ABSTRACT
Scallop shell powder produced by calcination process − the average diameter of the powder particles being 20 µm (SSP) − was further ground into nano-sized particles, with average diameter of 500 nm, here designated CaO-Nano. Solution of CaO-Nano could inactivate avian influenza virus within 5 sec, whereas the solution of SSP could not even after 1 hr incubation. CaO-Nano solution could also inactivate Newcastle disease virus and goose parvovirus within 5 sec and 30 sec, respectively. The virus-inactivating capacity (neutralizing index: NI>3) of the solution was not reduced by the presence of 20% fetal bovine serum. CaO-Nano solution seems to be a good candidate of materials for enhancement of biosecurity in farms.
Keywords: biosecurity, nano-sized scallop shell powder, virus inactivation
Highly pathogenic avian influenza (HPAI) is one of the most serious diseases in the poultry industry. Vaccinations of HPAI for poultry can be used to prevent onset of clinical signs, but vaccines cannot protect birds from infections [17]. In China, Egypt, Mexico and Vietnam where vaccination against HPAI has been used, the causative viruses could not be eradicated [2, 7, 10, 13]. In these days, fatal human cases appeared in China not only infected by HPAI virus H5N1, but also by low pathogenic avian influenza (LPAI) viruses of the subtypes H7N9 [6] and H10N8 [3], and all of which had a domestic poultry intermediate. In Korea, vaccination against LPAI virus H9N2 has been used since 2007, however, the virus underwent antigenic drift and evolved into distinct antigenic groups [11]. According to the strategy by the Office International des Epizooties (OIE), it is important to realize that vaccination alone is not considered the solution to the control of avian influenza (AI) whenever eradication is the desired result [14]. Without the application of monitoring systems, strict biosecurity and depopulation in the face of infection, there is the possibility that these viruses could become endemic in vaccinated poultry populations [5, 14].
Virulent type of Newcastle disease viruses (NDVs) was isolated from vaccinated chicken farms without typical signs of Newcastle disease (ND) [25]. This fact and HPAI conditions in countries using vaccines against HPAI suggest that virulent viruses may survive in the vaccinated flocks in the form of sub-clinical infection.
Enhancement of biosecurity at poultry farms is one of the most important strategies to control infectious diseases [14]. Organic solvents and detergents, such as sodium deoxycholate and sodium dodecylsulfate, can inactivate enveloped viruses, because these detergents can destroy lipid bilayers of viruses. However, non-enveloped viruses that are expected to have high resistance to disinfection, such as norovirus [15, 23] and especially parvovirus, the most resistant species identified, could not be inactivated by detergents [4, 15]. So far, many disinfectants have been applied for enhancement of biosecurity at poultry farms, however, most of them have disadvantages of diminished virus-inactivating ability in the presence of organic materials.
Calcinated calcium powder made by scallop shells, − the average diameter of the powder particles being 20 µm (SSP) − has been shown to possess virus-inactivating ability even in the presence of organic materials [24]. Bioceramic powder prepared from chicken feces was also shown to inactivate viruses in the presence of organic materials [19]. These powders can be used as “trapping” disinfection materials instead of slaked lime, because of their long lasting virus-inactivating ability [24]. Slaked lime became hardened in the shape of a board, but SSP remained as soft powder until the end of the experiment (8 months post-scattering) [24].
In the present study, SSP was further ground into nano-sized particles (the average size of which is 500 nm − thereafter called CaO-Nano), and CaO-Nano solution was evaluated for its virus-inactivating capacity against AIV, NDV and goose parvovirus (GPV).
Heat-treated scallop shell powder with average particle size of 20 µm (SSP) and 20% suspension (weight per volume, w/v) of CaO-Nano in redistilled water (dW2) were kindly supplied by C&C Co., Ltd. (Tokyo, Japan) and Takara Yojo Co., Ltd. (Kawasaki, Japan), respectively. Suspension of 3%, 6% and 10% (w/v) of SSP and 2% suspension of CaO-Nano were prepared in dW2. Just before use, these suspensions were centrifuged at 12,000 × g for 3 min, and the resulted supernatants were used as SSP solution (pH 12.3) or CaO-Nano solution (pH 13.1).
