Abstract
Guinea pigs are precocial rodents that show evidence of a selective attachment to the mother who, in turn, exhibits little active maternal care. Effects of separation in guinea pigs are, therefore, more likely to reflect the disruption of attachment than the removal of, or alterations in, patterns of maternal care. Here, effects in guinea pigs of the presence or absence of the mother on psychobiological endpoints and of maternal separation on depressive-like behavior are reviewed. It is argued that results with guinea pigs often align more closely with those of nonhuman primates than those of laboratory rats and mice, and that the guinea pig offers a valuable translational model for studies of the consequences of attachment and its disruption.
Keywords: guinea pig, attachment, attachment disruption, maternal separation, maternal buffering, stress buffering, depression, proinflammatory
Introduction
The guinea pig has a long history in the study of development. Sewall Wright’s breeding experiments with guinea pigs led to the discovery of the inbreeding coefficient and were instrumental in establishing the field of population genetics (Crow, 1994). W. C. Young and colleagues’ work with guinea pigs formed the empirical basis for the concept of organizational and activational effects of gonadal steroids (Phoenix, Goy, Gerall, & Young, 1959). Although guinea pigs are not the most commonly used rodent in biobehavioral research, aspects of their state of development at birth, high degree of sociality with complex social organization, and well studied behavior-endocrine interactions make them an excellent choice for addressing many questions central to developmental psychobiology today. Ongoing research programs with guinea pigs are, for instance, showing how social interactions during pregnancy and adolescence shape behavior development in ways that may be adaptive for predictive future social environments (Kaiser & Sachser, 2005; Lürzel, Kaiser, & Sachser, 2011), and how changes in photoperiod as early as the prenatal stage influence the timing of the onset of puberty (Trillmich, Mueller, Kaiser, & Krause, 2009). The present paper will focus on the epoch intervening between the prenatal and adolescent phases, that is, on the preweaning period. It is at this time that the maternal attachment figure predominates the social world of the guinea pig; her removal or presence in a threatening situation has widespread effects on the behavior and physiology of the young. Effects of maternal separation and maternal buffering will be described and comparisons of results in guinea pigs and non-human primates will be explored. It will be argued that studies of separation and maternal buffering with guinea pigs can provide information on the influence of attachment and its disruption that is difficult to glean from experiments with the more frequently used laboratory rodents—rats and mice. The paper will conclude by examining how the guinea pig might also be used as a model to help understand the consequences of early maternal separation for the development of depressive illness. The primary focus here will be to provide a very brief description of how studies with the guinea pig might afford a unique vantage point for characterizing complex developmental processes. More complete details of findings within each area can be found in the papers cited.
Maternal separation and social buffering: the role of attachment
While the mother is a central figure in the world of all newborn mammals, the nature, duration, and patterning of care she provides shows wide species variability. Similarly, the young of different species vary in such characteristics as overall development at birth, sensory capabilities, and emotional responsiveness. The sudden removal of the mother during the early postnatal period is a potentially life threatening event that can impact the infant on biological, emotional, and behavioral levels. Yet, the precise effects of separation will depend on what it is that is taken away, i.e., what the mother normally does or provides, and how sensitive the offspring is to the removal of these behaviors or functions. The most common laboratory rodents, Norway rats and house mice, are born in an extremely altricial state and must receive extensive maternal care in order to survive. Any separation, therefore, will deprive the infant of these caretaking activities for a period of time, which may account for observed effects of separation. In addition, the separation can alter care-taking activity upon return to the nest. Variation in these care-taking activities (e.g., licking, grooming) can profoundly affect developmental processes to shape the biobehavioral phenotype of the adult, and appear to be primary mediators of some of the lasting effects observed following the brief separation procedure known as “handling” (Parent, Zhang, Caldji, Bagot, Champagne, Pruessner, & Meaney, 2005). Further, as the work of Hofer and others have clearly documented, the physiological systems of immature offspring have evolved to depend on specific forms of stimulation provided by the mother to regulate physiological functions and foster normal development. Extended periods of separation remove these regulatory processes, so that changes in the infant’s biobehavioral activity reflect drift to the dysregulated state (Hofer, 1987; Levine, 2002).
