Abstract
The male and female reproductive tracts are complex microenvironments that have diverse functional demands. The immune system in the reproductive tract has the demanding task of providing a protective environment for a fetal allograft while simultaneously conferring protection against potential pathogens. As such, it has evolved a unique set of adaptations, primarily under the influence of sex hormones, which make it distinct from other mucosal sites. Here, we discuss the various components of the immune system that are present in both the male and female reproductive tracts, including innate soluble factors and cells and humoral and cell-mediated adaptive immunity under homeostatic conditions. We review the evidence showing unique phenotypic and functional characteristics of immune cells and responses in the male and female reproductive tracts that exhibit compartmentalization from systemic immunity and discuss how these features are influenced by sex hormones. We also examine the interactions among the reproductive tract, sex hormones and immune responses following HIV-1 infection. An improved understanding of the unique characteristics of the male and female reproductive tracts will provide insights into improving clinical treatments of the immunological causes of infertility and the design of prophylactic interventions for the prevention of sexually transmitted infections.
Keywords: female reproductive tract, genital immunity, HIV-1, male reproductive tract, mucosal immune responses, sex hormones, sexually transmitted infections
Introduction
The male and female reproductive tracts are integral to the inner mucosal lining of the human body, and similar to the gastrointestinal and lung mucosa, they are capable of mounting a full repertoire of immune responses. The immune system in the reproductive tract of both men and women has evolved distinct adaptations that meet the physiologically challenging demands of both successful reproduction and the maintenance of full protection against microbial invasion. The compartmentalization of systemic immunity and mucosal immune responses is a distinct feature of the male and female genital tracts. The outcome of immune responses in the reproductive tract is determined by interactions between the cells and components that make up the reproductive immune system and the local microenvironment that is dominated by sex hormones and a unique microbiome.1,2 Here, we review aspects of the biology of the male and female reproductive tract as it relates to the immunological demands on these tissues and discuss the innate and adaptive immune responses in homeostasis and following HIV infection and the regulation of these processes by sex hormones. The influence of the microbiome on the reproductive tract is an exciting emerging area that will significantly enhance our understanding of immune responses in the male and female reproductive tracts in the near future (recently reviewed by Brotman et al.).3
Female reproductive tract
Structure and morphology
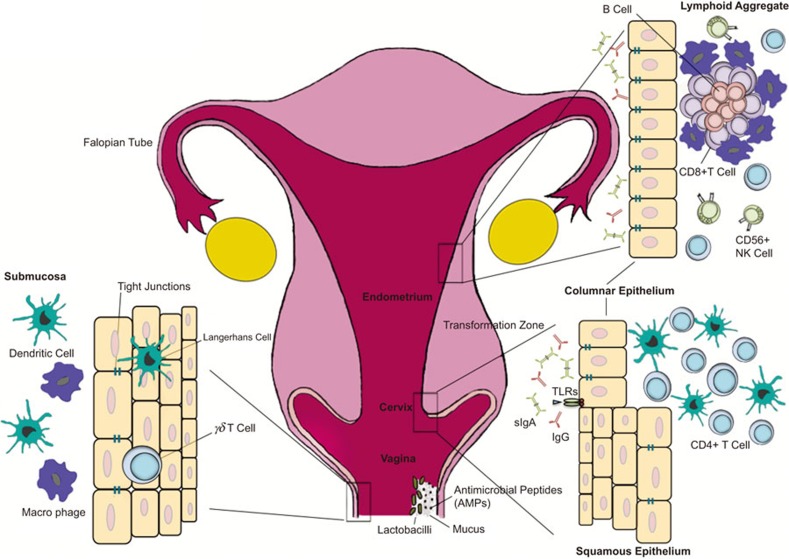
The female genital tract in humans is composed of the upper reproductive tract, which includes the fallopian tubes, uterus and endocervix, and the lower reproductive tract, which is composed of the ectocervix and vaginal tract (Figure 1). The upper reproductive tract is lined by a single layer of columnar epithelial cells joined together by tight junctions, which form a physical barrier that prevents the entry of microbes and other antigens present in the lumen.4,5 The lower reproductive tract lining is composed of stratified squamous epithelium that, unlike the upper reproductive tract, relies primarily on the presence of multiple layers to provide a protective barrier against the entry of organisms.5 The superficial layers of the lower reproductive tract are terminally differentiated and lack most intracellular organelles including nuclei, while the basal layers are metabolically active and undergo active proliferation.6 Consequently, the superficial layers of the lower genital tract are quite ‘leaky', allowing penetration by endogenous and pathogenic microbes and other mediators. The lamina propria beneath the epithelium in both the upper and lower reproductive tract is composed primarily of fibroblasts, scattered blood vessels lined with endothelium and a variety of immune cells. The proliferation and differentiation of reproductive tract tissue, including the epithelium, is regulated by the reproductive hormones estrogen and progesterone.7 Furthermore, these hormones also have a significant role in regulating immune cells and mediators throughout the reproductive tract.8,9 These features will be discussed in more detail below.
Figure 1.
Anatomical and immunological components of the female reproductive tract. The female reproductive tract consists of an upper (fallopian tubes, uterus and endocervix) and a lower (extocervix and vagina) tract. The vaginal epithelium has many innate immune-mediated protection mechanisms, such as tight junctions, AMPs and mucus, to neutralize, trap, and prevent the entry of potential pathogens. The vaginal lumen is colonized by commensal bacteria, mainly Lactobacilli spp., which help maintain a low pH environment and produce reactive oxygen species. Furthermore, innate immune cells, such as γδ T cells, DCs, and macrophages, are present beneath and between vaginal epithelial cells layer to survey the local environment for danger. The abrupt transition from keratinized squamous epithelial cells of the ectocervix to single columnar epithelial cells of the endocervix represents the transformation zone; this site has an abundance of HIV target cells (DCs and CD4+ T cells) and has been proposed to be one of the major sites of infection. Although traditional mucosal lymphoid structures are not found in the female reproductive tract, lymphoid aggregates in the endometrial tissue that are composed of B cells in the inner core and surrounded by CD8+CD4− T cells and an outer layer of macrophages have been described. Scattered CD56+ NK cells and CD4+ T cells can be found between lymphoid aggregates. The immune cells and functions of the female reproductive tract are regulated by sex hormones that orchestrate cyclical changes with the menstrual cycle. AMP, anti-microbial peptide; DC, dendritic cell; NK, natural killer.
Immune components in the female reproductive tract (FRT)
The immune system in the FRT is part of the mucosal immune system. As such, it has many characteristics that are defining features unique to the mucosal immune system and distinct from the systemic immunity, including mucosal homing markers, secretory immunoglobulins (Igs) and tissue-resident innate lymphocytes.10 In addition to the common features shared with other mucosal surfaces, the reproductive tract has unique adaptations in its immune system that have evolved primarily to protect the semi-allograft fetus from immune recognition and rejection. Unlike many other mammalian species for which coitus and the consequent risk of exposure to pathogens coincides with ovulation and female receptivity, the human immune system is required to confer protection against sexually transmitted pathogens throughout the entire menstrual cycle. This requires special adaptations in the immune system of the FRT, so that different components of the immune response, such as humoral and cell-mediated immunity, are differentially modulated to extend protection while facilitating fertilization and fetus implantation, if necessary. Sex hormones have been shown to be key regulators of immune cells and responses in the FRT and play an important role in cyclical changes in immunity.7,9 In addition to sex hormones, there is increasing evidence that the presence of a microbiome dominated by a specific bacterial species, such as Lactobacilli spp., is critical for the development and shaping of the reproductive tract innate and adaptive immune responses.3,11 In the following sections, we review the cells and mediators that play a dominant role in reproductive tract immunity.
Innate immunity
Anti-microbial peptides (AMPs)
AMPs are small proteins or peptides with anti-microbial properties that are secreted mainly by neutrophils and epithelial cells in the FRT. AMPs described in the FRT include defensins, secretory leukocyte protease inhibitor (SLPI), lysozyme, lactoferrin, elafin and cathelicidin. Both the columnar epithelium that lines the endometrium and the cervicovaginal epithelium have been shown to secrete a number of the AMPs, which are detectable in genital tract secretions and in epithelial cell cultures. Moreover, the secretion of AMPs has been shown to be regulated by the menstrual cycle. For a detailed review of AMP in the FRT, see a recent review by Wira et al.12
Human alpha- and beta-defensins are among the most well characterized and abundant AMPs present in the FRT.12 Of the six α-defensins and six β-defensins, human β-defensin (HBD)-1–4 and α-defensin 5 are expressed by endometrial epithelium, while α-defensins human neutrophil α-defensin-1–3 and HBD-2 are found in cervicovaginal secretions.1 Furthermore, many of the defensins are differentially regulated in the upper and lower reproductive tract during the menstrual cycle. Some reach their peak concentration during the proliferative phase, whereas others peak in the secretory phase. For example, HBD-2 is the highest in the upper reproductive tract during menstruation, but reaches its peak concentration in cervicovaginal secretions during the proliferative phase.13,14 The mechanism underlying this tissue-specific regulation has not been elucidated, although AMP levels and biological activity can be altered in response to changes in pH, semen and microbiota, in addition to sex hormones. Human defensins have been shown to have anti-bacterial properties against fungi, yeast, bacteria (Gram-negative and Gram-positive) and anti-viral activity, including activity against HIV-1.12
The second class of AMPs are the protease inhibitors, including serine protease inhibitors (serpins), SLPI, cystatins and elafins.15 In general, these protease inhibitors function as anti-inflammatory factors by inhibiting the proteases secreted by immune cells, such as neutrophils, which can trigger the activation of the complement system and secretion of other inflammatory mediators that can lead to tissue damage and severe inflammation.16 Protease inhibitors are expressed throughout the entire FRT, including in the vagina, cervix, uterus and even the fetal membranes. Cervical and vaginal epithelial cells have been shown to express most of these protease inhibitors. Other cells in the FRT, including granulocytes, macrophages, monocytes and dendritic cells (DCs), also produce protease inhibitors. In addition to anti-inflammatory properties, many protease inhibitors, such as SLPI and elafin, have been shown to have direct anti-bacterial, anti-fungal and anti-HIV properties.17 Others, including serpins and cystatins, have been shown to exert anti-HIV effects indirectly by controlling inflammation.16
Other secreted anti-microbial products in the FRT include calthelicidin LL37, lactoferrin and lysozyme and are present primarily in the lower genital tract.1,15 They are produced by neutrophils and lower genital tract epithelium and can be detected in vaginal and cervical secretions. Lactoferrin and lysozyme have been shown to exert anti-bacterial and anti-viral properties. Lactoferrin acts by sequestering iron required by microbes in the acidic environment of the lower reproductive tract, whereas lysozyme acts through the enzymatic digestion of bacterial cells walls.
