ABSTRACT
The presence of anti-erythrocyte autoantibodies in animals infected with various Babesia species is well reported. However, the pathogenesis of autoantibodies in babesiosis is poorly understood. Here, we demonstrated that anti-erythrocyte immunoglobulin (Ig) M and IgG were present in B. rodhaini-infected mice at 6 and 8 days after infection, respectively. Furthermore, we generated monoclonal antibodies against erythrocyte antigen from B. rodhaini-infected mice. Five clones were generated. By Western blotting analysis using whole erythrocyte antigens, one clone reacted with a broad band around 90–150 kDa, and the 2 clones reacted with a band larger than 150 kDa. B. rodhaini-infected mice and/or autoreactive monoclonal antibodies established in this study might be a powerful tool for in vivo pathogenesis studies of autoantibody development in infectious diseases.
Keywords: autoantibody, Babesiosis, erythrocyte antigen, immune-mediated anemia, monoclonal antibody
Babesiosis is a tick-borne hemoprotozoan disease of a wide range of mammalian hosts. Babesia spp.-infected animals sometimes develop severe anemia despite a relatively low parasitemia [8, 17]. This indicates that anemia caused by Babesia cannot only be explained by disruption of infected erythrocytes and that the destruction of non-infected erythrocytes is a critical feature of the disease. Many studies have demonstrated that anti-erythrocyte autoantibodies are generated during infection in canine and bovine babesiosis [1, 2, 7, 12, 13, 15, 16]. A major pathogenic autoantibody reported in canine autoimmune hemolytic anemia (AIHA), against erythrocyte membrane Band 3 molecule, was also reported in canine B. gibsoni infection [2]. Autoimmunity to erythrocytes in babesiosis has also been documented in humans [6, 20]. As a consequence of anti-erythrocyte autoantibodies, extravascular hemolysis, such as up-regulated erythrocyte phagocytosis and increased erythrocyte aggregation, in liver and spleen has also been reported in canines infected with B. gibsoni [13, 14]. These reports suggest anemia in babesiosis is partially caused by such autoantibodies. However, the role of autoantibodies in the pathogenesis of babesiosis is poorly understood. To investigate the in vivo pathogenesis of such autoantibodies, the passive transfer of autoantibodies to non-infected individuals can be the most simple and straightforward methodology. However, performing such experiments using domestic animals including dogs and cattle is unrealistic, and a small experimental animal model is required. Therefore, the present study investigated the properties of autoantibodies in B. rodhaini-infected mice.
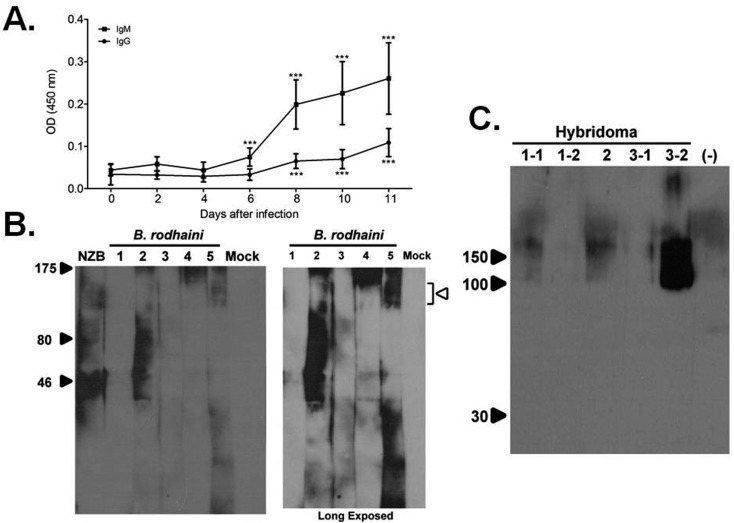
Infection of C57BL/6 mice (CLEA Japan Inc., Tokyo, Japan) with B. rodhaini and detection of autoantibodies were performed as reported previously with minor modifications [5]. Briefly, 106 parasitized erythrocytes were intravenously injected into 8-week-old female C57BL/6 mice, and serum samples were collected every other day. The experiments were performed in accordance with the Gifu University Animal Care and Use Committee guidelines. Autoantibodies reacting with erythrocytes were detected by enzyme-linked immunosorbent assay (ELISA) using erythrocyte membrane lysate as an antigen. To detect antigen-specific immunoglobulin (Ig) G and IgM, horseradish peroxidase (HRP)-conjugated rabbit-anti-mouse IgG or HRP-conjugated rabbit-anti-mouse IgM was used. After B. rodhaini infection, IgM reacting with erythrocytes was detected 6 days after infection (Fig. 1A). This indicates that primary immune responses to erythrocytes were activated at an early phase of B. rodhaini infection. IgG reacting with erythrocytes was also detected from 8 days after infection (Fig. 1A). The early presence of anti-erythrocyte IgG might be the result of an activated secondary immune response against erythrocytes. Natural autoantibodies against erythrocyte Band 3 and phosphatidylserine antigen were found in healthy animals, but at a low concentration [10]. B. rodhaini infection might reactivate B cell clones producing these autoantibodies.
