Summary
Patients with aggressive, BCL2 protein-positive (+) diffuse large B-cell lymphoma (DLBCL) often experience rapid disease progression that is refractory to standard therapy. However, there is potential for false-negative staining of BCL2 using the standard monoclonal mouse 124 antibody that hinders the identification of these high-risk DLBCL patients. Herein, we compare two alternative rabbit monoclonal antibodies (E17 and SP66) to the 124 clone in staining for BCL2 in formalin-fixed, paraffin-embedded DLBCL tissues. Overall, in two independent DLBCL cohorts E17 and SP66 detected BCL2 expression more frequently than 124. In the context of MYC expression, cases identified as BCL2 (+) with SP66 demonstrated the strongest correlation with worse OS. The 124 clone failed to detect BCL2 expression in the majority of translocation (+), amplification (+), and activated B-cell DLBCL cases in which high levels of BCL2 protein are expected. Using dual in-situ hybridization (Dual ISH) as a new tool to detect BCL2 translocation and amplification, we observed similar results as previously reported for fluorescence ISH for translocation but a higher amplification frequency, indicating that BCL2 amplification may be under-reported in DLBCL. Among the discrepant cases, phosphorylation of BCL2 at T69 and/or S70 was more common than in the concordant cases and may contribute to the 124 false-negatives, in addition to previously associated mutations within the epitope region. The accurate detection of BCL2 expression is important in the prognosis and treatment of DLBCL particularly with new anti-BCL2 therapies.
Keywords: diffuse large B-cell lymphoma, E17 and SP66 BCL2 antibodies, Dual in-situ hybridization (Dual ISH), BCL2 phosphorylation, BCL2 amplification and translocation
1. Introduction
BCL2 over-expression is associated with a poor response to therapy and shorter disease-free and overall survival (OS) in diffuse large B-cell lymphoma (DLBCL), the most common aggressive non-Hodgkin lymphoma in the United States [1–4]. This association is especially prevalent within the context of concurrent MYC expression [5,6]. On average, about 50% of DLBCL cases have detectable BCL2 protein using various cut-off values [5–11]. One study found as many as 71% BCL2 positive (+) DLBCL cases using a >10% cut-off [9]. When evaluated in the context of cell of origin (COO), similar percentages of BCL2 (+) cases in both the germinal center B-cell (GCB) subtype and activated B-cell (ABC) or non-GCB subgroups were found within small cohorts of DLBCL [8,9,11]. Studies conducted on larger cohorts, however, demonstrate that ABC cases are more often BCL2 (+) compared to GCB cases [2,5]. BCL2 translocations (t(14;18)) and amplifications (18q21) can contribute to these high levels of BCL2 expression. Approximately 20–30% of GCB-DLBCL cases are BCL2 translocation (+) [11–13] and up to 70% and 20% of ABC-DLBCL cases have BCL2 gene gains and amplification, respectively [2,10–12]. Yet, some translocation (+) DLBCL cases have low to no expression of BCL2 as detected by immunohistochemistry (IHC) using the standard clone 124 [7,9]. The lack of BCL2 detection is also reported in follicular lymphoma (FL), in which 10% of the t(14;18) positive cases stain BCL2 (−). In these FL cases, mutations within the flexible loop domain (FLD), which include the epitope region (amino acids 41–54) of clone 124, account for the false-negative staining as these cases will stain BCL2 (+) with whole protein-targeted antibody [15,16]. Similar studies have also demonstrated that mutations in BCL2 result in false-negative DLBCL cell lines that are expected to be BCL2 (+) due to the presence of the t(14;18) [17]. However, a recent study conducted in DLBCL cases suggest that mutations within BCL2 do not account for all of the false-negative cases [18].
The potential for false-negative BCL2 staining in DLBCL with clone 124 therefore demonstrates the need for a better antibody to accurately detect BCL2 expression. To date there is no comprehensive study of all relevant monoclonal BCL2 antibodies correlated with mRNA, gene status (amplification and translocation), MYC protein, and alternative mechanisms to mutations as the cause for the discrepant staining in DLBCL. In this study, we evaluated BCL2 staining with two new rabbit monoclonal antibodies (E17 and SP66) compared to clone 124 in DLBCL tissues. In cases with discrepant staining a new chromogenic BCL2 Dual ISH assay and previous gene expression profiling data was used to determine whether there is expected BCL2 expression based on the BCL2 gene status and COO subtype. We then investigated the presence of phosphorylation as a possible mechanism for the discordant detection of BCL2 expression.
2. Materials and Methods
2.1 DLBCL tissues
Two cohorts of DLBCL formalin-fixed, paraffin-embedded tissues (FFPET) were used for this study. One case series included pretreatment biopsies from in-house patient cases (N=5, University Medical Center, Tucson, AZ) and cases from the Leukemia/Lymphoma Molecular Profiling Project (LLMPP, N=89) [12,19]. Whole tissue sections were available for 24 of the cases and the remaining 70 were cores previously prepared on four different tissue microarrays (TMA). The second cohort of 144 DLBCL FFPET consisted of cases from the University of Nebraska Medical Center assembled on six different TMA [6]. Sections of normal tonsil served as control tissue. The use of human tissues and clinical data for this study was approved from the University of Arizona and the University of Nebraska Medical Center Institutional Review Boards in accordance with the Declaration of Helsinki.