Interferons (IFNs)
In addition to AMPs, cells of the FRT can produce IFNs that have a wide variety of immunomodulatory and antiviral effects. Type I IFNs (IFN-α, IFN-β) impede HIV replication by several mechanisms, including inducing the upregulation of restriction factors, such as apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G,18,19 tripartite motif 5α (TRIM5α),20 bone marrow stromal antigen 2 (also known as tetherin),21 SAM and human α-defensin (HD) domain 122,23 and myxovirus resistance 2 (also known as MxB).24 Interestingly, type I IFN has also been implicated as a contributor to HIV pathogenesis,25 and elevated type I IFN is a component of the signature associated with chronic immune activation.26 The potential benefit or harm of IFN responses most likely depends on the net outcome of a number of factors, including the stage of infection. Evidence from our lab suggests that in response to HIV-1 gp120, genital epithelial cells (GECs) markedly upregulate IFN-β, and neutralization of IFN-β resulted in enhanced induction of the HIV-long terminal repeat promoter in transfected Jurkat T cells (Nazli, Ferreira and Kaushic, unpublished results).
Two new mucosal IFN species have recently been described to show anti-HIV activity. Unlike other type I IFNs, IFN-ε is expressed constitutively in mucosal tissues, including the reproductive tract.27 Moreover, seminal plasma (SP) was also found to upregulate the expression of IFN-ε in cervicovaginal tissues,28 suggesting that IFN-ε may play a protective role in reproductive tissue. Interestingly, when IFN-ε was used in an intranasal/intramuscular heterologous HIV prime-boost immunization, elevated HIV-specific CD8+ T-cell responses were observed in the spleen, genitorectal draining lymph nodes and Peyer's patches.29 Furthermore, the recently described Type III IFN-λ (IL-28/29), which has similar antiviral properties to Type I IFN, has been shown to block HIV-1 infection in macrophages in vitro30,31 by inhibiting HIV-1 integration and post-transcriptional events.32 Interestingly, IFN-λ receptors are largely restricted to cells of epithelial origin. Together, these results suggest that IFN-ε and IFN-λ may play unique roles in protecting the genital mucosa.
Genital epithelial cell innate responses
The genital epithelium forms the primary barrier between the female reproductive tract and the external environment.33 In this role, cells in this tissue are the first responders to any incoming pathogens. These cells are dynamically active and play an important role in actively recognizing and tailoring a response to a wide variety of antigenic stimuli in the lumen of the FRT, including semen, sperm, semi-allogeneic fetus tissue, bacterial and viral pathogens. The presence of a wide repertoire of pattern recognition receptors (PRRs) expressed by the GECs facilitates their ability to recognize and differentially respond to various pathogens. The PRRs expressed by GEC include Toll-like receptors (TLRs) and NOD-like receptors, which allow the sensing of foreign microbes in the environment and the rapid transmission of messages to other innate and adaptive immune cells. Primary endocervical GECs express TLRs 1–3 and 6.1 Additionally, primary human uterine GECs express TLRs 1–9, indicating the potential to respond to a wide range of pathogens. The expression of NOD1 and NOD2 has also been detected in the human endometrium.34 PRR recognition of pathogens typically initiates an intracellular signaling cascade that results in the activation of transcription factors, such as NF-κB, and the production of a variety of cytokines and chemokines.35 For instance, the TLR-mediated activation of GECs can lead to the production of IL-6, IL-8, TNF-α, stromal derived factor-1 and β-chemokines, including macrophage inflammatory protein 1-α (MIP-1α), MIP-1β and regulated on activation, normal, T-cell expressed and secreted.35 Many studies, including ours, have reported that the GECs can respond to presence of viruses, such as Herpes simplex virus, type 2 (HSV-2), and bacteria, such as Neisseria gonorrhoea, by upregulating pro-inflammatory cytokines and chemokines.36,37 Interestingly, a number of TLR ligands (the TLR3 ligand Poly I∶C, the TLR9 ligand CpG and the TLR5 ligand flagellin) can be used to induce prophylactic anti-viral responses in the GECs.36 These innate anti-viral immune responses were correlated with protection against CMV and HSV-2 challenge.36,38 The protection was mediated primarily by the ability of TLR ligands to induce IFN-β responses. IFN-β responses are well known to correlate with anti-viral protection via the production of various IFN-inducible genes and proteins, such as 2′,5′-OAS, PKR, iNOS and MyxA, which either directly or indirectly inhibit viral replication, including HIV-1 replication.39
DCs and macrophages
Both DCs and macrophages are sentinels of the mucosal immune system that constantly survey and process antigens from the external environment, which provides important information and signals to the host immune system.40 DCs in particular serve the important function of bridging the innate responses with the initiation of adaptive immunity. Mucosal DCs are recognized for their unique ability to recognize and respond to antigens by inducing host immune responses that can range from tolerogenic to the induction of antigen-specific adaptive immunity.41 Typically, tissue-resident DCs, including those in the FRT, have an immature phenotype. Upon inflammatory or pathogen stimulation, tissue DCs can change their phenotype and function, becoming mature DCs with increased MHC Class II and costimulatory receptor expression and migrating into draining lymph nodes, where they can prime antigen-specific T-cell responses.42,43
A number of studies have examined the human FRT for the presence of antigen presenting cells, including myeloid DCs and macrophages.44 Because both DCs and macrophages originate from a common myeloid precursor, subsets of both cells share a number of surface markers, including CD14, CD11c, CD11b and MHC Class II. Furthermore, myeloid cells, including monocytes, can differentiate into macrophages or DCs depending on antigen stimulation and the cytokine microenvironment.45 Macrophages and DCs have been identified throughout the FRT, especially in the ecto- and endocervix.1,46,47 Macrophages are present in small numbers in the endometrium and are distributed throughout the uterine tissue. Furthermore, the macrophage population in the endometrium is likely regulated by sex hormones, as indicated by changes in immune cell populations that are correlated with menstrual cycle. Endometrial macrophages are most abundant in the mid-secretory phase of the cycle as part of the uterine lymphoid aggregates.48 DCs are present in smaller numbers and are typically localized to the sub-epithelial stroma of the endometrium.46 Compared with the endometrial APC populations, many more studies have examined these populations in the cervix and vagina, primarily to understand the role that these cells may play in immune responses to sexually transmitted pathogens, particularly HIV-1. Unlike the endometrium, CD1a+ Langerhans cells and other DC subsets are localized within the squamous epithelial layers and at the stromal/epithelial interface, both in the vagina and ectocervix.46 Other studies have examined the proportion of APCs in the FRT by flow cytometry and found that CD14+ cells, typically macrophages, are relatively abundant in the cervix.47 Most DC populations in the cervix were negative for CD103, a homing marker that is commonly expressed by other mucosal leukocytes, indicating a distinct profile of homing marker expression in the reproductive tract. Many DCs in the cervix were found to express DC—specific intercellular adhesion molecule-3-grabbing non-integrin, which is significant because of the possible role played by this molecule in the trans-infection of HIV in T cells by DCs.49,50
Neutrophils
Neutrophils are found throughout the FRT, although they are most abundant in the Fallopian tubes and are present in lower numbers in the upper and lower reproductive tracts.51 The GECs of both the upper and lower FRT produce abundant amounts of IL-8, which is a leukocyte chemoattractant factor. Under the influence of the IL-8 gradient, neutrophils cross the epithelium into the lumen to phagocytose sperm, microorganisms and any other cellular debris. Indeed, studies have shown that following coitus, there is a significant infiltration of neutrophils into the endometrium, which is part of a normal inflammatory response.52 Furthermore, the proportion of neutrophils also increases significantly in the endometrium prior to menses, again likely as part of the natural tissue breakdown and rebuilding process that is part of the normal menstrual cycle.1 Unlike the endometrium, the numbers of neutrophils in the lower genital tract remain stable throughout the cycle.
Natural killer (NK) cells
NK cells are an integral part of the innate immune system and play a key role in anti-viral and anti-tumor responses. When activated, NK cells function mainly by either killing virus-infected or tumor cells or producing large amounts of cytokines, particularly IFN-γ, to activate macrophages to kill intracellular bacteria.1 NK cells also mediate antibody-dependent cellular cytotoxicity as a consequence of expression of FcR by most NK cell subsets. NK cells are present throughout the reproductive tract in significant numbers, and previous studies have identified them as uterine large granular lymphocytes in the endometrium.53 They can make up anywhere from 10%–30% of leukocytes in the FRT of non-pregnant women.51 In the uterus, NK cell populations increase from the proliferative to the secretory stage.54 In the later stages of the secretory phase, NK cells can make up as much as 70% of the leukocytes in the endometrium.