Fig. 1.
Anti-erythrocyte autoantibodies generated by B. rodhaini infection. A. Development of anti-erythrocyte IgG (●) and IgM (■) after B. rodhaini infection (n=15 at 2, 4, 6, 8 and 10 days and n=14 at 11 days post-infection, ***P<0.0001: compared with day 0, by Student’s t test). B. Western blotting analysis using B. rodhaini-infected serum. As a negative control, mock-infected serum was used. (Open arrowhead: Band 3-like area, right panel: long exposed). C. Western blotting analysis using monoclonal antibodies produced by 5 hybridoma clones. As a negative control, supernatant of myeloma cell cultures was used (lane (−)). Whole erythrocyte lysate was used as an antigen.
We performed Western blotting analysis using erythrocyte membrane lysate as an antigen to identify the target antigen (s) on erythrocyte membranes. However, it was difficult to identify specific bands, because Western blotting analysis using serum samples from B. rodhaini-infected mice gave a wide molecular weight smear (Fig. 1B). Therefore, we generated anti-erythrocyte monoclonal antibodies to determine the autoantigens present in erythrocyte membranes. Three C57BL/6 mice were infected with B. rodhaini, and splenocytes from each infected mouse were isolated 11 days after infection. One billion splenocytes were cultured with 107 erythrocyte ghosts for 3 days in RPMI-1640 (supplemented with 15% fetal calf serum). After incubation, 1.5 × 108 splenocytes were fused with 3 × 107 myeloma cell (NS-1) by polyethyleneglycol (PEG) 4000 (Merck & Co., Whitehouse Station, NJ, U.S.A.). The hybridoma was then cultured in hypoxanthine-aminopterin-thymidine (HAT) culture medium (Sigma-Aldrich, St. Louis, MO, U.S.A.). As a control, a hybridoma culture was prepared from 3 non-infected C57BL/6 mice. Fourteen days after fusion, several wells of the culture plate showed B. rodhaini-infected or non-infected mouse-derived cell hybridoma growth (Table 1). At the first screening, 21 days after fusion, supernatants from each well showing colony growth were collected for ELISA to examine production of anti-erythrocyte IgG and IgM as described above. As a negative control, supernatant from un-fused myeloma cell culture was used. An optical density value higher than the mean of the negative control plus 3 standard deviations was defined as positive. Some supernatant samples obtained from B. rodhaini-infected mice were positive (Table 1). Hybridoma clones producing erythrocyte-reactive monoclonal IgM were not observed. After further re-cloning, we finally obtained 5 hybridoma clones stably producing erythrocyte-reactive monoclonal IgG (Table 1). Western blotting analysis was performed to evaluate which erythrocyte antigens were recognized by the hybridoma clones. Whole erythrocyte lysate prepared with a reported protocol [19] was used as an antigen. Briefly, heparinized whole blood from healthy mice was centrifuged, and then, the plasma and buffy coat were removed. Erythrocyte was washed with of 0.09% NaCl, hypotonic lysed with 10 mM Tris-HCl (pH=7.5) and incubated at 4°C for 10 min. The same volume of 0.25 M glucose was added into the lysate and incubated at 4°C for 5 min. After centrifuge, pale RBC ghost was collected from the bottom of tube. Fifty µl of erythrocyte ghost was incubated with 50 µl of SDS sample buffer at 98°C for 10 min and electrophoresed in a 10% SDS-PAGE (Atto co.). Culture supernatants, diluted twice, were used as the primary antibody. Secondary antibody was horseradish peroxidase (HRP)-conjugated rabbit-anti-mouse IgG. One hybridoma clone (clone 3-2) had monoclonal IgG reactivity recognizing a broad band of 90–150 kDa (Fig. 1C, lane 5). Two hybridoma clones (clones 1-1 and 2) secreted monoclonal IgG reactive with a band greater than 150 kDa (Fig. 1C, lanes 1 and 3). Two other hybridoma clones (clones 1-2 and 3-1) which showed positive results in ELISA, however, did not give a clear band under Western blotting analysis (Fig. 1C, lanes 2 and 4). New Zealand Black (NZB) mice, a mouse strain that naturally develops AIHA, produced autoreactive antibodies, of which the most common target is erythrocyte Band 3 [3, 9]. Band 3 usually gives a broad band around 90–100 kDa. Antigen (s) recognized by hybridoma clone 3-2 producing monoclonal IgG might contain erythrocyte Band 3. Among the major antigens contained in mouse erythrocyte membranes, only spectrin, an actin cross-linking protein, had a molecular weight greater than 150 kDa [3]. Thus, monoclonal antibodies produced by hybridoma clones 1-1 and 2 likely react with spectrin. It was reported that splenic T cells from mice with AIHA proliferated in response to mouse erythrocyte membrane fractions containing Band 3 or spectrin [3].