2.2 Determination of DLBCL cell of origin (COO) subtype
The COO was previously assigned by GEP for 83 cases as ABC (n=36), GCB (n=35), unclassifiable (n=6), and PMBL (n=6) [12,19]. There were 11 in-house cases or LLMPP cases without GEP and for 3 of these the Hans algorithm [20] was used to classify 2 as GCB and 1 as non-GCB. The COO was undetermined for the remaining 8 cases. The unknown COO and PMBL cases were only used in the overall DLBCL analyses.
2.3 BCL2 Dual ISH probe design
The target sequences for the BCL2 gene region were analyzed using an in-house bio-informatics software package (Ventana Medical Systems, Inc. (VMSI, Tucson, AZ). Subsequences within the BCL2 genomic target region were further tested using a Roche NimbleGen Comparative Genomic Hybridization (CGH) custom array (Roche, Indianapolis, IN). DNA target sequence hybridization to a human genomic probe and a Cot1 DNA probe were analyzed and sequences with high hybridization scores following the CGH analysis were eliminated. The specificity of each probe was confirmed on a metaphase spread, which resulted in BCL2 signals unique to chromosome 18.
2.4 BCL2 automated Dual in situ hybridization (Dual ISH) assay and evaluation
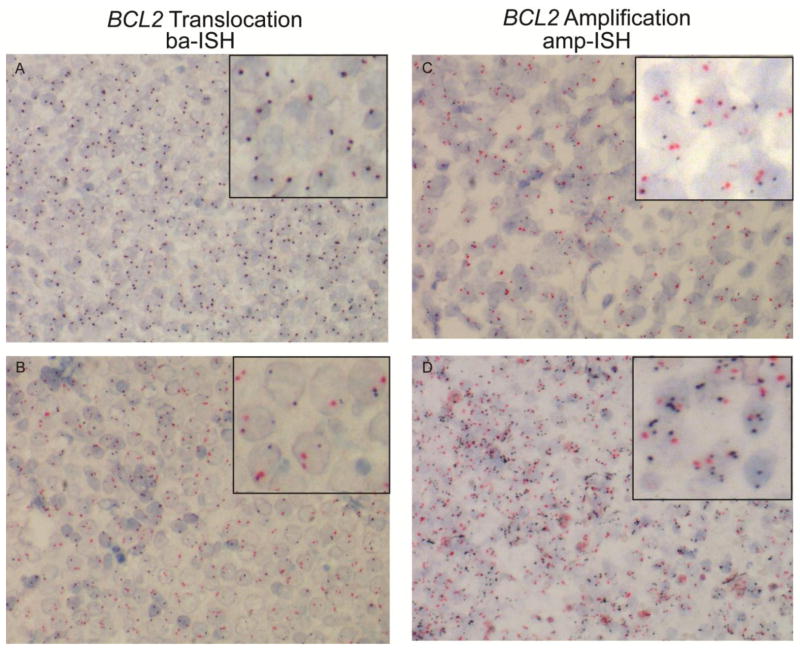
The Dual ISH assays were developed on the BenchMark® XT/BenchMark® Ultra automated staining system (VMSI) [21,22]. Sections were prepared with deparraffinization, cell conditioning, and denaturation. For detection of the translocation, break-apart (ba)-ISH DNP-5′-BCL2 and DIG-3′-BCL2 probes were hybridized to targets then detected using ultraView Dual ISH DNP and ultraView RED ISH DIG detection kits, respectively (VMSI). The BCL2 amplification was measured with the same detection method using DNP- BCL2 and DIG-D18Z1 for detection of chromosome 18 (amplification (amp)-ISH). A FL whole tissue section and TMA was stained as a positive control for ba-ISH and two tonsil TMA cores served as negative controls for the BCL2 ba-ISH and amp-ISH assays. Stained FFPET were viewed, blinded to FISH or IHC results, using an Olympus BX-61 microscope system (Olympus America, Inc., Center Valley, PA) at 10x, 40x, and 60x magnification (Plan Fluor, Nikon Instruments, Inc., Melville, NY). Images were captured with a Nikon DS-Fi1 digital CCD camera and DS-L2 imaging controller (Nikon). Cases were scored translocation (+) if at least 15% of cells within a 20 informative cell count displayed distinctly separate black (DNP, 5′ end of BCL2) and red (DIG, 3′ end of BCL2) signals [21]. According to previously published guidelines, a case was considered positive for BCL2 amplification if at least 15% of cells within a 20 informative cell count consisted of ≥4 BCL2 gene signals (DNP, black) with a normal chromosome 18 ploidy (2 DIG, red signals) [22–24]. Similarly, chromosome 18 polysomy was defined as greater than 4 red signals. An informative cell consisted of the presence of at least two of each signal (one set per copy of the gene, two DNP and two DIG Dual ISH signals). At minimum, 20% of the cases were independently evaluated by at least two reviewers (SK, TMG, LMR).