Although it is unclear whether this increase in numbers is a consequence of either local proliferation or recruitment from peripheral blood, the significant accumulation of NK cells late in the menstrual cycle is likely associated with preparation for the extensive uterine remodeling that occurs at implantation and during the first trimester of pregnancy. NK cells in the uterus (uNK) have a distinct phenotype compared with those found in the blood.55,56 The uNK cells express CD56 and very little CD16, in contrast to blood NK cells. They also express CD9 and CD69, and microarray studies have indicated that they have a distinct gene profile that is very different from blood NK cells.57 Nevertheless, similar to blood NK cells, uNK cells in the endometrium of non-pregnant women have been shown to have cytolytic function in vitro and to produce significant amounts of IFN-γ, GM-CSF, IL-10, transforming growth factor-β2 (TGF-β) and IL-8.1 The uNK cells require IL-15 to be produced constitutively by endometrial stromal cells to survive and proliferate. Both CD56+CD16− and CD56−CD16+ NK cells have been reported in the ectocervix and endocervix, with no significant differences in their proportions correlated with menstrual cycle.47
Adaptive immunity
T-cell immunity
CD4+ and CD8+ T cells are found throughout the FRT. In the upper reproductive tract, CD8+ T cells are more frequent than are CD4+ T cells. Yeaman et al.48 showed that the uterine endometrium contains organized lymphoid aggregates (LAs) that are located in the stratum basalis layer (Figure 1). These structures have a B-cell core that is surrounded by CD8+ T cells, with an outer halo of macrophages. Although the LA can be found throughout the menstrual cycle, they number the fewest (approximately 300 cells) during the proliferative stage and the most (2000–3000 cells) during the secretory phase of the menstrual cycle. Other studies from the same group demonstrated that CD3+ T cells from the FRT have cytolytic functions.58 High levels of cytolytic activities were observed in CD3+ T cells isolated from the cervix and vagina, independent of the stage of the menstrual cycle. However, in the uterus, the greatest cytolytic potential of CD3+ T cells was observed during the proliferative phase, while little or no cytolytic activity was observed during the secretory stage. Because the decrease in cytolytic activity of T cells correlated with an increased number of LA T cells, it is unclear whether the LA CD8+ T cells have any cytolytic functions.
Interestingly, CD3+ T cells from the uterus of post-menopausal women show consistently high cytolytic activity.58 A more recent study further characterized the phenotype and functionality of T cells from the endometrium and endocervix of normal cycling women during the secretory phase and found that, compared with T cells from blood, endometrial and endocervical CD4+ T cells had increased CCR5 expression and were enriched for an activated, effector memory phenotype, suggesting that they could be more susceptible to HIV-1 infection.59 Other studies have also examined the proportions and localization of T-cell populations in the cervix and vagina of non-pregnant women. Pudney et al.46 performed an extensive immunohistological characterization to show that the T cells were the most prevalent in the cervical transformation zone and surrounding tissue, whereas the vaginal tissue contained few T cells. Most T cells in the lower genital tract were localized at the stroma/epithelial interface. However, significant numbers of CD8+ T cells were interspersed in the vaginal and ectocervical squamous epithelium (intra-epithelial lymphocytes). Interestingly, the ectocervix contained significantly higher numbers of CD4+ intra-epithelial lymphocytes cells and inflamed vaginal and cervical samples contained higher concentrations of intra-epithelial lymphocyte populations compared with non-inflamed tissue. No changes in T-cell populations were found to correlate with the menstrual cycle. The presence of the highest number of T cells in the transformation zone is significant because of the finding that this area can be particularly susceptible to HIV infection. A more recent study examined T-cell populations in the endo- and ectocervix by flow cytometry and found that the ectoervix of premenopausal women contained significantly more CD4+ T cells, CD8+ T cells and B cells than did the endocervix.47
Recent studies have shown that regulatory T (Treg) cells and T-helper 17 (Th17) cells can be present in the FRT. Typically, the presence of these cells has been noted following exposure to inflammatory or immunoregulatory conditions.60,61 For example, Treg cells were found to be induced in both experimental mouse models and humans following exposure to seminal plasma, a rich source of TGF-β.61 Similarly, a number of studies have described an increase in the numbers and distribution of Th17 cells following genital tract infection, including N. gonorrhoea, Chlamydia and HIV-1 infections.60
Humoral immunity
The female reproductive tract adaptive immune system displays unique characteristics that are distinct from other mucosal surfaces.62,63 For example, it lacks permanent and organized lymphoid structures that are capable of inducing immune responses. The LA found in uterine endometrium do not have the same composition as those found in other organized lymphoid follicles, such as intestinal Peyer's patches, because the T cells found in LAs are primarily CD8+ T cells that express activation and memory markers, and the associated B cells primarily express CD5, a marker that is typically associated with B-1 cells.64 Nevertheless, resident IgA plasma cells have been identified in the FRT, especially in the cervix.63 Therefore, while a number of studies have shown that the FRT is an effector site, the ability of the FRT to induce local immune responses remains unclear. Studies have examined the antibody responses to infections in the genital tract and have provided evidence for the presence of consistent but weak IgG and IgA responses that have been predominantly detected in cervical secretions against Neisseria gonorrhoea, Chlamydia, HSV-2 and HIV-1.62 Experimental evidence from mouse models has shown that intravaginal and intranasal immunization, especially with live organisms, can induce robust IgG and IgA antibody responses.9,65 Furthermore, immune responses can be induced in splenectomized LT-a knockout mice, which lack any secondary lymphoid organs, following genital immunization that resulted in protective anti-viral immunity.66 However, further investigations are needed to prove the inductive capability of FRT.
Immunohistochemical studies that have examined the FRT for the presence of antibody-secreting cells found that the endocervix contains the most IgG and IgA antibody-producing and antibody-containing cells compared with the ectocervix, vagina and fallopian tubes.62 Unlike the intestinal tract, the FRT is unique in that the dominant antibody response in genital secretions is IgG instead of IgA, although IgA is present. Both IgA1 and IgA2 are found in cervical secretions; interestingly, while cervical mucus contains approximately 70% of IgA in its polymeric form, the vaginal secretions have approximately equal proportion of pIgA and mIgA.63 The transport of IgA and IgG into the lumen of the FRT is dependent on the expression of their respective transporter proteins, pIgR (polymeric immunoglobulin receptor) and FcRn (neonatal Fc receptor) on the genital epithelium. The pIgR has been studied in detail for its ability to transport IgA, and its expression on epithelial cells is regulated by sex hormones, such that estradiol increases expression on uterine epithelium, whereas progesterone reverses this effect.67 The source of IgG in the genital tract is likely to be from both IgG in circulation and local production, as secreted by plasma cells that reside in the FRT. Recent studies have identified FcRn expressed on GECs as the primary receptor that transports IgG across the epithelium into the lumen.68
Regulation of innate and adaptive immune responses by female sex hormones
It has been well documented that both tissue and immune cells in the female genital tract are regulated by the sex hormones, estradiol and progesterone. Menstrual cycle-mediated changes in innate and adaptive immunity in the reproductive tract have been demonstrated by many studies and have been reviewed in detail elsewhere.7,9 In previous sections of this article, we briefly reviewed the changes in cells and immune responses associated with the menstrual cycle. In this section, we discuss some of the experimental evidence demonstrating the regulation of immune responses by female sex hormones. We also examine clinical data that show changes in the immune system with hormonal contraceptives. This is clinically important because of its relevance to immune protection against sexually transmitted pathogens.
The treatment of animals and humans with progesterone-based contraceptives can have significant effects on the immune response to sexually transmitted infections (STIs). We have previously reported that mice treated with Depo-Provera have decreased levels of HSV-2-specific mucosal immune responses after intravaginal immunization with the attenuated strain of HSV-2 (TK-HSV-2).69,70 Consequently, these mice fail to develop protective immune responses against subsequent WT HSV-2 challenge. DMPA, a progesterone-based hormonal contraceptive used worldwide, also exerts many effects on T cell-mediated immunity, including the inhibition of cytotoxic T lymphocyte activity and blocking perforin expression in T cells.9,71
Progesterone also influences cytokine production, generally favoring Th2-type immune responses.72 A more recent study reported that progesterone treatment, at concentrations achieved during hormonal therapy, decreases the proliferation and Th1-type cytokine production of varicella-zoster virus-specific CD8+ and CD4+ T cells, and this effect was exacerbated in cells obtained from HIV-infected individuals.73 Progesterone can also significantly affect the infiltration of lymphocytes, NK cells, and macrophages into the female genital tract.74,75 Additionally, progesterone can also impair NK cell function and FcR expression on monocytes, thereby reducing the two arms of antibody-dependent cell cytotoxicity.76,77 A recent study indicated that medroxyprogesterone, the active compound of DMPA, could inhibit TLR9-induced IFN-β production by human and mouse plasmacytoid DCs.78 Because plasmacytoid DCs have been shown to play an important role in detecting the pathogen-associated molecular patterns expressed by sexually transmitted viruses, such as HSV-2, through TLR9 expression on their surface and the production of IFN-β in response,79 it is possible that this inhibition of IFN-β production by progesterone may impair an important aspect of the innate antiviral immune response. Taken together, the reductions in antibody production, cytotoxic T lymphocyte activity, IFN-β production and antibody-dependent cell cytotoxicity activity observed in women using progesterone-based contraceptives may contribute to the increased susceptibility and shedding of HIV-1 observed in women using these hormonal therapies.80,81
The effects of estrogen on the immune system of women are also well documented. Depending on the concentration, estrogen can evoke either pro- or anti-inflammatory effects.82 At low concentrations, estrogen induces TNF-α, IL-6 and IL-1α expression, inhibits Th2-type cytokines and increases the migration of leukocytes to sites of inflammation.82 Other studies have shown that estrogen can also inhibit the production of TNF-α, IL-1α and IL-6 by T cells, macrophages and DCs and induce the Th2-type cytokines IL-4, IL-10 and TGF-β, resulting in anti-inflammatory effects.83 At higher concentrations, estrogen inhibits cell-mediated immunity and decreases the expression of numerous activation markers.73 Estradiol also has varying effects in the genital tract. In the uterus of ovariectomized rats, estradiol increases the levels of IgG, IgA and the pIgR. However, the levels of these antibodies are reduced in cervicovaginal secretions in response to estradiol treatment.9 Estradiol treatment also influences the activity of many types of immune cells. For instance, the treatment of rats with estradiol was correlated with reduced antigen presentation in the vagina, likely as a consequence of increased local production of TGF-β in response to estradiol treatment.84 Estradiol also downregulated cytotoxic T lymphocyte activity.58,83 Additionally, high levels of estrogen may result in the decreased migration of inflammatory T cells and macrophages into the genital tract because of the downregulation of ICAM-1, E-selectins and VCAM-1,82 which could contribute to the decreased risk of HIV acquisition in response to estrogen treatment.