For precocial laboratory species, such as the guinea pig, the situation is very different. The guinea pig pup emerges from the birth canal fully furred with eyes and ears open. It can stand and locomote shortly thereafter and can nibble solid food and sip from a water spout within a day. Thermoregulatory ability is well developed. These differences from rats and mice in physical independence of the pups is paralleled by differences in the solicitousness of the mothers. Lactating guinea pigs do not build nests. They will move towards and call in response to pup calls (Naguib, Kober, & Trillmich, 2010), but they do not retrieve pups. In fact, with the exception of some limited licking of the pups, mainly during the first few days of life, guinea pig mothers initiate no obvious active maternal behavior (Hennessy & Jenkins, 1994; König, 1985). Thus, if one tests guinea pigs beyond a week of age, there is negligible pup-directed maternal behavior to be removed by maternal separation or to be altered upon return of the pup to the home cage. For this reason, maternal separation effects in guinea pigs are not likely to be mediated by the absence of maternal care during separation or by alterations in maternal behavior upon mother-pup reunion. In like fashion, physiological systems in the physically mature guinea pig pup appear largely, if not entirely, self-regulating, so that there is little or no need for external regulation furnished by maternal stimulation. Separation effects, therefore, would not be expected to reflect the removal of maternal regulation.
Though physically independent and mobile, guinea pig pups clearly are drawn to the mother. Indeed, it is only the strong attraction the pups evince for her that maintains mother-pup proximity. Pups initiate nursing bouts, which mothers simply accommodate with their body position and immobility (Hennessy & Jenkins, 1994). The attraction of pups for the mother can be viewed in terms of filial attachment. If one applies the basic criteria that have been used to define attachment in primates, guinea pigs do quite well. They readily approach and follow the mother; they show evidence of recognition and preference for her; they appear to use her as a secure base for exploration; and they show clears signs of distress when separated from her (Hennessy, 2003; Jäckel & Trillmich, 2003; Pettijohn, 1979). In sum, effects of maternal separation in the guinea pig are unlikely to be the result of disrupted or altered maternal behavior or the removal of maternal regulation of immature physiological systems of the young. Instead, separation is much more likely to reveal the impact of removing the object of emotional attachment.
If an infant guinea pig is removed from the home cage and isolated in an unfamiliar environment, it shows rapid activation of behavioral and physiological responses associated with stress. Pups emit classic distress vocalizations, which for the guinea pig are audible high-pitched whistles (Pettijohn, 1979). Pups tend to increase locomotor activity and there is activation of both the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (Hennessy & Moorman, 1989; Hennessy et al., 1989). The vocalizing and locomotor activity are relatively short-lived, typically declining after about 30 min. At about this time a second, passive phase of response emerges (Fig. 1). The pup adopts a characteristic crouched stance with partially or fully closed eyes and extensive piloerection (Hennessy et al., 1995). All of these responses—HPA, sympathetic, and active and passive behavior—are greatly suppressed or entirely eliminated if the mother is placed with the pup in the novel environment (Hennessy & Moorman, 1989; Hennessy et al., 1989; Hennessy & Morris, 2005).
Figure 1.
Median frequency of active behaviors (top row) and median number of 1-min intervals of passive behaviors (bottom row) shown by young guinea pigs isolated in a novel environment. Each observation period was for 30 min (adapted from Hennessy et al., 1995).
Moreover, there is selectivity in the ability of the mother to suppress both the HPA and behavioral responses. Numerous studies over the years have found pups to show little or no elevation of plasma cortisol levels when tested with the mother in a novel enclosure (e.g., Hennessy & Ritchey, 1987; Hennessy & Moorman, 1989; Sachser, Dürschlag, & Hirzel, 1998). This effect is not due simply to the presence of a familiar social partner because when tested under identical conditions, but with a littermate present, cortisol levels are as high as when the pup is tested alone (Ritchey & Hennessy, 1987). Other adult animals sometimes have a moderating effect on the HPA responses of preweaning and early post-weaning guinea pigs, but overall the mother has a more-consistent and potent ameliorating influence (Hennessy, Kaiser, & Sachser, 2009). Behaviors, particularly the passive responses of the second stage of separation, also are selectively buffered by the mother. In one study, the presence of the mother, but not an unfamiliar nonlactating female, significantly reduced the crouching, eye-closing, and piloerection of preweaning guinea pig pups (Hennessy & Morris, 2005). More recently, the mother, but not a litter-mate or unfamiliar adult male, was found to reduce the passive behavior in a novel environment (Hennessy, Schiml, Watansriyakul, Rubinson, Willen, & Johnson, unpublished).