LPAI virus A/duck/Aomori/395/04 (H7N1) [9], NDV strain Sato [21] and GPV strain IHC [20] were propagated in 10-day-old embryonated chicken eggs for AIV and NDV or Muscovy duck embryo fibroblasts (MDEF) prepared from 14-day-old Muscovy duck embryos for GPV. After aliquot, these viruses were kept at −80°C until used. AIV was titrated in Madin-Darby canine kidney (MDCK) cells as described [8], NDV in chicken embryo fibroblasts (CEF) prepared from 10-day-old chicken embryos and GPV in MDEF as described [20, 21]. Viruses were diluted in serial-10 fold dilution in Eagle’s minimum essential medium (MEM, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 0.3% of tryptose phosphate broth, penicillin 100 units/ml, streptomycin 100 µg/ml, amphotericin B 0.5 µg/ml and 4 mM L-glutamine, and 100 µl of each dilution were inoculated onto susceptible cells seeded in 96 well cell-culture plates (4 wells per dilution, 200 µl final volume in each well). For AIV, 2 µg/ml (final concentration) trypsin (Trypsin, from bovine pancreas 10,000BAEE units/mg protein, Sigma, St. Louis, MO, U.S.A.) was added to each well. The plates were incubated at 37°C in the presence of 5% CO2 and observed for virus-induced cytopathic effect (CPE). At 3 days-post inoculation (dpi) for AIV and at 5 dpi for NDV, hemagglutinin (HA) activity of the culture supernatant was checked with 0.5% chicken red blood cells (CRBCs) [21]. For GPV, at 7 dpi, an endpoint cell viability was assayed by crystal-violet staining [18]. Titers were calculated by Behrens-Kaerber’s method [8] with HA results for AIV and NDV and with result of crystal violet for GPV and expressed as 50% tissue culture infectious dose (TCID50).
Four hundred fifty micro-liters of SSP or CaO-Nano solutions were mixed with 100 µl of virus in a microtube, incubated at indicated time (5, 15, 30 and 60 sec) and then neutralized with 450 µl of 1M Tris-HCl (pH 7.2). The neutralized samples were titrated immediately for remained viruses in each sensitive cell. To evaluate virus-inactivating activity of the solutions with organic materials, 200 µl of fetal bovine serum (FBS) was added to 100 µl of viruses, mixed with 450 µl of solutions in a microtube and then neutralized with 250 µl of 1M Tris-HCl (pH 7.2). To confirm the effect of 1 M Tris-HCl (pH 7.2) for neutralizing the tested solutions, 1 M Tris-HCl (pH 7.2) was added to each solution before adding viruses (treatment for zero second). All the virus-inactivating activity tests were repeated at least 3 times and conducted at room temperature, namely 25°C ± 1. A numerical method (neutralizing index: NI) was used to express the ability of an agent to inactivate viruses as previously described [12]. The NI of virus inactivation is calculated using the following equation:
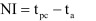 |
where tpc is the titer converted into an index in log10 of the positive control, and ta is the converted titer of the recovered virus from the agent-treated tube. Inactivation of viruses was considered effective when NI >3.0 as described [12, 22].
As shown in Table 1, CaO-Nano solution inactivated AIV within 5 sec to undetectable level, and this ability was not affected by the presence of organic materials. When CaO-Nano solution was neutralized with 1 M Tris-HCl (pH 7.2), AIV was not inactivated. The high pH 13.1 of CaO-Nano solution seemed to be one of the main virus-inactivating mechanisms, because the ability of the solution was diminished after neutralization of pH with 1 M Tris-HCl (pH 7.2). SSP solution at 10% (pH 12.3) could not inactivate AIV even after 1 hr incubation (data not shown). To inactivate AIV, high pH − namely more than 12 − was required [26]. In water, calcium oxide (CaO) is converted to calcium hydroxide (Ca (OH)2), which is sparsely soluble in water at 0.15% [1]. In nanoparticle, CaO-Nano may have more solubility in water than SSP, and that solubility makes the pH as high as pH 13.1.
Table 1. Inactivation of viruses with CaO-Nano solution.
| Virus | FBS a) | tpcb) | ta at incubation period (sec) c) | ||||
|---|---|---|---|---|---|---|---|
| 0 d) | 5 | 15 | 30 | 60 | |||
| AIV | + | 7.50 | 7.50 | 3.75 | <3.50 | <3.50 | <3.50 |
| – | 7.50 | 7.50 | <3.50 | <3.50 | <3.50 | <3.50 | |
| NDV | + | 9.00 | 9.00 | 5.00 | 4.75 | 4.50 | 4.25 |
| – | 9.00 | 9.00 | 4.50 | 4.50 | 4.50 | <3.50 | |
| GPV | + | 8.00 | 8.00 | NT e) | NT | NT | 3.75 |
| – | 8.00 | 8.00 | 7.00 | 5.75 | 4.25 | <3.50 | |
a) Fetal bovine serum (FBS: final concentration in the reaction micro-tube was 20%) was added to viruses before mix with CaO-Nano solution (+), not added (–). b) tpc is the titer converted into an index in log10 of the positive control. c) ta is the titer converted into an index in log10 of the recovered virus from the CaO-Nano-treated tube. d) CaO-Nano solution was neutralized with 1 M Tris-HCl before add viruses. e) NT: Not tested.