HIV infection in the FRT
According to a WHO report in 2012, the total number of new cases of the four most common treatable STIs in adults in 2008 was estimated to be 498.9 million. These infections included Chlamydia trachomatis, Neisseria gonorrhea, Trichomonas vaginalis and syphilis. Of these, the first three are more prevalent in women, whereas syphilis is more common in men. Among chronic viral infections, human papilloma virus, HSV-2 and HIV-1 are the most common infections that affect women in disproportionately high numbers.85 Among the 40 million HIV-1-infected individuals, women now constitute >50% of the population worldwide. Although vaginal transmission is estimated to have a lower risk per exposure event, recent estimates show that 40% of HIV infections are initiated in the FRT.86
Recent observations from studies conducted using in vitro cell cultures, ex vivo cervicovaginal tissues and non-human primates have provided some insights into the acute transmission events in the female genital tract.3,4 These studies indicate that HIV-1 transmission can occur in both the upper and lower female genital tract. A number of different mechanisms have been proposed regarding the mechanism by which HIV can cross the FRT mucosal barrier, including microtears in the squamous epithelium and direct infection, transcytosis and sequestration of the virus in the columnar epithelium that lines the upper tract. Some of the clearest evidence comes from studies examining acute simian immunodeficiency virus (SIV) infection in non-human primates.4,5 These studies clearly indicate that when inoculated intravaginally at high doses, SIV preferentially crossed the epithelial barrier in the endocervix, within hours and established small foci of infection within 48–72 h. These observations are consistent with studies that demonstrated that HIV infection in infected hosts can be traced back to small (between 1 and 5) founder virus populations. In the macaque SIV infection study, the small foci underwent local amplification in the genital mucosa in the first few days prior to systemic dissemination.4,5 Based on the elegant studies by Hladik et al.86 that have been confirmed by others, there is a general consensus that HIV-1 replication in the FRT occurs primarily in the target T cells. While some subsets of DCs can be both productively infected by HIV-1 and play a role in trans-infection of T cells, their contribution to the local amplification of HIV-1 in the genital tract is likely limited.3,4 The subset of T cells that are most susceptible to HIV-1 and the role played by various subsets of DCs in trans-infection versus viral replication are still being investigated, and the progress made in these areas has been previously reviewed.7,8
Before HIV-1 can establish a productive infection in the FRT, it must overcome a number of mechanical, chemical and biological barriers. The mucus, AMP and Type 1 IFNs secreted by the epithelial and innate immune cells significantly impede the ability of HIV to establish infection in the FRT. Many of the cytokines and chemokines found in the FRT have the ability to directly interfere with viral infections, including HIV. The chemokine stromal derived factor-1 (or CXCL12), which is found within the subepithelial layer of the cervix, is able to competitively inhibit X4 strains of HIV.87 Similarly, the β-chemokines, MIP-1α, MIP-1β and the regulated on activation, normal, T-cell expressed and secreted are all secreted by cells of the upper and lower genital tracts constitutively and under infectious conditions88,89,90 and, as natural ligands for the CCR5 receptor, may also play a role in preventing R5-tropic viruses from establishing an infection. In our recent studies, we have shown that primary human GECs directly interact with HIV-1 surface glycoprotein gp120, leading to the production of an array of pro-inflammatory cytokines.91 Among these cytokines, TNF-α production induced a rapid decrease in trans-epithelial resistance, a measure of epithelial barrier integrity. Disruption of the barrier was accompanied by increased mucosal permeability and consequent bacterial and viral translocation across the epithelium. Thus, increased mucosal permeability and microbial translocation can result directly from early interactions between HIV-1 and the genital epithelium, leading to the initiation of microbial translocation and immune activation. Further studies have since revealed that the gp120-mediated activation of pro-inflammatory cytokine pathways in GECs utilizes TLR2 and TLR4 in addition to cell surface heparin sulfate moieties.92 Furthermore, the presence of seminal plasma and viral and bacterial co-infections was associated with increased innate inflammation and increased HIV-1 replication.
The adaptive immune responses in the FRT to HIV infection are not completely understood because of the limited availability of mucosal tissues for analysis. Recent studies have identified a subset of activated T helper cells in the cervix that express α4β7, CCR5, IL-17A and IFN-γ and are highly susceptible to HIV-1 infection. These cells were completely depleted in HIV-1-infected women, indicating that this subset of CD4+ T cells may play a key role in susceptibility to HIV-1 infection in the FRT. Other studies have shown that HIV-1 infection leads to the induction of CD8+ T-cell responses in the cervical mucosa. Interestingly, studies have also indicated that there was no correlation between blood and cervical T cell responses. However, cervical T-cell responses correlate positively with significantly higher levels of pro-inflammatory cytokines in cervical secretions, especially in women who have cervical viral shedding.
Male reproductive tract
Structure and morphology of the human male reproductive tract (MRT)
Urethral tract
Compared with the FRT, much less information is available regarding the immune response in the MRT in the homeostatic state or following pathogen infection. This is partly a consequence of limitations in sampling techniques and the availability of samples.93,94 Urethral secretion sampling by swabs or lavages can be painful, and the surgical collection of MRT tissues is not performed routinely, compared with that in the FRT (e.g., hysterectomies or tubal ligations). However, more recent techniques for sampling urethral secretions, including the collection of pre-ejaculatory fluid, expressed prostate secretions, and first-catch urine, and the availability of male genital epithelial cell (EC) lines have had a positive impact on the field.94,95,96,97
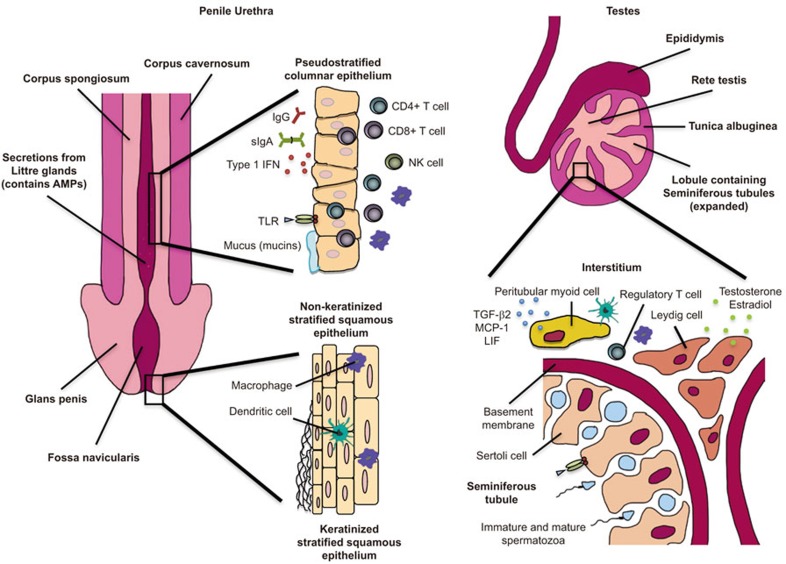
The penile urethra is the main site of many STIs in men.93,95 Analogous to the upper and lower FRT, different regions of the urethral mucosa consist of different microenvironments that are composed of various EC types. The urethral opening is similar in morphology to the transformation zone in the FRT, where the keratinized stratified squamous epithelium abruptly transitions into non-keratinized stratified squamous epithelium in the fossa navicularis33 (Figure 2). Subsequently, as the non-keratinized stratified squamous epithelium enters the shaft of the penis, it transitions into pseudostratified glandular columnar epithelium, which lines the length of the penile urethra.93 Within the epithelium of the penile urethra, deep invaginations, known as Littre glands, are responsible for the secretion of viscous fluid, such as the pre-ejaculate, which serves as lubrication during sexual intercourse and assists in neutralizing any residual urine in the urethra.93
Figure 2.