In all, studies of maternal separation and buffering in the guinea pig have produced results more in line with those nonhuman primates than of altricial rodent species. That is, it has long been known that not only do the infants of many nonhuman primate species form strong filial attachments, but maternal separation produces HPA and autonomic stress responses, as well as a two-stage, active/passive behavioral response (Mineka & Suomi, 1978). These findings also are consistent with what has been observed in our own species (Gunnar, Talge, & Herrera, 2009; Hill-Soderlund, Mills-Koonce, Propper, Calkins, Granger, Moore, Gariepy, & Cox, 2008; Spitz, 1946), though results are often less clear-cut, likely due at least in part to constraints inherent in research with children. Of particular concern in the human literature, however, is the ever increasing evidence that early life experiences such as prolonged maternal separation, neglect, and abuse promote a host of deleterious long-term psychological and physical outcomes (Agid, Shapira, Zislin, Ritsner, Hanin, Murad, Troudart, Bloch, Heresco-Levy, & Lerer, 1999; Coelho, Viola, Walss-Bass, Brietzke, & Brassi-Gliveira, 2013; Teicher & Samson, 2013). While these effects are often attributed to “attachment disruption” the degree to which effects in children result from disruption of attachment per se, versus the absence of appropriate maternal care is not easily parsed (Gunnar, 2005). This being the case, the guinea pig would seem to afford a valuable translational rodent model for examining effects due to attachment figure disruption rather than altered maternal care. Among the most pernicious consequences of early separation, neglect, or abuse in humans is increased risk for developing major depressive disorder.
Modeling effects of maternal separation on the development of depression
Depressive reactions to prolonged maternal separation were first documented in institutionalized children in the 1940s and 50s (e.g., Spitz, 1946). This work prompted studies of maternal separation in macaque monkeys, which were found to show similar depressive-like reactions to prolonged maternal separation in the laboratory. After a period of vocalizing and other signs of increased arousal, a portion of the monkey infants would quiet, adopt a hunched stance, appear disinterested in their surroundings, and display facial expressions suggesting grief (Kaufman & Rosenblum, 1967; Mineka & Suomi, 1978). Though the infant monkey separation paradigm became an accepted animal model for studying depressive illness (Gilmer & McKinney, 2003), it was never widely adopted due in large part to practical considerations, such as the expense and specialized resources required for nonhuman primate research, and the time needed to complete studies with animals taking so long to mature and having such lengthy intervals between births.
The two-stage response of guinea pig infants to a several-hour period of isolation in an unfamiliar environment mirrors the reaction of separated macaque monkey infants, but with a much compressed time scale. Authors of early human and monkey studies noted that separated infants conveyed the appearance of physical illness (Kaufman & Rosenblum, 1967; Spitz, 1946), as do the isolated guinea pigs. In fact, the passive behaviors these pups exhibit appear to be mediated by inflammatory-related mechanisms. This claim is based on three lines of evidence. First, injection of lipopolysaccharide, which induces a systemic inflammatory reaction, produces the same behavior constellation of crouching, eye-closure, and piloerection as does separation (Hennessy, Deak, Schiml-Webb, Wilson, Greenlee, & McCall, 2004). In addition, separation has been found to elicit physiological signs of an inflammatory reaction, specifically an increase in expression of the proinflammatory cytokine, tumor necrosis factor-alpha, in spleen as well as a transitory fever (Hennessy, Deak, Schiml-Webb, & Barnum, 2007; Schneider, Schiml, Deak, & Hennessy, 2012). Finally, several anti-inflammatory agents have been shown to reduce the passive, depressive-like behavioral response (e.g., Perkeybile, Schiml-Webb, O’Brien, Deak, & Hennessy, 2010).