NDV and GPV were also inactivated by CaO-Nano within 5 sec and 30 sec, respectively. In the presence of organic materials, CaO-Nano was effective against NDV and GPV (NI >3), yet, these viruses were not completely inactivated even after 60 sec incubation (Table 1)
SSP slurry (0.2% w/v) has been shown to possess bacteria-inactivation ability [16]. For enhancement biosecurity in farms, slaked lime has been used in Japan as a “trapping” disinfection material. We have shown the efficacy of alternative materials, such as bioceramics derived from chicken feces [19] and scallop shell powder [24], but all are powder type. SSP has been shown to have advantage over slaked lime, because of its lasting softness under field conditions [24]. SSP and CaO-Nano are derived from the same material, namely scallop shell, but the differences of their particle diameter brought about different solubility in water and different pH (SSP: pH 12.3 and CaO-Nano: pH 13.1). Here, CaO-Nano that can be used in liquid form has been shown as another candidate material for the enhancement of biosecurity in farms. CaO-Nano also has the excellent merit that can keep the virus-inactivating ability even in the presence of organic materials. To combat against pathogens, thick protective barrier with different forms of “trapping” disinfection materials is necessary.
REFERENCES
- 1.Bassett H.1954. Solubility of calcium hydroxide in water. pp. 630–632. In: Solubilities of Inorganic and Metal Organic Compounds, 4th ed. (Linke, W. F. ed.), American Chemical Society, Washington. [Google Scholar]
- 2.Chen H.2009. H5N1 avian influenza in China. Sci. China C Life Sci. 52: 419–427. doi: 10.1007/s11427-009-0068-6 [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Yuan H., Gao R., Zhang J., Wang D., Xiong Y., Fan G., Yang F., Li X., Zhou J., Zou S., Yang L., Chen T., Dong L., Bo H., Zhao X., Zhang Y., Lan Y., Bai T., Dong J., Li Q., Wang S., Zhang Y., Li H., Gong T., Shi Y., Ni X., Li J., Zhou J., Fan J., Wu J., Zhou X., Hu M., Wan J., Yang W., Li D., Wu G., Feng Z., Gao G. F., Wang Y., Jin Q., Liu M., Shu Y.2014. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet online Feb 5. http://dx.doi.org/ . [DOI] [PubMed] [Google Scholar]
- 4.Eterpi M., McDonnell G., Thomas V.2009. Disinfection efficacy against parvoviruses compared with reference viruses. J. Hosp. Infect. 73: 64–70. doi: 10.1016/j.jhin.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 5.FAO/OIE. 2005. Regional Meeting on Avian Influenza control in Asia. [Google Scholar]
- 6.Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K., Xu X., Lu H., Zhu W., Gao Z., Xiang N., Shen Y., He Z., Gu Y., Zhang Z., Yang Y., Zhao X., Zhou L., Li X., Zou S., Zhang Y., Li X., Yang L., Guo J., Dong J., Li Q., Dong L., Zhu Y., Bai T., Wang S., Hao P., Yang W., Zhang Y., Han J., Yu H., Li D., Gao G. F., Wu G., Wang Y., Yuan Z., Shu Y.2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med.. doi: 10.1056/NEJMoa1304459 [DOI] [PubMed] [Google Scholar]
- 7.Hafez M. H., Arafa A., Abdelwhab E. M., Selim A., Khoulosy S. G., Hassan M. K., Aly M. M.2010. Avian influenza H5N1 virus infections in vaccinated commercial and backyard poultry in Egypt. Poult. Sci. 89: 1609–1613. doi: 10.3382/ps.2010-00708 [DOI] [PubMed] [Google Scholar]
- 8.Jahangir A., Ruenphet S., Hara K., Shoham D., Sultana N., Okumura M., Nakamura M., Takehara K.2010. Evaluation of human intestinal epithelial differentiated cells (Caco-2) for replication, plaque formation and isolation of avian influenza viruses. J. Virol. Methods 169: 232–238. doi: 10.1016/j.jviromet.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 9.Jahangir A., Ruenphet S., Shoham D., Okamura M., Nakamaura M., Takehara K.2010. Haemagglutinin and neuraminidase characterization of low pathogenic H5 and H7 avian influenza viruses isolated from Northern pintail (Anas acuta) in Japan, with special reference to genomic and biogeographical aspects. Virus Genes 40: 94–105. doi: 10.1007/s11262-009-0423-5 [DOI] [PubMed] [Google Scholar]