Anatomical and immunological components of the male reproductive tract. The MRT is composed of two main parts—the penile urethra and the testes. The opening of the penile urethra transitions from keratinized stratified squamous epithelium to non-keratinized stratified squamous epithelium in the fossa navicularis. As the non-keratinized stratified squamous epithelium enters the shaft of the penis, it transitions into the pseudostratified glandular columnar epithelium, which lines the length of the penile urethra. Within the epithelium of the penile urethra are deep invaginations, known as Littre glands, which provide lubricating pre-ejaculate for sexual intercourse and contribute to innate immunity by producing AMPs. Urethral pseudostratified columnar epithelium is an active immune microenvironment that contains CD8+ and CD4+ T cells, NK cells, dendritic cells and resident macrophages, which have been found to be key targets for HIV infection in the penile urethra. IgG, IgA and IgM immunoglobulins are also secreted from the urethra epithelium, along with Type 1 IFN, mucins and AMPs. The testes are the main site of spermatogenesis, which begins in the rete testes and seminiferous tubules; mature spermatozoa enter the epidiymis. Seminiferous tubules, located within the lobules of the testes, are made up of Sertoli cells that are surrounded by a basement membrane. Sertoli cells aid in spermatogenesis but also express TLRs. Within the interstitial space of the seminiferous tubules are Leydig cells, which produce testosterone and estradiol, and PMCs, which regulate testes development and spermatogenesis by secreting TGF-β2, MCP-1 and LIF. Resident ED2+ macrophages, which have an attenuated inflammatory function, are found within the interstitial spaces of the MRT along with regulatory T cells—both cell types contribute to the immunoregulatory microenvironment of the testes. AMP, anti-microbial peptide; IFN, interferon; Ig, immunoglobulin; LIF, leukemia inhibitory factor; MCP, monocyte chemoattractant protein; MRT, male reproductive tract; NK, natural killer; PMC, peritubular myoid cell; TGF, transforming growth factor; TLR, Toll-like receptor.
Testes
The testes, an immune privileged site, represents a unique immunological environment; this was validated experimentally when allografts were able to survive indefinitely when placed in the interstitial space of rat testes.98 The testes exhibit both aspects of immune privilege in mammals: it induces immune tolerance when transplanted into an allogeneic recipient, and it readily accepts a transplant without inducing immune responses that cause rejection.99 Previously, it has been hypothesized that immune privilege in testes was primarily a consequence of the sequestration of antigens from systemic immune system, which is mediated by the blood–testis barrier in the seminiferous epithelium.100 However, recent evidence has shown that the combination of the unique physical structure of the testes, the properties of local cells, and paracrine and autocrine cytokines all contribute to the immune privilege of the testes.101,102
The human testes are structured into two discrete regions: the interstitial spaces between the tubules and the seminiferous tubules100 (Figure 2). The testis is responsible for the generation of sperm (spermatogenesis), and the production of sex steroid hormones, primarily testosterone (steroidogenesis).100,103 Spermatogenesis occurs in the coiled seminiferous tubules, which begin and end at the rete testis.99,104,105 The seminiferous tubules are made up of columnar Sertoli cells (SCs) that surround spermatogenic cells and stem cells.100,106,107 In addition to providing essential nutrients and growth factors, SCs play an important role in the morphological changes for sperm because it undergoes spermatogenesis, as demonstrated in Nectin-2 knockout mice.108 Previous experiments involving allograft and xenograft cotransplantation have shown SCs to have inherent immunosuppressive capabilities.109,110,111,112
Peritubular myoid cells (PMCs) surround the seminiferous tubules, providing support for the integrity of the tubules.104 PMCs assist in the contractile motion needed to facilitate movement and maturation of spermatozoa within the seminiferous tubules and into the epididymis.113 PMCs can indirectly regulate spermatogenesis and testis development via secreted factors such as TGF-β2, monocyte chemoattractant protein-1 and leukemia inhibitory factor.114 Human myeloid peritubular cells express tumor-necrosis factor-alpha (TNF-α) receptor 1 and 2, which mediate the expression of other inflammatory molecules, such as interleukin 6 (IL-6) and cyclooxygenase 2 (COX-2).115
The majority of the cell populations within the interstitial compartment of the testes are Leydig cells, which are responsible for the synthesis of testosterone and small amounts of estradiol under the stimulus of luteinizing hormone secreted by the pituitary gland.104 Leydig cells have been shown to regulate the expansion of testicular macrophages and lymphocytes in the testes of rats; however, these cells have been shown to exert weak antiviral responses in humans.116,117,118
Innate immunity
PRRs
Similar to the FRT (discussed above) and all other mucosal tissues of the body, PRRs, similar to TLRs, bind to pathogen-associated molecular patterns and activate initial response genes associated with innate immunity. Recent studies have suggested that TLRs not only are involved in disease and inflammation but also play an important role in the basic pathology and physiology of reproduction.100,103,119,120 To date, 10 functional TLRs (TLRs 1–10) have been identified in humans, and 13 TLRs have been identified mice.121 A previous study by Nishimura and Naito122 reported the expression of mRNA transcripts for TLRs 1–10 and their respective adaptor proteins in the human prostate and testes. Additionally, Pudney and Anderson123 reported a detailed histological survey of TLR expression in the MRT, showing no TLR expression on the epithelium or in immune cells of the rete testis, vas deferens, or foreskin tissue, including macrophages inside the efferent ducts and epididymis.93,123,124 Only TLR-1, which recognizes bacterial lipoproteins, is present in immune cells in the testis, efferent ducts, epididymis and seminal vesicles. Epithelial cells in the prostate and penile urethra also express TLR-3 and TLR-8 or TLR-9, respectively.100,123,125
Different TLRs are able to recognize highly conserved bacterial, fungal, or viral components (e.g., peptidoglycan, lipopolysaccharide, dsRNA, ssRNA, Poly I∶C or flagellin). After binding their respective ligands, the common transduction pathway involving cytoplasmic TLR/IL-1 (TIR) is activated.121 TIR domains interact with intracellular factors, such as myeloid differentiation primary activation protein 88, TIRAP/Mal, TIR domain-containing adaptor inducing IFN-β and TIR domain-containing adaptor inducing IFN-β-related adaptor molecule.120,121 These interactions lead to the activation of NF-κB, which can translocate to the nucleus and stimulate the transcription of different cytokines and chemokines in response to infections and spermatogenesis under normal conditions.120,126,127,128
Mucus
As part of the biological barrier, mucus serves as one of the first lines of immune defense against pathogens by trapping and eliminating them before they can reach the epithelial surface. Previous studies have shown that membrane-associated mucins (i.e., MUC1, MUC3, MUC4, MUC13, MUC15, MUC17 and MUC20) are expressed on the apical surface of epithelial cells throughout the MRT.129 Functional studies of the cervicovaginal mucus showed MUC4 and MUC5b expression, which impedes pathogen penetration through their surface charge and associated antibodies (e.g., IgM and IgA).130 Thus, one could reasonably conclude that similar functions are present in the MRT. Membrane-associated mucins not only provide a physical barrier and lubrication, but they also serve as signal transducers through their juxtamembrane regions.131
AMPs
AMPs are constitutively expressed by cells in the mucosal epithelia, including DCs, macrophages, neutrophils, NK cells and ECs.132 Many of these immune-related secretions have been shown to play an important role in inhibiting infection by bacteria, fungi and viruses in both the FRT and MRT.132,133 Defensins, a family of small cationic proteins, are often divided into categories based on the cell type that produces them. HBD-1, -2 and -3, and HD-5 and -6 are primarily expressed by epithelial cells, whereas human neutrophil α-defensins 1–4 are predominantly expressed by granulocytes.93,134 Porter et al.132 showed that the secretion and upregulation of HD-5 in the urethral secretions occurs as a propeptide in Chlamydia trachomatis and Neisseria gonorrhoea infections. Furthermore, the expression of HBD-1 was detected by reverse transcription polymerase chain reaction in RNA isolates from the testes and prostate, whereas HBD-4 was abundantly found in the testis.135,136 HBDs have been shown to neutralize a broad range of pathogens in vivo, such as multidrug-resistant Enterococcus faecium, Escherichia coli, Candida species and Pseudomonas aeruginosa.137,138,139
Several other classical AMPs have also been documented, such as lysozyme expression in the glands of Littre, intraepithelial cells, epididymis, prostate, testis and seminal fluid.140 SLPI, a highly cationic single-chain protein, is expressed abundantly in seminal plasma, ECs and lamina propria of the prostate, seminal vesicles, epididymis, as well as the columnar epithelium of the urethral mucosa.120,141 Similarly, columnar epithelial cells of the urethra consistently express lactoferrin, a protein that is commonly found in semen, tears, and breast milk. Lactoferrin is a significant epididymal secretory protein that binds sperm and restricts the availability of iron, copper, zinc, manganese, and other metal ions.120,142 Das et al.143 have previously described the influence of estrogen in lactoferrin in the uterus; however, steroid hormone regulation in the MRT has not been investigated, and the roles of lactoferrin in the epididymis and on the surface of spermatozoa remain to be elucidated. A variety of combinatorial AMPs could be expressed on the mucosal surface. Singh et al.144 demonstrated that the triple combination of lactoferrin, lysozyme and SLPI showed greater synergistic killing of E. coli compared with any single- or double-combination of AMPs.