Table 1. Number of anti-erythrocyte antibody secreting hybridoma clones.
| Primary Screening | Secondary Screening | |||
|---|---|---|---|---|
| Mouse | No. of clone | α RBC clonea) | α RBC cloneb) | |
| (after re-cloning) | ||||
| B. rodhaini infected | A | 25 | 4 | 3 |
| B | 21 | 5 | 0 | |
| C | 15 | 4 | 2 | |
| Mock | A | 11 | 0 | 0 |
| B | 5 | 0 | 0 | |
| C | 4 | 0 | 0 | |
a) Total number of well showed hybridoma growth. All established clones were proceeded to ELISA test for detecting erythrocyte-reacting clones. b) Number of clone reacting with erythrocyte antigen.
However, due to the unstable nature of hybridoma, the ability of antibody secretion gradually decreased after serial passages. To obtain enough amount of antibody for further study, artificial synthesis of antibody can be performed. After the hybridoma from B. rodhaini-infected mice was established, total RNA of hybridoma could be extracted and mRNA sequence of antibody Fab region could then be analyzed [4]. Antibody against erythrocyte then could be artificially synthesed based on the mRNA sequence.
To our knowledge, our data show for the first time that B. rodhaini-infected mice produced autoreactive IgG with similar reactivity to IgG produced in B. gibsoni-infected dogs and mice with AIHA [2, 3]. Thus, B. rodhaini-infected mice might be a potential model to study the pathogenesis of Babesia-associated autoreactive antibodies. However, clinical symptoms of B. rodhaini-infected mice were different from those of canine or bovine babesiosis. Although extravascular hemolysis is observed in canine and bovine babesiosis [7, 16, 18], B. rodhaini-infected mice developed severe intravascular hemolysis associated with extremely high parasitemia [11]. The relatively larger effect of proliferating B. rodhaini merozoites likely masked the pathogenesis of the autoreactive antibodies. Therefore, B. rodhaini-infected mice might not be suitable for investigating canine or bovine babesiosis. Passive transfer of autoreactive antibodies harvested from B. rodhaini-infected mice into non-infected individuals might be a useful model to determine the pathogenesis of antibodies independent to the direct effect of proliferating merozoites. However, it is difficult to obtain purified autoreactive antibodies from small amounts of mouse serum. In contrast, monoclonal antibodies can be stably produced. Autoreactive monoclonal antibodies established in this study will be a powerful tool for passive-transfer experiments to examine the properties of babesia-associated autoreactive antibodies in vivo.
Acknowledgments
This study was supported by a Corporation Research Grant (25-Joint-5) of National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine.
REFERENCES
- 1.Adachi K., Tateishi M., Horii Y., Nagatomo H., Shimizu T., Makimura S.1994. Elevated erythrocyte-bound IgG value in dogs with clinical Babesia gibsoni infection. J. Vet. Med. Sci. 56: 757–759. doi: 10.1292/jvms.56.757 [DOI] [PubMed] [Google Scholar]
- 2.Adachi K., Tateishi M., Horii Y., Nagatomo H., Shimizu T., Makimura S.1995. Immunologic characteristics of anti-erythrocyte membrane antibody produced in dogs during Babesia gibsoni infection. J. Vet. Med. Sci. 57: 121–123. doi: 10.1292/jvms.57.121 [DOI] [PubMed] [Google Scholar]
- 3.Barker R. N., Shen C. R., Elson C. J.2002. T-cell specificity in murine autoimmune haemolytic anaemia induced by rat red blood cells. Clin. Exp. Immunol. 129: 208–213. doi: 10.1046/j.1365-2249.2002.01917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourne Y., Renault L., Essono S., Mondielli G., Lamourette P., Boquet D., Grassi J., Marchot P.2013. Molecular characterization of monoclonal antibodies that inhibit acetylcholinesterase by targeting the peripheral site and backdoor region. PLoS ONE 8: e77226. doi: 10.1371/journal.pone.0077226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiou S. P., Yokoyama N., Igarashi I., Kitoh K., Takashima Y.2012. Serum of Babesia rodhaini infected mice down regulates catalase activity of healthy erythrocytes. Exp. Parasitol. 132: 327–333. doi: 10.1016/j.exppara.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Evenson D. A., Perry E., Kloster B., Hurley R., Stroncek D. F.1998. Therapeutic apheresis for babesiosis. J. Clin. Apher. 13: 32–36. doi: [DOI] [PubMed] [Google Scholar]