2.5 Immunohistochemical (IHC) staining and evaluation
Paraffin slides were prepared from blocks cut at 4 microns and stained using the BenchMark® XT instrument. The BCL2 primary antibodies, VMSI Clone 124 (mouse, Cat# 790-4464, N terminus, amino acids 41–54) and VMSI Clone SP66 (rabbit, Cat# 790-4604, N terminus, amino acids 40–75) were used as supplied while the Epitomics Clone E17 (rabbit, Cat# 1017-1, Epitomics, Burlingame, CA, N terminus, amino acids 61–76) and phospho-S70 (rabbit, Cat# ab28819, Abcam) were used at a 1:50 dilution. The phospho-T69 (rabbit, Cat# ab59397, Abcam, Cambridge, MA) was used at a 1:100 diultion. Each slide was deparaffinized with a 56 min (E17, SP66, and phospho-T69), 16 min (phospho-S70) or 60 min (124) CC1 cell conditioning regimen and optimized for darkest staining. The E17, SP66, phospho-S70 and –T69 antibodies were incubated for 32 min at 37°C and detected with the OptiView DAB IHC Detection kit (Cat# 760-700, VMSI). The OptiView Amplification kit (Cat# 760-099, VMSI) was used with the S70 antibody IHC. The 124 antibody was detected using the iView DAB Detection kit (Cat# 760-091, VMSI) with a 36 min, 37°C incubation. A subset of cases was also stained with 124 using the OptiView detection kit using the 56 min CC1 treatment. All slides were counterstained with hematoxylin for 4 min. MYC IHC was performed as previously described [6].
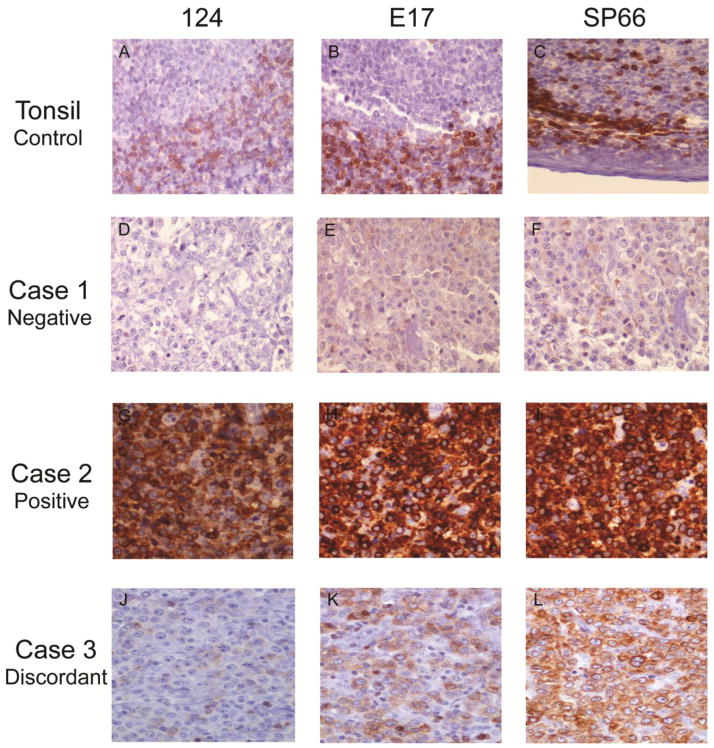
SLK and LR evaluated all of the BCL2 stained slides and any discrepancies were resolved by using a multi-head microscope and a third reviewer, LMR. Cases were examined individually and evaluated at high power (400–600X) as previously described for Dual ISH evaluation. As shown in Fig. 1, a case with ≥ 30% of cells (within a 150 cell count) with moderate to strong DAB brown staining in the most highly stained region of the tissue was scored positive as previously described [2]. Only malignant B-cells were included in the count. Reactive T-cells and histiocytes were excluded, but used as an internal positive control for the degree of staining required for malignant cells to be positive.
Fig. 1. Comparison of BCL2 IHC with the 124, E17, and SP66 antibodies.
A FFPET section of a normal tonsil was assayed with all three antibodies as a positive control (A–C). Three representative DLBCL FFPET sections from three individual cases were compared for negative (Case 1, D–F), positive (Case 2, G–I), and discrepant (Case 3, negative with 124 (J) and positive with E17 (K) and SP66 (L)) staining with all three antibodies. 100X magnification.
2.6 Statistical analysis
All statistical tests were performed using the GraphPad Prism software, version 4.0 (La Jolla, CA). The Fisher’s exact and chi-square tests were used to determine significant associations when P <0.05. OS was estimated with the Kaplan-Meier method using the date of lymphoma diagnosis and differences were determined using the log-rank test. A multivariate analysis was conducted using Cox regression for BCL2 and MYC combination groups as previously described [6].
3. Results
3.1 Clinical correlation
We stained 94 DLBCL cases independently with the standard monoclonal mouse BCL2 antibody (124), and two alternative monoclonal rabbit antibodies (E17 and SP66) (Fig. 1). All three antibodies highlighted the BCL2 (+) B cells in the normal control tonsil’s mantle zone and interfollicular T cells, while leaving the germinal center B-cells unstained (Fig. 1. A–C). Using a ≥30% cut-off, BCL2 expression detected with 124, E17, or SP66 did not significantly correlate with any IPI factors (Supplemental Table S1). There was a borderline association in DLBCL as a whole entity as well as in the ABC/non-GCB subgroups, of increasing age and BCL2 positivity with the SP66 antibody, and to a lesser extent with E17.
Dual ISH assays were successfully performed on 83 of the 94 cases with 73 and 61 evaluable cases for BCL2 translocation (ba-ISH) and amplification (amp-ISH), respectively (Fig. 2). Overall, there were no significant correlations between BCL2 translocation/amplification and any IPI factors (Supplemental Table S2). In the ABC subtype alone (P=0.038) or with unclassifiable and non-GCB cases (P=0.020) BCL2 gene amplification correlated with a LDH ratio above one (9/9; 100%) compared to amplification (−) cases (6/11; 55%).