In addition to AMPs, cells within the MRT are capable of producing Type 1 interferons (IFN-α and -β), which have immunomodulatory and anti-viral effects.145,146 Type 1 interferon-producing cells were commonly observed in the lamina propria and are occasionally observed in the epithelial layer of the urethral tract.93 Moreover, the basal layer of epithelial cells in the fossa navicularis was consistently positive for IFN-β.93
Adaptive immunity
Cellular immunity
There are many types of immune cells in the urethra and testes of the MRT, with a predominance of macrophages that are commonly found in the interstitial spaces.100,147 Previous studies have identified at least two subsets of macrophages in the testes. Testicular macrophages display a reduced capacity for both producing inflammatory factors and possessing immunosuppressive properties compared with macrophages in other tissues.148,149 Resident macrophages, representing approximately 80% of testicular macrophages, are classified by the surface expression of ED2.100,150 Conversely, ED1-positive macrophages are recruited to the testes from the circulation as monocytes.100 ED1+ and ED2+ macrophages have inherent differences in their ability to initiate inflammatory responses. A previous study by Gerdprasert et al.151 showed that ED2+ macrophages lack the ability to induce inflammatory cytokines in response to lipopolysaccharide challenge in rats. Previous studies have demonstrated that the balance between these two types of macrophage can be disrupted in cases of infection (e.g., orchitis).150,152 ED1+ macrophages are recruited to the testes during acute and chronic inflammation; however, the influx of these macrophages only lasts a couple of days in acute cases.153
DCs represent a small cell population within the testes and comprise approximately 10% of the size of the macrophage population.102 Because of the small population size, a limited number of studies have examined the role of DCs within the MRT. However, in the experimental model of autoimmune orchitis, the number of DCs increased significantly, suggesting that DCs may play a role in immune regulation of the testes.154
Mast cells have been shown to regulate steroidogenesis by Leydig cells.155 An increase in the number of mast cells has been associated with infertility in men. In addition to releasing serine protease tryptase to promote proliferation of fibroblasts and collagen, mast cells upregulate monocyte chemoattractant protein-1 and thereby recruit macrophages into the testes.156 The presence of NK cells in the mucosa of the urethra has been reported.124 However, further studies into their functions in immune responses in the MRT need to be carried out because their role in the MRT remains unclear.
T lymphocytes are abundant in all areas of the urethra and account for approximately 15% of testicular immune cells in adult rats.118,124 Both CD8+ and CD4+ T cells are present in the epithelium and lamina propria of the urethra; however, similar to the testes, CD8+ T cells are more abundant.93,118,124 Moreover, Treg cells are found within the testicular interstitium under physiological conditions, which may be responsible for the immunosuppressive characteristics of the testes.157 A small population of naïve T lymphocytes expressing CD45RA was also found in the urethra, primarily in the lamina propria.93,103,118 The majority of T cells are CD45RO (memory T cells) in both the epithelium and lamina propria, and they express α4β7 integrin (a mucosal homing receptor).93,124 Based on recent studies, it is clear that the lower MRT is an immunologically competent site that is capable of generating both cellular and humoral immunological responses.
Humoral immunity
The penile urethra contains numerous IgA and J chain-positive plasma cells, particularly in the lamina propria.93,158 There are fewer IgA+ plasma cells in the urethra than there are IgG+ plasma cells. IgM-positive plasma cells were also present, and their expression was correlated with that of IgA-positive cells. Most of the IgG+ population appeared to be derived from the serum, whereas the IgA+ population appeared to be produced locally.
Composition of semen
Semen contains many diverse bioactive factors that contribute to its function as a support medium for spermatozoa and as a primer of the FRT for the initiation of conception. When deposited into the FRT, high concentrations of certain immunoregulatory factors in semen, primarily prostaglandin E (PGE) and TGF-β, modulate immunity in the FRT microenvironment.159 Seminal prostaglandins, in the form of 16-hydroxy PGE, exert their immunoregulatory role in the FRT by inhibiting the function and clonal expansion of NK and T cells and can polarize CD4+ T cells towards a Th-2 phenotype.160 Additionally, PGE induces tolerance by promoting a switch from IL-12 to IL-10 cytokine production in DCs.160,161,162 While this immunosuppressive property of PGE, in tandem with IL-10, is important for the survival of spermatozoa, it could be detrimental in the context of sexually transmitted infections, such as HIV, HSV and human papilloma virus, because it would divert cell-mediated immune responses.
TGF-β is a potent immunoregulatory cytokine that has a key function in mediating tolerance to male antigens in the FRT. Its concentration in semen is one of the highest measured in biological fluids, occurring at up to five times the amount present in serum and at levels comparable to the colostrum.104,163,164 The prostate has been identified as the major source of TGF-β in men, whereas the seminal vesicle is the primary source of TGF-β in mice.163 When deposited and activated in the FRT, TGF-β that is present in semen elicits a transient pro-inflammatory response that leads to the activation and recruitment of neutrophils, macrophages and DCs into the FRT, culminating in the induction of a tolerogenic phenotype in these cells.163,164 Seminal TGF-β also increases the expression of pregnancy-supporting cytokine production from FRT epithelial cells.164,165,166,167 Our lab previously showed that TGF-β1 levels in human SP differed significantly depending on the presence and stage of HIV infection, which also leads to differential cytokine responses in FRT epithelial cells.168 Acute HIV infection leads to a moderate increase in TGF-β levels, whereas chronic infection significantly increased TGF-β in our therapy-naive cohort. Furthermore, TGF-β expression in HIV-uninfected and HIV-infected men is compartmentalized between the blood and SP (Kafka et al., unpublished data), and sCD14, an immune activation marker, was negatively correlated with active TGF-β1 expression in seminal plasma of chronic therapy-naive men. These findings suggested that activated TGF-beta could play a role in regulating chronic immune activation (Kafka et al., unpublished data).
In addition to a diverse array of cytokines, growth factors and leukocytes, several Ig isotypes have also been detected in semen. The presence of IgG, IgA and IgM has been documented; however, their respective levels and molecular properties vary across studies, possibly because of different collection protocols and standards used in detection assays. Studies have shown that IgG, rather than IgA, is the most abundant immunoglobulin present in the semen of healthy men.94,169 Furthermore, the distribution of IgA subclasses indicated the presence of secretory IgA, polymeric IgA and monomeric IgA.94
Effects of hormones on testicular immune responses
Androgens, namely testosterone, have immunosuppressive functions and have been shown to reduce TLR-4 expression in testicular macrophages.170,171 High local testosterone concentrations are likely to be involved in the maintenance of testicular immune privilege. Testosterone plays an immunomodulatory role by regulating the balance between pro- and anti-inflammatory cytokine expression in SCs, Leydig cells and MPCs, but it likely does not directly affect testicular leukocytes because androgen receptor expression levels have not been found in testicular immune cells. In a study by Meng et al.,172 androgens were shown to regulate the permeability of the blood–testes barrier by regulating the expression of Claudin 3, a tight junction protein found in SC.
Luteinizing hormone may control the proliferation of spermatozoa in the testis during puberty and the maintenance of macrophages in the adult testis by acting on Leydig cells.117 Follicle-stimulating hormone regulates the maturation of testicular macrophages via SCs.173 Moreover, gonadotropin-releasing hormone antagonists have been shown to reduce the percentage of Treg cells and increase the number of NK cells in healthy men.174
STIs in the MRT
Bacterial and viral infection of the MRT has been associated with infertility. Chlamydia trachomatis and Neisseria gonorrhoea are the most prevalent STIs among sexually active men under the age of 35. Moreover, among the chronic viral sperm infections, HIV), hepatitis B virus and hepatitis C virus are the most detrimental.175 Inflammation of the testis from acute epididymitis caused by ascending infection can persist even after treatment. As a result, the chronic inflammation, even post-treatment, could affect the functions and structural integrity of the seminiferous tubules and cause infertility.103,104,116
UNAIDS has estimated that there are more than 35 million people living with HIV worldwide in 2012, 43%–48% of whom are men.176 Among the 2.3 million new infections each year, approximately 60%–90% result from sexual contact, with semen being the main vector involved in HIV transmission.89,177 Previous studies have shown that HIV causes chronically low genitourinary tract inflammation and impairs the motility, morphology and function of sperm cells, thereby reducing fertility in males.178
It was previously thought that the only source of HIV in semen was from the lymphocytes and macrophages that arrived in the tissue from blood, but this is not the case. HIV in semen has been shown to evolve separately from viruses in the blood and other tissues.179,180,181,182 The blood–testis barrier makes it likely that the testis serves as a virus reservoir, which may be protected against anti-viral treatments.107,116 Infected leukocytes have been detected in MRT tissues of HIV-infected men and can migrate into semen, where they can be key contributors to HIV transmission.158,183 Preliminary studies in vivo suggest that semen enhancer of viral infection can facilitate the transmission of low doses of SIV.184
