L. J., Tajara E. H., Gutkind J. S., Curti C., Leopoldino A. M.2014. Stable SET knockdown in head and neck squamous cell carcinoma promotes cell invasion and the mesenchymal-like phenotype in vitro, as well as necrosis, cisplatin sensitivity and lymph node metastasis in xenograft tumor models. Mol. Cancer 13: 32. doi: 10.1186/1476-4598-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Lindern M., van Baal S., Wiegant J., Raap A., Hagemeijer A., Grosveld G.1992. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol. Cell. Biol. 12: 3346–3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westermarck J., Hahn W. C.2008. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 14: 152–160. doi: 10.1016/j.molmed.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Huang Q., Lu Y., Li X., Huang S.2012. Reactivating PP2A by FTY720 as a novel therapy for AML with C-KIT tyrosine kinase domain mutation. J. Cell. Biochem. 113: 1314–1322. doi: 10.1002/jcb.24003 [DOI] [PubMed] [Google Scholar]
- 20.Yu G., Yan T., Feng Y., Liu X., Xia Y., Luo H., Wang J. Z., Wang X.2013. Ser9 phosphorylation causes cytoplasmic detention of I2PP2A/SET in Alzheimer disease. Neurobiol. Aging 34: 1748–1758. doi: 10.1016/j.neurobiolaging.2012.12.025 [DOI] [PubMed] [Google Scholar]