Fig. 2. BCL2 Dual ISH assays for detection of gene translocation and amplification.
Representative DLBCL FFPET sections stained with a break-apart probe set (A and B) specific for the 3′ and 5′ end of the BCL2 gene or an amplification probe set specific for chromosome 18 centromere and the BCL2 gene (C and D) shown as red and black signals, respectively. Overlapping red and black signals indicates the absence of translocation (A), while a “free”, non-overlapped red signal indicates translocation (B). For the amplification ISH, more than 2 black signals in the presence of 2 red signals indicates amplification. Inset is a portion of the section at high power. 400X magnification.
3.2 Antibodies targeted at different epitopes detect varying levels of BCL2 expression
Overall, the SP66 (75/94 cases, 80%) and E17 (58/94 cases, 62%) antibodies detected significantly (P<0.0001) more frequent BCL2 expression than the 124 (32/94 cases, 34%) (Table 1). BCL2 staining across all three antibodies agreed in only 53% of the DLBCL cases with 19% (18/94) BCL2 (−) (“Case 1”, Fig. 1D–F) and 34% (32/94) BCL2 (+) (“Case 2”, Fig. 1G–I). In contrast, 47% (44/94) cases were discrepant with 124 staining BCL2 (−) and E17 and/or SP66 staining BCL2 (+) as shown in “Case 3” (Fig. 1J–K) (Table 2). Specifically, there were 28% (26/94) discrepant cases between 124 and E17 and 46% (43/94) between 124 and SP66. Staining with SP66 and E17 disagreed in 20% of cases (19/94) with 18 cases BCL2 (−) by E17, but BCL2 (+) by SP66 and 1 case was E17 (+) while SP66 (−). The discrepant staining also occurred within the DLBCL subtypes where 124 did not distinguish the previously reported higher incidence of BCL2 expression in ABC/non-GCB cases (Table 1). We confirmed the previously reported higher BCL2 mRNA level in the ABC subtype (7.7 log2 transformed array signal intensity) relative to the GCB subtype (6.5 log2 transformed array signal intensity) using prior gene expression data for a subset of the cases (Supplemental Fig. S1). To further test whether the 124 and E17/SP66 discrepant cases would be expected to have BCL2 expression (ie. harbor mechanisms for BCL2 up-regulation, such as translocation or amplification) and determine if the positive staining may be false-positives, we used new Dual ISH assays to detect BCL2 translocation and amplification in the DLBCL cases.
Table 1.
Comparison of BCL2 protein expression by three different antibodies in DLBCL.
| BCL2 Positivea n (%) |
P | |||
|---|---|---|---|---|
| 124 | E17 | SP66 | ||
| DLBCL, all subtypes (n=94) | 32 (34.0) | 58 (61.7) | 75 (79.8) | <0.0001 |
| ABC (n=36) | 13 (36.1) | 26 (72.2) | 32 (88.8) | <0.0001 |
| Non-GCBb (n=43) | 16 (37.2) | 33 (76.7) | 39 (90.7) | <0.0001 |
| GCB (n=38) | 14 (36.8) | 18 (47.4) | 27 (71.1) | 0.009 |
| BCL2gene status | ||||
| Translocation (+) (n=14) | 9 (64.3) | 11 (78.6) | 13 (92.9) | 0.183 |
| Amplification (+) (n=28) | 10 (35.7) | 22 (78.6) | 26 (92.9) | <0.0001 |
| Chrs. 18 polysomy (+) (n=6) | 1 (16.7) | 4 (66.7) | 4 (66.7) | 0.135 |
defined as ≥ 30% of the cells stained positive (medium to dark brown) in a 150 cell count
non-GCB subtype includes ABC and unclassifiable as determined by GEP and non-GCB as determined by IHC according to Hans classification (15).
Table 2.
Characterization of the 124-E17/SP66 Discordant DLBCL Cases.
| BCL2 Discordanta n (%) |
BCL2 (−)Concordant n (%) |
BCL2 (+)Concordant n (%) |
Pb | |
|---|---|---|---|---|
| DLBCL, all subtypes (n=94) | 44 (46.8) | 18 (19.1) | 32 (34.1) | |
| BCL2gene status | (n=39) | (n=12) | (n=27) | |
| Translocation (+) | 4 (10.3) | 1 (8.3) | 9 (33.3) | |
| Amplification (+) | 17 (43.6) | 1 (8.3) | 10 (37.0) | |
| Chrs. 18 polysomy (+) | 3 (7.7) | 2 (16.7) | 1 (3.7) | |
| > 1 chromosomal abnormality | 22 (56.4) | 4 (33.3) | 16 (59.3) | |
| BCL2 phophorylation status | ||||
| Phospho-T69 (+) (n=37; 8; 25) | 13 (35.1) | 0 (0.0) | 5 (20.0) | 0.030 |
| Phospho-S70 (+) (n=27; 8; 20) | 13 (48.1) | 3 (37.5) | 9 (45.0) | 0.789 |
| Dual site (n=27; 8; 19) | 3 (11.1) | 0 (0.0) | 4 (21.1) | 1.000 |
| Single site (n=27; 8; 19) | 23 (85.2) | 3 (37.5) | 10 (52.6) | 0.008 |
defined as BCL2 (−) with 124 and BCL2 (+) with E17 and/or SP66
P-value only calculated for BCL2 phosphorylation status as the other variables are not directly compared for significance testing. Concordant (−) and (+) cases were pooled to form one collective group for statistical analysis to compare concordant and discordant cases.