Behavioral reactions induced by inflammatory processes are commonly referred to as “sickness behaviors”. These behaviors represent components of the acute phase response, the immune system’s broad first line of defense during exposure to pathogens (Baumann & Gauldie, 1994). Sickness behaviors are adaptive. They conserve energy and slow body heat loss to support fever and thereby bolster the immune system’s response to the pathogen (Hart, 1988). Aspects of the acute phase response, including sickness behaviors, can also be induced by some stressors (Maier & Watkins, 1998). It appears then that the behaviors of the second, passive stage of the separation response in guinea pigs are examples of such stress-induced sickness behaviors, with the stressor being separation from the attachment object in a novel environment. These observations are relevant for understanding the relation between attachment disruption and depression because of the now abundant evidence that inflammatory processes contribute to forms of depressive illness in humans (e.g., Dantzer, O’Connor, Lawson, & Kelley, 2011), particularly, perhaps, depression triggered by stress (Miura, Ozaki, Sawada, Isobe, Ohta, & Nagatsu, 2008). And while sickness behaviors are adaptive in the face of pathogen exposure, they also are thought to reflect the underlying inflammatory processes that can lead to depression (Dantzer et al., 2011). Thus, the guinea pig results point to the possibility that inflammatory mechanisms may also contribute to the depressive reaction of nonhuman primates and possibly humans during separation.
Of greater clinical concern than depression occurring during an episode of separation, however, is the lasting effect by which early attachment disruption increases susceptibility of vulnerable individuals to developing depressive disorders in later life (e.g. Teicher & Samson, 2013). These effects are often conceptualized in terms of the stress diathesis hypothesis in which the exposure to early-life trauma sensitizes some aspect of stress physiology so that when exposed to stressors at a later age the individual responds with a prolonged, excessive, and unregulated stress response that underlies or actually constitutes the depressive episode (Gold, Goodwin, & Chrousos, 1988). One aspect of stress physiology that sensitizes may be inflammatory mechanisms (Hennessy, Deak, & Schiml-Webb, 2010). Indeed, early-life maltreatment and harsh childhood environments have been associated not only with later psychopathology, such as depression, but also with increased inflammatory responses in adolescence and adulthood (Coelho et al., 2013; Miller & Chen, 2010). Further, the association between depression and inflammation may be strongest for those adults who had experienced childhood maltreatment (Danese, Moffitt, Pariante, Ambler, Poulton, & Caspi, 2008).
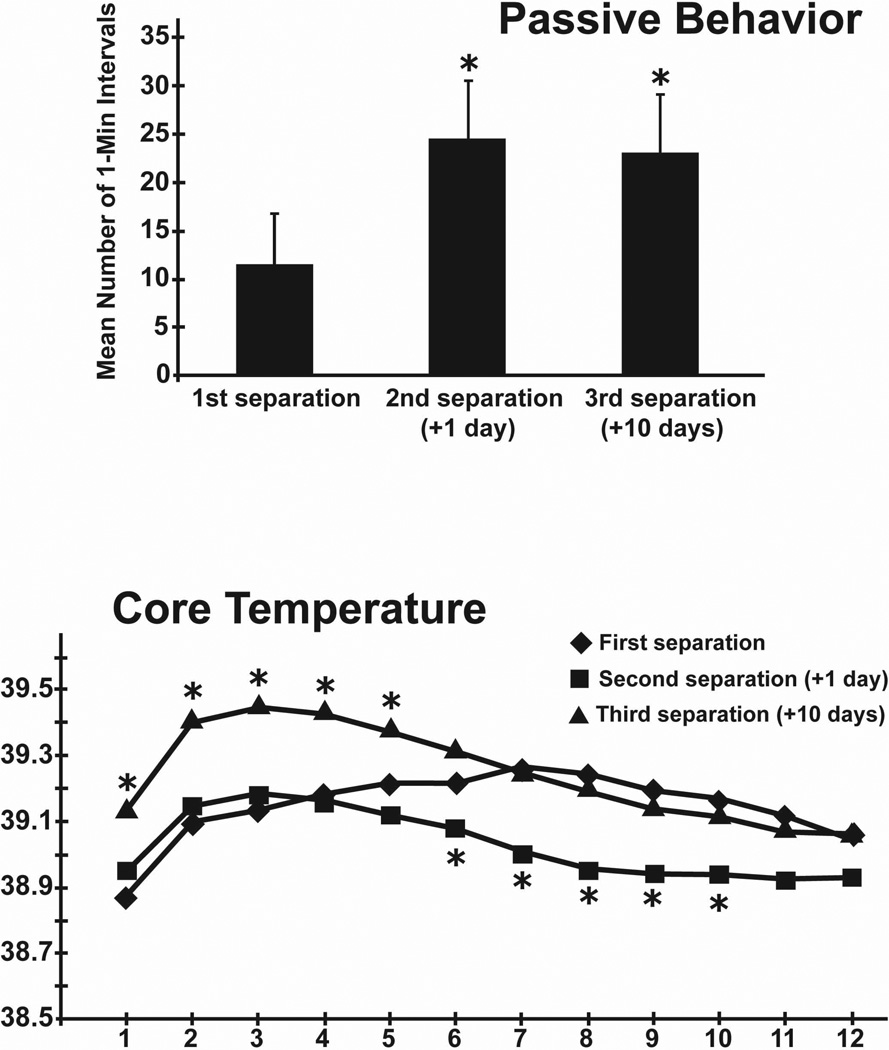
Results with guinea pigs are consistent with these findings. Even a single 3-hr separation increases the level of passive, but not active, behaviors observed during separation the following day (Schneider et al., 2012). If 3-week-old pups are then separated a third time 4 or 10 days after the first separation, the same heightened level of passive behavior is observed (Hennessy, Paik, Caraway, Schiml, & Deak, 2011; Schneider et al., 2012; Yusko, Hawk, Schiml, Deak, & Hennessy, 2012; Fig. 2). This sensitization of depressive-like behavior is accompanied by a sensitization of the febrile response to the separation procedure. That is, Increases in core temperature during subsequent separations are greater and occur more rapidly than during the initial separation (Schneider et al., 2012; Yusko et al., 2012; Fig. 2). These increases are not associated with increases in locomotor activity, and neither the behavioral nor the core temperature change can be accounted for by the increasing age of the animals (Schneider et al., 2012; Yusko et al., 2012). The sensitization of the fever response implies a sensitization of inflammatory mechanisms, though this remains to be directly tested.