- 10.Lee C. W., Senne D. A., Suarez D. L.2004. Effect of vaccine use in the evolution of Mexican lineage H5N2 avian influenza virus. J. Virol. 78: 8372–8381. doi: 10.1128/JVI.78.15.8372-8381.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D. H., Song C. S.2013. H9N2 avian influenza virus in Korea: evolution and vaccination. Clin. Exp. Vaccine Res. 2: 26–33. doi: 10.7774/cevr.2013.2.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombardi M. E., Ladman B. S., Alphin R. L., Benson E. R.2008. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 52: 118–123. doi: 10.1637/8055-070907-Reg [DOI] [PubMed] [Google Scholar]
- 13.Long N. T., Thanh T. T., van Doorn H. R., Vu P. P., Dung P. T., Dung T. T. K., Tien T. N., Thao D. T. T., Hung P., Quang N. V., Hoa N. T., Bryant J. E., Boni M. F.2011. Recent avian influenza virus A/H5N1 evolution in vaccinated and unvaccinated poultry from farms in southern Vietnam, January–March 2010. Transbound. Emerg. Dis. 58: 537–543. doi: 10.1111/j.1865-1682.2011.01229.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OIE. 2007. The global strategy for prevention and control of H5N1 highly pathogenic avian inlufenza. http://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Global_Strategy_fulldoc.pdf
- 15.Sanekata T., Fukuda T., Miura T., Morino H., Lee C., Maeda K., Araki K., Otake T., Kawahata T., Shibata T.2010. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci. 15: 45–49. doi: 10.4265/bio.15.45 [DOI] [PubMed] [Google Scholar]
- 16.Sawai J.2011. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 16: 95–102. doi: 10.4265/bio.16.95 [DOI] [PubMed] [Google Scholar]
- 17.Sims L. D.2007. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 51: 174–181. doi: 10.1637/7637-042806R.1 [DOI] [PubMed] [Google Scholar]
- 18.Smee D. F., Morrison A. C., Barnard D. L., Sidwell R. W.2002. Comparison of colorimetric, fluorometric, and visual methods for determining anti-influenza (H1N1 and H3H2) virus activities and toxicities. J. Virol. Methods 106: 71–79. doi: 10.1016/S0166-0934(02)00137-4 [DOI] [PubMed] [Google Scholar]
- 19.Takehara K., Chinen O., Jahangir A., Miyoshi Y., Ueno Y., Ueda S., Takada Y., Ruenphet S., Mutoh K., Okamura M., Nakamura M.2009. Ceramic Powder Made from Chicken Feces: Anti-Viral Effects Against Avian Influenza Viruses. Avian Dis. 53: 34–38. doi: 10.1637/8382-062008-Reg.1 [DOI] [PubMed] [Google Scholar]
- 20.Takehara K., Hyakutake K., Imamura T., Mutoh K., Yoshimura M.1994. Isolation, identification, and plaque titration of parvovirus from muscovy ducks in Japan. Avian Dis. 38: 810–815. doi: 10.2307/1592118 [DOI] [PubMed] [Google Scholar]
- 21.Takehara K., Shinomiya T., Kobayashi H., Azuma Y., Yamagami T., Yoshimura M.1987. Characterzation of Newcastle disease viruses isolated from field cases in Japan. Avian Dis. 31: 125–129. doi: 10.2307/1590784 [DOI] [PubMed] [Google Scholar]
- 22.Takehara K., Yamazaki K., Miyazaki M., Yamada Y., Ruenphet S., Jahangir A., Shoham D., Okumura M., Nakamura M.2010. Inactivation of avian influenza virus H1N1 by photocatalyst under visible light irradiation. Virus Res. 151: 102–103. doi: 10.1016/j.virusres.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Terpstra F. G., van den Blink A. E., Bos L. M., Boots A. G., Brinkhuis F. H., Gijsen E., van Remmerden Y., Schuitemaker H., van’t Wout A. B.2007. Resistance of surface-dried virus to common disinfection procedures. J. Hosp. Infect. 66: 332–338. doi: 10.1016/j.jhin.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 24.Tsujimura M., Thammakarn C., Yamada Y., Satoh K., Hasegawa T., Ruenphet S., Takehara K.2012. Antiviral activity of scallop-shell powder against avian influenza virus and goose parvovirus. Trans. Mater. Res. Soc. Jpn. 37: 567–570. doi: 10.14723/tmrsj.37.567 [DOI] [Google Scholar]
- 25.Umali D. V., Ito H., Suzuki T., Shirota K., Katoh H., Ito T.2013. Molecular epidemiology of Newcastle disease virus isolates from vaccinated commercial poultry farms in non-epidemic areas of Japan. Virol. J. 10: 330. doi: 10.1186/1743-422X-10-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou S., Guo J., Gao R., Dong L., Zhou J., Zhang Y., Dong J., Bo H., Qin K., Shu Y.2013. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol. J. 10: 289. doi: 10.1186/1743-422X-10-289 [DOI] [PMC free article] [PubMed] [Google Scholar]