3.3 Dual ISH assays identify BCL2 gene translocation and amplification in the 124 discrepant cases and an overall higher amplification frequency then previously reported for DLBCL
The 124 clone detected BCL2 protein expression in only 64% (9/14) translocation (+), 36% (10/28) amplification (+), and 17% (1/6) chromosome 18 polysomy (+) cases compared to E17 and SP66 (Table 1). In the discrepant cases, 56% (22/39) were positive for BCL2 translocation, amplification, chromosome 18 polysomy, or a combination (Table 2). The entire DLBCL cohort was also evaluated for frequency of BCL2 gene abnormalities. There was 21% (15/73) BCL2 translocation (+) DLBCL cases overall with 36% (10/28) and 7% (2/30) in the GCB and ABC subgroups, respectively (Table 3). The remaining 3 BCL2 translocation (+) cases were non-GCB (n=1) or of unknown COO (n=2). For BCL2 amplification, we found 48% (29/61) of the cases were positive with polysomy for chromosome 18 in 13% (8/61) indicating that in some cases BCL2 amplification may be due to polysomy (Table 3). Specifically, the majority of BCL2 amplification occurred in the ABC (52% (12/23)) and GCB subtypes (42% (10/24)) with 7 cases representing the nonGCB (n=3), PMBL (n=1), and unknown (n=2) classifications. We compared the ba-ISH to the current standard, FISH, in a subset of 32 DLBCL cases. The ba-ISH and FISH had comparable failure rates of 19% (6/32) with only one case failing both assays and 90% (19/21) agreement on BCL2 translocation status (data not shown). The 2 discrepant cases were ba-ISH (−) and FISH (+).
Table 3.
Frequency of BCL2 gene abnormality with copy number and mRNA levels in DLBCL.
| BCL2 Gene Status n (%) |
P | |||
|---|---|---|---|---|
| Translocation(+)a | Amplification(+)a | Chrs. 18 Polysomy(+)a | ||
| DLBCL, all subtypes | 15/73 (20.5) | 29/61 (47.5) | 8/61 (13.1) | |
| ABC | 2 (6.7) | 12 (52.2) | 3 (13.0) | 0.564c |
|
| ||||
| Non-GCBb | 3 (8.6) | 16 (53.3) | 3 (10.0) | |
| GCB | 10 (35.7) | 10 (41.7) | 4 (16.7) | 0.009d |
|
| ||||
| BCL2 mRNA level | ||||
| Average log2 transformed | 5.9 | 7.1 | 5.5 | 0.049 |
Total “n” differs for the translocation and amplification/polysomy categories due to the different pass rates for each of the Dual ISH assays. The “n” for each variable is as follows: ABC, n=30 (translocation (+)) and 23 (amplification/polysomy (+)); Non-GCB, n=35 and 30; and GCB, n=28 and 24.
non-GCB subtype includes ABC and unclassifiable as determined by GEP and non-GCB as determined by IHC according to Hans classification (15).
P-values calculated for difference between translocation(+)c and amplification(+)d cases in the ABC and GCB subtypes.
To determine any correlations between mechanism of BCL2 deregulation and gene expression, we evaluated mRNA levels from previous GEP studies [12,19] of the translocation (+), amplification (+), and translocation/amplification (−) cases. The amplification (+) cases were moderately associated (P = 0.049) with a higher average log2 transformed mRNA level (7.1) compared to translocation (+) cases (5.9) (Supplemental Fig. S2 and Table 3).
3.4 Clone 124 fails to detect differences in OS according to BCL2 expression alone, but maintains previously reported prognostic significance in the context of MYC expression
The impact of BCL2 on OS is somewhat controversial in the literature, but recent reports demonstrate the importance of concurrent BCL2 and MYC expression in clinical outcome [5,6]. We first correlated the BCL2 staining from 124, E17, and SP66 with the survival of a subset (n=44) of the 94 DLBCL patients (excludes PMBL) treated with the current standard of therapy for DLBCL, R-CHOP (rituximab- cyclophosphamide, hydroxydoxrubicin, oncovin, prednisone). BCL2 expression detected by SP66 trended towards worse OS for BCL2 (+) cases compared to BCL2 (−) cases (Supplemental Fig. S3A). We were unable, however, to detect this difference with the 124 and E17 antibodies in this current cohort (Supplemental Fig. S3A). This could be due to a lower case number as well as a different cut-off (≥30% rather than ≥50% previously reported) although using a ≥50% cut-point for BCL2 positivity did not change our findings (Supplemental Fig. S3B). Second, we correlated BCL2 staining with all three antibodies in the context of previously determined MYC status in a separate cohort of 144 DLBCL cases in which 116, 117, or 121 cases successfully stained with 124, E17, and SP66, respectively. There was a similar 124-E17/SP66 discrepancy rate of 38% (44/116) compared to the initial examination of 94 DLBCL cases and failure of 124 to detect worse OS at 30% and 50% cut-offs (Supplemental Fig. S4). The SP66 antibody demonstrated a trend towards worse OS at the 50% cut-off (Supplemental Fig. S4). BCL2 expression with all three antibodies significantly correlated with worse OS in MYC (+) cases. SP66 demonstrated the strongest correlation (P<0.001) with a shorter median survival compared to 124 (P=0.001) and E17 (P=0.002) (Supplemental Fig. S5). As previously reported in the literature, the ABC cases within these cohorts had worse OS as compared to the GCB cases (Supplemental Fig. S6 and [6]). With the inability to detect differences in OS or BCL2 expression in translocation/amplification (+) and ABC-like DLBCL tumors using 124, we explored the possible mechanisms responsible for the false-negative IHC.