Figure 2.
Mean number of 1-min intervals of passive, depressive-like behavior exhibited by guinea pig pups during repeated separations. Vertical bars represent standard errors of the means. *p < 0.05 versus first separation (top). Mean core temperature of pups undergoing repeated separation. *p < 0.05 versus each of the other separations (bottom) (adapted from Schneider et al., 2012).
The characterization of the role of inflammatory mechanisms in human depression has led to the suggestion of using anti-inflammatory agents as antidepressants (Müller & Schwarz, 2008). If the increased vulnerability for developing depressive illness following early-life attachment disruption and other trauma involves sensitization of inflammatory mechanisms, then anti-inflammatory compounds administered prior to the onset of depression—or even at the time of the early trauma—might, in principle, be expected to mitigate the long-term adverse outcomes. While studies examining these possibilities would be especially challenging to carry out in human populations, experiments in guinea pigs might provide some insight.
To examine this question, the anti-inflammatory cytokine Interleukin-10 (IL-10) or vehicle was administered through an intracerebroventricular cannula to guinea pig pups prior to an initial separation, and the pups were separated on two consecutive days. While the vehicle controls showed the expected sensitization of depressive-like behavior across separations, infusion of IL-10 prior to the first separation prevented sensitization the following day (Hennessy et al., 2011Fig. 3). Presumably, inhibition of underlying proinflammatory processes during the first separation prevented their sensitization and the resulting enhancement of the depressive-like behavioral response during separation the next day. A more-recent study asked whether this effect might be extended over a longer inter-separation interval. In this case, pups were administered naproxen, a cycloxygenase inhibitor that readily crosses the blood-brain barrier. Naproxen blocks synthesis of prostaglandins, which serve as downstream mediators of proinflammatory cytokine actions. Naproxen also has been shown to reduce sickness behavior in other contexts (e.g., Johnson, Curtis, Dantzer, & Kelley, 1993). Pups receiving three, daily injections of naproxen terminating an hour prior to an initial separation showed significantly reduced levels of passive, depressive-like behavior relative to controls during the initial separation, separation the next day, and separation 10 days after the first (Hennessy, Stafford, Schiml, Xanthos, & Deak, 2013). Thus, the guinea pig work suggests further research into a possible prophylactic effect of a common anti-inflammatory drug on the depressive reactions induced by earlier attachment-figure separation.
Figure 3.
Median number of 1-min intervals in which pups infused centrally with either IL-10 or vehicle prior to a first separation exhibited passive, depressive like behavior during two separations on consecutive days. *p < 0.05 versus Separation 1 (reprinted from Hennessy et al., 2011).