- 7.Góes T. S., Góes V. S., Ribeiro M. F., Gontijo C. M.2007. Bovine babesiosis: anti-erythrocyte antibodies purification from the sera of naturally infected cattle. Vet. Immunol. Immunopathol. 116: 215–218. doi: 10.1016/j.vetimm.2006.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Groves M. G., Dennis G. L.1972. Babesia gibsoni: field and laboratory studies of canine infections. Exp. Parasitol. 31: 153–159. doi: 10.1016/0014-4894(72)90057-4 [DOI] [PubMed] [Google Scholar]
- 9.Hall A. M., Ward F. J., Shen C. R., Rowe C., Bowie L., Devine A., Urbaniak S. J., Elson C. J., Barker R. N.2007. Deletion of the dominant autoantigen in NZB mice with autoimmune hemolytic anemia: effects on autoantibody and T-helper responses. Blood 110: 4511–4517. doi: 10.1182/blood-2007-06-094383 [DOI] [PubMed] [Google Scholar]
- 10.Hod E. A., Arinsburg S. A., Francis R. O., Hendrickson J. E., Zimring J. C., Spitalnik S. L.2010. Use of mouse models to study the mechanisms and consequences of RBC clearance. Vox Sang. 99: 99–111. doi: 10.1111/j.1423-0410.2010.01327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda H., Hasegawa K., Kozaki S.1987. Development of anti-erythrocyte antibodies in mice infected with Babesia rodhaini. Zentralbl. Bakteriol. Mikrobiol. Hyg. [A] 266: 543–551 [DOI] [PubMed] [Google Scholar]
- 12.Morita T., Saeki H., Imai S., Ishii T.1995. Reactivity of anti-erythrocyte antibody induced by Babesia gibsoni infection against aged erythrocytes. Vet. Parasitol. 58: 291–299. doi: 10.1016/0304-4017(94)00726-S [DOI] [PubMed] [Google Scholar]
- 13.Murase T., Maede Y.1990. Increased erythrophagocytic activity of macrophages in dogs with Babesia gibsoni infection. Nippon Juigaku Zasshi 52: 321–327. doi: 10.1292/jvms1939.52.321 [DOI] [PubMed] [Google Scholar]
- 14.Murase T., Ueda T., Yamato O., Tajima M., Maede Y.1996. Oxidative damage and enhanced erythrophagocytosis in canine erythrocytes infected with Babesia gibsoni. J. Vet. Med. Sci. 58: 259–261. doi: 10.1292/jvms.58.259 [DOI] [PubMed] [Google Scholar]
- 15.Onishi T., Suzuki S., Horie H., Hashimoto M., Kajikawa T., Ohishi I., Ejima H.1993. Serum hemolytic activity of Babesia gibsoni-infected dogs: the difference in the activity between self and nonself red blood cells. J. Vet. Med. Sci. 55: 203–206. doi: 10.1292/jvms.55.203 [DOI] [PubMed] [Google Scholar]
- 16.Orinda G. O., Commins M. A., Waltisbuhl D. J., Goodger B. V., Wright I. G.1994. A study of autoantibodies to phosphatidyl-serine in Babesia bovis and Babesia bigemina infections in cattle. Vet. Immunol. Immunopathol. 40: 275–281. doi: 10.1016/0165-2427(94)90025-6 [DOI] [PubMed] [Google Scholar]
- 17.Saleh M. A.2009. Erythrocytic oxidative damage in crossbred cattle naturally infected with Babesia bigemina. Res. Vet. Sci. 86: 43–48. doi: 10.1016/j.rvsc.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Solano-Gallego L., Baneth G.2011. Babesiosis in dogs and cats–expanding parasitological and clinical spectra. Vet. Parasitol. 181: 48–60. doi: 10.1016/j.vetpar.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 19.Tomoda A., Kodaira K., Taketo A., Tanimoto K., Yoneyama Y.1984. Isolation of human erythrocyte membranes in glucose solution. Anal. Biochem. 140: 386–390. doi: 10.1016/0003-2697(84)90182-9 [DOI] [PubMed] [Google Scholar]
- 20.Wolf C. F., Resnick G., Marsh W. L., Benach J., Habicht G.1982. Autoimmunity to red blood cells in babesiosis. Transfusion 22: 538–539. doi: 10.1046/j.1537-2995.1982.22683068624.x [DOI] [PubMed] [Google Scholar]