3.5 Phosphorylation of BCL2 may contribute to the false-negative IHC with clone 124
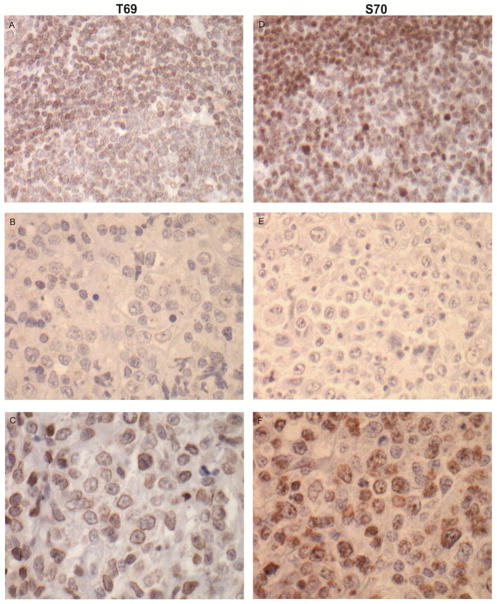
Phosphorylation of BCL2 at sites within the FLD, T69, S70, and S87, increase the pro-survival function of BCL2 through a conformational change that prevents the binding of p53 (aa32–68) and promotes the interaction with BAX to inhibit cell death [25,26]. We performed IHC on the discrepant cases to determine if phosphorylation at the most common sites, T69 and S70, could account for the failed 124 detection of BCL2 at aa41–54. Figure 3 shows representative T69 (A–C) and S70 (D–E) staining in a control tonsil tissue (top panel, A and D) and in DLBCL tissues, negative cases (middle panel, B and E), and positive cases (bottom panel, C and F).
Fig. 3. Comparison of BCL2-phospho IHC with the T69 and S70 antibodies.
IHC for phosphorylated BCL2 at either the T69 (A–C) or S70 (D–F) site was applied to a FFPET section of a normal tonsil (A and D). Two representative DLBCL FFPET sections from two individual cases were compared for negative (B and E) and positive (C and F) staining with the antibodies. 400X magnification.
Of the 44 discrepant cases, 37 cases were successfully stained with T69 and 27 cases with S70. There were 7 dual failures (tissue fell off slide) and for the additional S70 failures, 8 failed to stain with the protocol and 1 was un-interpretable. There was a significant (P=0.008) increase in phosphorylation in the discrepant cases, 85% (23/27), with 13 cases T69(+) or S70 (+) and 3 cases positive for both (Table 2). All of the BCL2 (−) concordant cases (n=16) were T69 (−) and 37.5% (3/8, 8 cases failed) were positive for S70. For the BCL2 (+) concordant cases, only 53% (10/19) were T69 (+) and/or S70 (+). To our knowledge this is the first report of BCL2 phosphorylation status in DLBCL, thus, the entire DLBCL cohort was also assessed for incidence of phosphorylation. Overall, phosphorylation at the S70 (46%, 25/55) position was more common (P=0.009) than at T69 (23%, 18/77) with 66% (36/55) of DLBCL cases consisting of at least single site phosphorylation (Table 4). When examined in the context of COO, the ABC cases exhibited a higher incidence (81%, 17/21; P=0.004) of phosphorylation at either T69 or S70 compared to GCB cases (65%, 11/29).
Table 4.
IHC detection of phosphorylation of BCL2 at positions T69 and S70 in DLBCL.
| BCL2 Phosphoryalation
Status n(%) |
P | ||||
|---|---|---|---|---|---|
| T69(+) | S70(+) | Dual site | Single site | ||
| DLBCL, all subtypes (n=77) | 18 (23.4) | 25 (45.5) | 7 (12.7) | 36 (65.5) | 0.009a |
| ABC (n=28) | 10 (35.7) | 10 (45.5) | 3 (14.3) | 17 (81.0) | 0.004a |
| GCB (n=29) | 4 (14.3) | 9 (50.0) | 2 (11.8) | 11 (64.7) | |
P-value calculated for difference between ABC and GCB subtypes for overall phosphorylation (Single site).