Conclusion
Mice and rats are by far the most commonly used laboratory rodents for behavioral neuroscience research due in large part to the enormous amount of background data regarding their physiology, anatomy, behavior, and life histories that is available to undergird any new experimental approach. Yet, differences among rodents in these same factors can inform choices of models for examining particular experimental questions. While research with mice and rats has been invaluable as the source of much of what we know about mother-infant interactions and effects of early experience, studies in guinea pigs afford a somewhat different perspective that appears especially useful for furthering our understanding of the psychobiological effects of filial attachment and the consequences of its disruption.
Acknowledgment
Preparation of the paper and unpublished work were supported by grants IOS-1120932 from the National Science Foundation and MH068228 from the National Institute of Mental Health. The author has no conflict of interest to report.
References
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Molecular Psychiatry. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunology Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Coelho R, Viola TW, Waiss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta psychiatrica Scandinavica, Advance online publication. 2013 doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- Crow JF. National Academy of Sciences. Washington D.C: 1994. Sewall Wright: a biographical memoir. [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry. 2008;65:409–416. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynerenine. Pscyhoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. Journal of Affective Disorders. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress (Part 2) New England Journal of Medicine. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- Gunnar MR. Attachment and stress in early development: does attachment add to the potency of social regulators of infant stress? In: Carter CS, Ahnert L, Grossman KE, Hrdy SB, Lamb ME, Porges SW, Sachser N, editors. Attachment and Bonding: a new synthesis. Cambridge MA: The MIT Press; 2005. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: what does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of behavior of sick animals. Neuroscience and Biobehavioral Reviews. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Enduring maternal influences in a precocial rodent. Developmental Psychobiology. 2003;42:225–336. doi: 10.1002/dev.10095. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA. Early attachment-figure separation and increased risk for later depression: potential mediation by proinflammatory processes. Neuroscience and Biobehavioral Reviews. 2010;34:782–790. doi: 10.1016/j.neubiorev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Barnum CJ. Immune influences on behavior and endocrine activity in early-experience and maternal separation paradigms. In: Czerbska MT, editor. Psychoneuroendocrinology Research Trends. Hauppauge, NY: Nova Science Publishers; 2007a. pp. 293–319. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiology and Behavior. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Jenkins R. A descriptive analysis of nursing behavior in the guinea pig (Cavia porcellus) Journal of Comparative Psychology. 1994;108:23–28. doi: 10.1037/0735-7036.108.1.23. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Long SJ, Nigh CK, Williams WT, Nolan DJ. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF activity mediate behavior of guinea pig pups during isolation? Behavioral Neuroscience. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Moorman L. Factors influencing cortisol and behavioral responses to maternal separation in guinea pigs. Behavioral Neuroscience. 1989;103:378–385. doi: 10.1037//0735-7044.103.2.378. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Morris A. Passive responses of young guinea pigs during exposure to a novel environment: influences of social partners and age. Developmental Psychobiology. 2005;46:86–96. doi: 10.1002/dev.20045. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Paik KD, Caraway JD, Schiml-Webb PA, Deak T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behavioral Neuroscience. 2011;125:426–433. doi: 10.1037/a0023559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Ritchey RL. Hormonal and behavioral attachment responses in infant guinea pigs. Developmental Psychobiology. 1987;20:613–625. doi: 10.1002/dev.420200607. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Stafford NP, Schiml PA, Xanthos E, Deak T. Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience. Online; 2013. Anti-infammatory treatment prevents lasting depressive-like effect of early maternal separation in guinea pigs. Program No. 121.03. [Google Scholar]