4. Discussion
Within the past decade, the extensive focus on the role of BCL2 in chemotherapy resistance has led to the development of various anti-BCL2 therapies. The accurate identification of patients with BCL2 (+) tumors at time of diagnosis will become critical for administration of such agents. Here, we demonstrate the standard BCL2 124 antibody routinely used in clinical laboratories fails to detect BCL2 expression in a significant proportion of DLBCL cases, particularly those with known mechanisms for BCL2 up-regulation (ie. translocation and amplification) or expected high levels of BCL2 (ie. ABC/non-GCB subtype). Alternative rabbit monoclonal antibodies, E17 and SP66, more reliably detected BCL2 expression in these cases and correlated with known genetic aberrations and expression levels. Similar to studies that also detected BCL2 false-negative cases, this further supports the 124 false-negatives are not artifact [15–17]. Whether this is directly and highly associated with the incidence of mutations within the 124 BCL2 epitope region as discovered for the majority of the pseudo-negative follicular lymphomas [15,16] and DLBCL cell lines [17] remains unclear. A recent publication addressing this issue in DLBCL demonstrated that the FLD wherein the epitope region is located is highly mutated [18]. However, the authors concluded that there were no mutations to account for the 124 and E17 discrepant staining they observed, which was minimal (10% compared to 28% in our cases). The variability in tumor content that often occurs in FFPET sections could account for the increased false-negative BCL2 staining we observed. We attempted to eliminate this variable by confirming >80% tumor content within the scored sections and restricting cell count to malignant DLBCL cells. In addition, BCL2 staining can be highly variable among laboratories as previously recognized [27] and the antibody staining method, selection of BCL2 (+) cut-point (both percentage of cells and intensity of staining considered “positive”), as well as field choice for evaluation could also lead to differences from previous studies [5, 18]. Accordingly, we also stained a subset of the cases with 124 using the OptiView Detection kit. There were no changes to the 124 scoring, although, some cases displayed a slightly higher intensity stain compared to staining with the iView Detection kit. Our IHC scoring bias was towards highly BCL2 (+) stained regions of the tissue based on the rationale that any BCL2 (+) portion of the tumor will contribute to the poor response to therapy and OS of the patient. This may also account for the differences in our OS observations in comparison to previous findings [5,6].
In addition to mutations within the epitope recognition site, phosphorylation may also induce conformational changes in protein structure that may inhibit antibody binding. BCL2 phosphorylation induces a conformational change and prevents p53 binding to the negative regulatory domain within the FLD further enhancing BCL2 activity [26]. Conversely, p53 binding upstream of the T69, S70, and S87 sites of BCL2 inhibits phosphorylation and potent activation of BCL2 [26]. Both the binding of p53, or any other BCL2-binding protein, and phosphorylation within the FLD may affect the ability of 124 antibody-epitope recognition. Consistent with this, there was a high percentage (85%) of phospho-T69 (+) and/or S70 (+) DLBCL cases that stained 124 (−), but E17 (+) and/or SP66 (+). Similar to the caveat that the high incidence of mutations within the epitope region does not account for all of the 124 false negative cases, phosphorylation of BCL2 may only explain a portion of the discrepant cases. Although phosphorylation occurs at a significant rate in the discrepant cases, 124 did detect BCL2 in some phosphorylation positive cases. We also found a high incidence of BCL2 phosphorylation in DLBCL as a whole entity (70%) with the majority of these staining positive for S70. Interestingly, most of the phospho-BCL2 (+) cases were of the ABC subtype that has worse OS. This raises the question of whether phosphorylation of BCL2 confers a worse response to therapy and may directly correlate with poor OS. With the limited number of cases we were unable to determine any association and further analysis is required with a larger cohort.
In our correlations of the IHC staining with presence of BCL2 gene translocation and amplification, we used new Dual ISH assays recently developed, which allowed for the concurrent visualization of morphology and chromosomal abnormalities in FFPET. We observed an incidence of BCL2 translocation consistent with established literature, but a higher frequency of amplification (48% compared to 20%) than previously reported for DLBCL. The assessment of BCL2 translocation or amplification was straightforward, highly reproducible among observers, could be evaluated directly within cells of the tumor and gave comparable results as obtained with standard FISH. Furthermore, the assays provide a more robust clinical assay as many of the limitations of FISH (rapid signal decay, fluorescence microscope, and inability to visualize tissue architecture) are overcome with using chromogenic based probes.
Taken together, our data suggests BCL2 protein (+) and amplification (+) cases may be underreported in DLBCL with the current methods. In addition, the elevated frequency of BCL2 phosphorylation in the 124 and E17/SP66 discrepant cases indicates this post-translational modification may contribute to the false-negative IHC. In support of the recently demonstrated clinical importance of BCL2 expression concurrent with MYC, high BCL2 levels detected by all three antibodies in combination with high MYC levels confer a poor prognosis [5,6]. Although 124 distinguished the worse OS for patients with double BCL2/MYC (+) DLBCL, the ability for SP66 to identify more of the BCL2 (+) cases allowed for a more significant prognostic distinction. The alternative and most likely more accurate rabbit antibodies provide new, effective tools for studying BCL2 expression in DLBCL. Further studies in larger cohorts are needed before recommendations can be confidently made regarding appropriate antibody choice for clinical use. The potential false-negative staining with 124, however, supports the need for a better antibody or possibly an antibody cocktail for BCL2 detection during diagnosis and stratification of patients for therapy.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA157581), the National Institutes of Health and the Lymphoma Research Foundation (Samantha Kendrick fully supported by CA009213 and the Lymphoma Research Foundation). We thank Dr. Kandeval Shanmugam and Dr. Nelson Alexander from VMSI for contributing to the initial set-up and design of the collaboration between VMSI and the Rimsza Lab and the design of the BCL2 Dual ISH probes, respectively.