- Hennessy MB, Tamborski A, Schiml P, Lucot J. The influence of maternal separation on plasma concentrations of ACTH, epinephrine, and norepinephrine in guinea pig pups. Physiology and Behavior. 1989;45:1147–1152. doi: 10.1016/0031-9384(89)90101-7. [DOI] [PubMed] [Google Scholar]
- Hill-Soderlund AL, Mills-Koonce WR, Propper C, Calkins SD, Granger DA, Moore GA, Gariepy J-L, Cox MJ. Parasympathetic and sympathetic responses to the strange situation in infants and mothers from avoidant and securely attached dyads. Developmental Psychobiology. 2008;50:361–376. doi: 10.1002/dev.20302. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Shaping forces within early social relationships. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal Development: a psychobiological perspective. Academic Press, Orlando; 1987. pp. 251–274. [Google Scholar]
- Jäckel M, Trillmich F. Olfactory individual recognition of mothers by young guinea pigs (Cavia porcellus) Ethology. 2003;109:197–208. [Google Scholar]
- Johnson RW, Curtis SE, Dantzer R, Kelley K. Central and peripheral prostaglandins are involved in sickness in birds. Physiology and Behavior. 1993;53:127–131. doi: 10.1016/0031-9384(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Sachser N. The effects of prenatal social stress on offspring development: mechanisms and function. Neuroscience and Biobehavioral Reviews. 2005;29:283–294. doi: 10.1016/j.neubiorev.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosomatic Medicine. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- König B. Maternal activity budget during lactation in two species of Caviidae (Cavia porcellus and Galea musteloides) Zeitschrift für Tierpsychologie. 1985;68:215–230. [Google Scholar]
- Levine S. Regulation of the hypothalamic-pituitary-adrenal axis in the neonatal rat: the role of maternal behavior. Neuotoxicity Research. 2002;4:557–564. doi: 10.1080/10298420290030569. [DOI] [PubMed] [Google Scholar]
- Lürzel S, Kaiser S, Sachser N. Social interaction decreases stress responsiveness during adolescence. Psychoneuroendocrinology. 2011;36:1370–1377. doi: 10.1016/j.psyneuen.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychological Bulletin. 1978;85:1376–1400. [PubMed] [Google Scholar]
- Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. COX-2 inhibition in schizophrenia and major depression. Current Pharmaceutical Design. 2008;14:1452–1465. doi: 10.2174/138161208784480243. [DOI] [PubMed] [Google Scholar]
- Naguib M, Kober M, Trillmich F. Mother is not like mother: concurrent pregnancy reduces lactating guinea pigs’ responsiveness to pup calls. Behavioural Processes. 2010;83:79–81. doi: 10.1016/j.beproc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Parent C, Tie-Yuan Z, Caldji C, Bagot R, Champagne FA, Pruessner J, Meaney MJ. Maternal care and individual differences in defensive responses. Current Directions in Psychological Science. 2005;14:229–233. [Google Scholar]
- Perkeybile AM, Schiml-Webb PA, O’Brien E, Deak T, Hennessy MB. Anti-inflammatory influences on behavioral, but not cortisol, responses during maternal separation. Psychoneuroendocrinology. 2009;34:1101–1108. doi: 10.1016/j.psyneuen.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn TF. Attachment and separation distress in the infant guinea pig. Developmental Psychobiology. 1979;12:73–81. doi: 10.1002/dev.420120109. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Ritchey RL, Hennessy MB. Cortisol and behavioral responses to separation in mother and infant guinea pigs. Behavioral and Neural Biology. 1987;48:1–12. doi: 10.1016/s0163-1047(87)90514-0. [DOI] [PubMed] [Google Scholar]
- Sachser N, Dürschlag M, Hirzel D. Social relationships and the management of stress. Psychoneuroendocrinology. 1998;23:891–904. doi: 10.1016/s0306-4530(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Schneider RL, Schiml PA, Deak T, Hennessy MB. Persistent sensitization of depressive-like behavior and thermogenic response during maternal separation in pre-and post-weaning guinea pigs. Developmental Psychobiololgy. 2012;54:514–522. doi: 10.1002/dev.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz RA. Anaclitic depression: an inquiry into the genesis of psychiatric conditions in early childhood: II. Psychoanalytical Study of the Child. 1946;2:313–342. [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinincally and neurobiologically distinct subtypes. American Journal of Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillmich F, Mueller B, Kaiser S, Krause J. Puberty in female cavies (Cavia aperea) is affected by photoperiod and social conditions. Physiology and Behavior. 2009;96:476–480. doi: 10.1016/j.physbeh.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Yusko B, Hawk K, Schiml PA, Deak T, Hennessy MB. Sensitization of depressive-like behavior during repeated maternal separation is associated with more-rapid increase in core temperature and reduced plasma cortisol levels. Physiology and Behavior. 2012;105:861–867. doi: 10.1016/j.physbeh.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]