Footnotes
Disclaimer: Authors, Andrea Muranyi, Leigh A. Henricksen, Stacey Stanislaw, and Thomas M. Grogan are employees of Ventana Medical Systems, Inc., Tucson AZ. All other authors declare no potential conflict of interest in connection with this submitted manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iqbal J, Meyer PN, Smith L, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and Rituximab. Clin Cancer Res. 2011;17:7785–95. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal J, Neppalli VT, Wright G, et al. BCL2 expression is a prognostic markers for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961–8. doi: 10.1200/JCO.2005.03.4264. [DOI] [PubMed] [Google Scholar]
- 3.Jovanovic MP, Jakovic L, Bogdanovic A, Markovic O, Martinovic VC, Mihaljevic B. Poor outcome in patients with diffuse large B-cell lymphoma is associated with high percentage of bcl-2 and Ki 67-positive tumor cells. Vojnosanit Pregl. 2009;66:738–43. doi: 10.2298/vsp0909738p. [DOI] [PubMed] [Google Scholar]
- 4.Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Scie USA. 2012;109:3879–84. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–9. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry AM, Alvarado-Bernal Y, Laurini JA, et al. MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol. 2014;165:382–91. doi: 10.1111/bjh.12763. [DOI] [PubMed] [Google Scholar]
- 7.Skinnider BF, Horsman DE, Dupuis B, Gascoyne RD. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol. 1999;30:803–8. doi: 10.1016/s0046-8177(99)90141-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–90. doi: 10.1182/blood.v99.7.2285. [DOI] [PubMed] [Google Scholar]
- 9.Iqbal J, Sanger WG, Horsman DE, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165:159–66. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierlamm J, Murga Penas EM, Bentink S, et al. Gain of chromosome region 18q21 including the MALT1 gene is associated with the activated B-cell-like gene expression subtype and increased BCL-2 gene dosage and protein expression in diffuse large B-cell lymphoma. Haematologica. 2008;93:688–96. doi: 10.3324/haematol.12057. [DOI] [PubMed] [Google Scholar]
- 11.Bea S, Zettl A, Wright G, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106:3183–90. doi: 10.1182/blood-2005-04-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105:13520–5. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bairey O, Zimra Y, Shaklai M, Okon E, Rabizadeh E. Bcl-2, Bcl-2X, Bax, and Bak Expression in Short-and Long-Lived Patients with Diffuse Large B-Cell Lymphomas. Clinical Cancer Res. 1999;5:2860–6. [PubMed] [Google Scholar]
- 14.Barrans LS, Carter I, Owen GR, et al. Germinal center phenotype and bcl-2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood. 2002;99:1136–43. doi: 10.1182/blood.v99.4.1136. [DOI] [PubMed] [Google Scholar]
- 15.Schraders M, de Jong D, Kluin P, Groenen P, van Krieken H. Lack of Bcl-2 epxression in follicular lymphoma may be casued by mutations in the BCL2 gene or by absence of the t(14;18) translocation. J Pathol. 2005;205:329–35. doi: 10.1002/path.1689. [DOI] [PubMed] [Google Scholar]
- 16.Adam P, Baumann R, Schmidt J, et al. The BCL2 E17 and SP66 antibodies discriminate 2 immunophenotypically and genetically distinct subgroups of conventionally BCL2-“negative” grade 1/2 follicular lymphomas. Human Pathol. 2013;44:1817, 26. doi: 10.1016/j.humpath.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Masir N, Campbell LJ, Jones M, Mason DY. Pseudonegative BCL2 protein expression in a t(14;18) translocation positive lymphoma cell line: a need for an alternative BCL2 antibody. Pathology. 2010;42:212–16. doi: 10.3109/00313021003631296. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz JM, Johnson NA, Morin RD, et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia. 2012;26:1383–90. doi: 10.1038/leu.2011.378. [DOI] [PubMed] [Google Scholar]
- 19.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–47. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 20.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–7. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, et al. Development of Automated Brightfield Double In Situ Hybridization (BDISH) Application for HER2 Gene and Chromosome 17 Centromere (CEN 17) for Breast Carcinomas and an Assay Performance Comparison to Manual Dual Color HER2 Fluorescence In Situ Hybridization (FISH) Diagnostic Pathology. 2008;3:41. doi: 10.1186/1746-1596-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitta H, Zhang W, Kelly BD, et al. Automated Brightfield Break-apart in Situ Hybridization (ba-ISH) Application: ALK and MALT1 Genes as Models. Methods. 2010;52:352–58. doi: 10.1016/j.ymeth.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Valentino C, Kendrick S, Johnson N, et al. Colorimetric in situ hybridization identifies MYC gene signal clusters correlating with increased copy number, mRNA, and protein in diffuse large B-cell lymphoma. Am J Clin Pathol. 2013;139:242–54. doi: 10.1309/AJCP2Z0TAGMUYJEB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stasik C, Nitta H, Zhang W, et al. Increased MYC gene copy number correlates with increased mRNA levels in diffuse large B-cell lymphoma. Hematologica. 2010;95:597–603. doi: 10.3324/haematol.2009.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Deng X, Carr B, May WS. Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem. 1997;272:11671–3. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 26.Deng X, Gao F, Flagg T, Anderson J, May WS. Bcl2’s Flexible Loop Domain Regulates p53 Binding and Survival. Mol and Cell Biol. 2006;26:4421–34. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong D, Rosenwald A, Chhanabhai M, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications-a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25:805–12. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.