Poor correlations between blood and seminal viral load, phenotype and genotype in the literature suggest a viral compartmentalization phenomenon in HIV infected men. Viral RNA levels in the seminal plasma have been shown to be more variable than those found in blood plasma (BP).185 There are considerable discrepancies in the relative concentrations of HIV in the SP and BP, both between and within studies. In most studies, men with undetectable viral loads in the SP also had undetectable viral loads in the BP.185,186,187,188 However, some studies have shown that SP has an equal or greater viral load than BP, even in patients who had undetectable blood plasma viral loads. As an example, in a small prospective study by Sheth et al.,188 the authors reported substantial inter-individual heterogeneity in the SP viral load (as high as 5000 copies/ml), despite an undetectable BP viral load. This observation is independent of anti-retroviral activity or regiment. Thus, other contributing factors, such as co-infections (e.g., HSV-2 or human papilloma virus), and the stage of HIV infection should be closely examined in the future.187
Several endocrine and testicular dysfunctions have been reported in HIV-infected men at various stages of infection.189 Men during early stages of HIV infection have high levels of testosterone; however, men with AIDS were shown to exhibit lower levels of testosterone. It has been hypothesized that the testosteronemia results both from lymphocyte infiltration and fibrosis of the interstitial tissue and a reduction in the number of Leydig cells.189,190,191,192
In an effort to curb the transmission of HIV globally, many epidemiological studies, systemic reviews and a meta-analysis have been conducted to assess the benefits of circumcision for HIV prevention.193 Most of the evidence indicates a protective effect of male circumcision on heterosexual HIV acquisition.194,195 This positive correlation could be explained by the fact that the outer surface of the penile shaft and the foreskin are covered by a keratinized stratified squamous epithelium.196,197 This provides a protective barrier against HIV infection. However, the inner surface of the foreskin is not keratinized and is abundant in Langerhans cells with HIV receptors that are likely to be the primary point of viral entry into the penis of an uncircumcised individual.196,198 The foreskin is pulled back down the shaft of the penis, exposing the mucosa of the inner foreskin (the site of many antigen presenting cells) to vaginal secretions during heterosexual intercourse, thereby providing a large area where HIV transmission could occur.198
HIV infection in the MRT has a profound effect on semen composition, leading to altered cytokine profiles that can modulate HIV replication, promote viral shedding and cause local target cell activation.168,199 We previously showed that primary FRT epithelial cells exposed to SP from acutely infected men produced increased levels of pro-inflammatory cytokines, which lead to increased HIV-long terminal repeat activation in an infected T cell line.80 Several studies have shown that semen can also facilitate or inhibit HIV infection. Factors in semen, such as clusterin and mucin-6, can compete with HIV as a DC-specific intercellular adhesion molecule-3-grabbing non-integrin ligand.167,200,201,202 Cationic peptides, such as semenogelin and prostatic acid phosphatase, can also contribute to anti-HIV activity.203 In vitro studies have reported the presence of amyloid fibrils, formed by amyloidogenic fragments of prostatic acid phosphatase and semenogelins, which might increase the infectivity of HIV by several orders of magnitude by facilitating virion attachment to cells.204,205,206,207,208
The main HIV receptor, CD4, is absent in spermatozoa; however, several other alternative receptors for HIV have been described, including GalAAG, a glycolipid related to galactosylceramide that binds to gp120, the HIV coreceptor CCR5, and the mannose receptor.209,210 Pudney and Anderson158 previously reported the expression of the CD4 receptor on lymphocytes and macrophages infiltrating the testis, which represents a potential target for HIV infection. Similar to other mucosal sites, the MRT is populated by mucosal memory T cells,124,211 which are depleted during acute infection in humans and macaques.212,213 Semen contains CD4+ T cells and macrophages, both of which are target cells for HIV infection.211,214,215 Interestingly, seminal mucosal CD4+ T cells were restored following the initiation of anti-retroviral therapy.213 A recent study showed that primary SIV infection induced a strong inflammatory response, which was associated with increased numbers of leukocytes in primate semen.214 Lymphocytes in the semen showed a mucosal phenotype and were productively infected both in vitro and at all stages of SIV infection in vivo.214
Summary and conclusion
The male and female genital tracts are highly complex mucosal sites where structural tissue cells, immune cells and resident microbial flora interact in a microenvironment regulated by male or female sex hormones, respectively. Because of the diverse physiological demands on these mucosal sites, the immune system in either reproductive site is highly compartmentalized and has developed unique adaptations. In the FRT, innate immune cells, soluble factors, and adaptive immune cells undergo cyclical changes with the menstrual cycle, which are orchestrated by the female sex hormones, estradiol and progesterone. The male reproductive tract is characterized by the unique blood–testes barrier that provides an immune-privileged site for spermatogenesis. Studies have shown that most innate immune cells, including DCs and NK cells in the FRT and macrophages in the MRT, have distinct phenotypic and functional adaptations that are specific to the reproductive tract environment. Similarly, there is a distinct compartmentalization of adaptive immune cells, such that CD8+ T cells are the main T-cell population in the FRT and IgG is the dominant immunoglobulin in both the male and female genital tracts. Innate factors in the reproductive tract provide the first line of protection against sexually transmitted pathogens. These include the physical barrier conferred by the epithelial lining and the mucus and anti-microbial factors that are present in the lumen of the reproductive tract. Furthermore, the epithelial and innate immune cells induce inflammatory cytokines, chemokines and Type I IFN upon recognition of microbial pathogen-associated molecular patterns that initiate innate and antigen-specific adaptive immune responses.
Among sexually transmitted infections, HIV-1 has unique adaptations that exploit the host immune responses to establish successful infection. The inflammatory anti-viral innate immune responses in the reproductive tract facilitate HIV-1 infection and replication. The adaptive immune responses to HIV in the reproductive tract are handicapped by the preferential infection of activated CD4+ T cells. CD8+ T-cell responses and antibody responses are induced, but correlations with protective immunity have not been established to date. An improved understanding of the unique characteristics and functions of the immune system in the male and female reproductive tracts will help improve clinical interventions for the treatment of infertility and the design of better prophylactic measures to prevent sexually transmitted infections.
Acknowledgments
This work was supported by research grants from the Ontario HIV Treatment Network (OHTN), the Canadian Foundation of AIDS Research (CANFAR) and the Canadian Institutes of Health Research (CIHR). CK is the recipient of an Applied HIV Research Chair Award from the OHTN. VHF is a recipient of an OHTN Studentship Award and currently holds a CIHR Frederick Banting and Charles Best Canada Graduate Scholarship Doctoral Award. The authors would like to thank the members of Kaushic Lab for the research contributions described in this review.
References
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Ferreira VH, Kafka JK, Nazli A. HIV infection in the female genital tract: discrete influence of the local mucosal microenvironment. Am J Reprod Immunol. 2010;63:566–575. doi: 10.1111/j.1600-0897.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Brotman RM, Ravel J, Bavoil PM, Gravitt PE, Ghanem KG. Microbiome, sex hormones, and immune responses in the reproductive tract: challenges for vaccine development against sexually transmitted infections. Vaccine. 2014;32:1543–1552. doi: 10.1016/j.vaccine.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VH, Kafka JK, Kaushic C. Influence of common mucosal co-factors on HIV infection in the female genital tract. Am J Reprod Immunol. 2014;71:743–554. doi: 10.1111/aji.12221. [DOI] [PubMed] [Google Scholar]
- Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71:618–623. doi: 10.1111/aji.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Richardson J, Prabhala R.Endocrine regulation of mucosal immunity: effect of sex hormones and cytokines on the afferent and efferent arms of the immune system in the female reproductive tractIn: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR (ed.)Handbook of Mucosal Immunology San Diego, CA: Academic Press; 1994705–718. [Google Scholar]
- Wira CR, Crane-Godreau MA, Grant-Tschudy KS.Endocrine regulation of the mucosal immune system in the female reproductive tractIn: Lamm ME Mestecky J, Strober W, Bienenstock J, McGhee JR and Mayer L (ed.).Mucosal Immunology San Diego, CA: Elsevier Academic Press; 20051661–1676. [Google Scholar]
- Kaushic C, Roth KL, Anipindi V, Xiu F. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol. 2011;88:204–209. doi: 10.1016/j.jri.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol. 2005;53:208–214. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–195. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. Am J Obstet Gynecol. 2002;187:561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- Aboud L, Ball TB, Tjernlund A, Burgener A. The role of serpin and cystatin antiproteases in mucosal innate immunity and their defense against HIV. Am J Reprod Immunol. 2014;71:12–23. doi: 10.1111/aji.12166. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2009;29:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer I, Vieillard V, de Maeyer E. Retrovirally mediated IFN-beta transduction of macrophages induces resistance to HIV, correlated with up-regulation of RANTES production and down-regulation of C–C chemokine receptor-5 expression. J Immunol. 2000;164:1582–1587. doi: 10.4049/jimmunol.164.3.1582. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature. 2013;502:563–566. doi: 10.1038/nature12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JJ, Woods M, Lindsay RJ, Doyle EH, Griesbeck M, Chan ES. Higher expression of several interferon-stimulated genes in HIV-1-infected females after adjusting for the level of viral replication. J Infect Dis. 2013;208:830–838. doi: 10.1093/infdis/jit262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy GA, Sieg S, Rodriguez B, Anthony D, Asaad R, Jiang W. Interferon-alpha is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS ONE. 2013;8:e56527. doi: 10.1371/journal.pone.0056527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MP, Owczarek CM, Jermiin LS, Ejdeback M, Hertzog PJ. Characterization of the type I interferon locus and identification of novel genes. Genomics. 2004;84:331–345. doi: 10.1016/j.ygeno.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- Xi Y, Day SL, Jackson RJ, Ranasinghe C. Role of novel type I interferon epsilon in viral infection and mucosal immunity. Mucosal Immunol. 2012;5:610–622. doi: 10.1038/mi.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Wang X, Ye L, Zhou L, Yang ZQ, Riedel E. Lambda interferon inhibits human immunodeficiency virus type 1 infection of macrophages. J Virol. 2009;83:3834–3842. doi: 10.1128/JVI.01773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MQ, Zhou DJ, Wang X, Zhou W, Ye L, Li JL. IFN-lambda3 inhibits HIV infection of macrophages through the JAK–STAT pathway. PLoS ONE. 2012;7:e35902. doi: 10.1371/journal.pone.0035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian RR, Guo HX, Wei JF, Yang CK, He SH, Wang JH. IFN-lambda inhibits HIV-1 integration and post-transcriptional events in vitro, but there is only limited in vivo repression of viral production. Antiviral Res. 2012;95:57–65. doi: 10.1016/j.antiviral.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- King AE, Horne AW, Hombach-Klonisch S, Mason JI, Critchley HO. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Mol Hum Reprod. 2009;15:311–319. doi: 10.1093/molehr/gap020. [DOI] [PubMed] [Google Scholar]
- Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A, Yao XD, Smieja M, Rosenthal KL, Ashkar AA, Kaushic C. Differential induction of innate anti-viral responses by TLR ligands against Herpes simplex virus, type 2, infection in primary genital epithelium of women. Antiviral Res. 2009;81:103–112. doi: 10.1016/j.antiviral.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Ferreira VH, Nazli A, Khan G, Mian MF, Ashkar AA, Gray-Owen S. Endometrial epithelial cell responses to co-infecting viral and bacterial pathogens in the genital tract can activate the HIV-1 LTR in an NFκB and AP-1 dependent manner. J Infect Dis. 2011;204:299–308. doi: 10.1093/infdis/jir260. [DOI] [PubMed] [Google Scholar]
- Harwani SC, Lurain NS, Zariffard MR, Spear GT. Differential inhibition of human cytomegalovirus (HCMV) by toll-like receptor ligands mediated by interferon-beta in human foreskin fibroblasts and cervical tissue. Virol J. 2007;4:133. doi: 10.1186/1743-422X-4-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Iijima N, Thompson JM, Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 2008;1:451–459. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- Trifonova RT, Lieberman J, van Baarle D. Distribution of immune cells in the human cervix and implications for HIV transmission. Am J Reprod Immunol. 2014;71:252–264. doi: 10.1111/aji.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol. 1997;61:427–435. [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, et al. DC-SIGN, a dendritic cell-specific HIV-1 binding protein that enhances trans-infection of T-cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci. 2007;85:E36–E44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
- Moffett-King A, Entrican G, Ellis S, Hutchinson J, Bainbridge D. Natural killer cells and reproduction. Trends Immunol. 2002;23:332–333. doi: 10.1016/s1471-4906(02)02261-5. [DOI] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur J Immunol. 2001;31:3121–3127. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Meadows SK, Wira CR, Sentman CL. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-beta. J Leukoc Biol. 2004;76:667–675. doi: 10.1189/jlb.0204090. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA. CD3+CD8+ CTL activity within the human female reproductive tract: influence of stage and menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- Shanmugasundaram U, et al. Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women. Am J Reprod Immunol. 2014;71:95–108. doi: 10.1111/aji.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon LR, Nyanga B, Chege D, Izulla P, Kimani M, Huibner S. Characterization of a human cervical CD4+ T cell subset coexpressing multiple markers of HIV susceptibility. J Immunol. 2011;187:6032–6042. doi: 10.4049/jimmunol.1101836. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69:315–330. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Mestecky J, Wira CR.Mucosal immunity in the human female reproductive tractIn: Lamm ME Mestecky J, Strober W, Bienenstock J, McGhee JR, Mayer L (ed.)Mucosal Immunology San Diego, CA: Elsevier Academic Press; 20051631–1646. [Google Scholar]
- Mestecky J, Moldoveanu Z, Smith PD, Hel Z, Alexander RC. Mucosal immunology of the genital and gastrointestinal tracts and HIV-1 infection. J Reprod Immunol. 2009;83:196–200. doi: 10.1016/j.jri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Roth KL, Bhavanam S, Jiang H, Gillgrass A, Ho K, Ferreira VH. Delayed but effective induction of mucosal memory immune responses against genital HSV-2 in the absence of secondary lymphoid organs. Mucosal Immunol. 2013;6:56–68. doi: 10.1038/mi.2012.48. [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses. 1998;14 Suppl 1:S51–S55. [PubMed] [Google Scholar]
- Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci USA. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol. 2008;181:969–975. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Enomoto LM, Kloberdanz KJ, Mack DG, Elizabeth D, Weinberg A. Ex vivo effect of estrogen and progesterone compared with dexamethasone on cell-mediated immunity of HIV-infected and uninfected subjects. J Acquir Immune Defic Syndr. 2007;45:137–143. doi: 10.1097/QAI.0b013e3180471bae. [DOI] [PubMed] [Google Scholar]
- Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by estrogen and progesterone. Biol Reprod. 1999;61:85–90. doi: 10.1095/biolreprod61.1.85. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kanzaki H, Imai K, Narukawa S, Katsuragawa H, Watanabe H. Progesterone stimulates the induction of human endometrial CD56+ lymphocytes in an in vitro culture system. J Clin Endocrinol Metab. 1996;81:1502–1507. doi: 10.1210/jcem.81.4.8636358. [DOI] [PubMed] [Google Scholar]
- Scanlan JM, Werner JJ, Legg RL, Laudenslager ML. Natural killer cell activity is reduced in association with oral contraceptive use. Psychoneuroendocrinology. 1995;20:281–287. doi: 10.1016/0306-4530(94)00059-j. [DOI] [PubMed] [Google Scholar]
- Yovel G, Shakhar K, Ben-Eliyahu S. The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol. 2001;81:254–262. doi: 10.1006/gyno.2001.6153. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- Mostad SB. Prevalence and correlates of HIV-1 shedding in female genital tract. AIDS Res Hum Retroviruses. 1999;1998:S11–S15. [PubMed] [Google Scholar]
- Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Zang YC, Halder JB, Hong J, Rivera VM, Zhang JZ. Regulatory effects of estradiol on T cell migration and cytokine profile: inhibition of transcription factor NF-kappa B. J Neuroimmunol. 2002;124:106–114. doi: 10.1016/s0165-5728(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Wira CR, Rossoll RM, Kaushic C. Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells. Endocrinology. 2002;141:2877–2885. doi: 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- Brabin L. Interactions of female hormonal environment, susceptibility to viral infection and disease progression. AIDS Patient Care STDS. 2002;16:211–221. doi: 10.1089/10872910252972267. [DOI] [PubMed] [Google Scholar]
- Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agace WW, Amara A, Roberts AI, Pablos JL, Thelen S, Uguccioni M. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–328. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Abdelwahab SF, Cocchi F, Bagley KC, Kamin-Lewis R, Gallo RC, DeVico A. HIV-1-suppressive factors are secreted by CD4+ T cells during primary immune responses. Proc Natl Acad Sci USA. 2003;100:15006–15010. doi: 10.1073/pnas.2035075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol. 2013;191:4246–4258. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- Pudney J, Anderson D. Innate and acquired immunity in the human penile urethra. J Reprod Immunol. 2011;88:219–227. doi: 10.1016/j.jri.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu Z, Huang WQ, Kulhavy R, Pate MS, Mestecky J. Human male genital tract secretions both mucosa and systemic immune compartments contribute to the humoral immunity. J Immunol. 2005;175:4127–4136. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- Anderson D, Politch JA, Pudney J. HIV infection and immune defense of the penis. Am J Reprod Immunol. 2011;65:220–229. doi: 10.1111/j.1600-0897.2010.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbschat A, Paulus P, Wiegratz I, Beschmann H, Hadji P, Hofmann R.Macrophage metalloelastase-12 is detectable in human seminal plasma and represents a predictor for inflammatory processes in the male genital tract Andrologia 2014. in press. [DOI] [PubMed]
- Smelov V, Eklund C, Bzhalava D, Novikov A, Dillner J. Expressed prostate secretions in the study of human papillomavirus epidemiology in the male. PloS ONE. 2013;8:e66630. doi: 10.1371/journal.pone.0066630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983;36:423–431. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Bacher M, Metz C, Bucala R, Wreford N, Lan H. Local regulation of macrophage subsets in the adult rat testis—examination of the roles of the seminiferous tubules, testosterone, and macrophage-migration inhibitory factor. Biol Reprod. 1998;59:371–378. doi: 10.1095/biolreprod59.2.371. [DOI] [PubMed] [Google Scholar]
- Filippini A, Riccioli A, Padula F, Lauretti P, D'Alessio A, De Cesaris P. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–449. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- Bronson R. Biology of the male reproductive tract: its cellular and morphological considerations. Am J Reprod Immunol. 2011;65:212–219. doi: 10.1111/j.1600-0897.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011;32:625–640. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Lycke NY. Immunology of the human genital tract. Curr Opin Infect Dis. 2003;16:43–49. doi: 10.1097/00001432-200302000-00008. [DOI] [PubMed] [Google Scholar]
- Mital P, Hinton BT, Dufour JM. The blood–testis and blood–epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–858. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Rosenquist TA, Takai Y, Bronson RA, Wimmer E. Loss of nectin-2 at Sertoli–spermatid junctions leads to male infertility and correlates with severe spermatozoan head and midpiece malformation, impaired binding to the zona pellucida, and oocyte penetration. Biol Reprod. 2003;69:1330–1340. doi: 10.1095/biolreprod.102.014670. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol. 1996;14:1692–1695. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes. 2000;49:1810–1818. doi: 10.2337/diabetes.49.11.1810. [DOI] [PubMed] [Google Scholar]
- Wyatt C, Law L, Magnuson J, Griswold M, Magnuson N. Suppression of lymphocyte proliferation by proteins secreted by cultured Sertoli cells. J Reprod Immunol. 1988;14:27–40. doi: 10.1016/0165-0378(88)90033-2. [DOI] [PubMed] [Google Scholar]
- de Cesaris P, Filippini A, Cervelli C, Riccioli A, Muci S, Starace G. Immunosuppressive molecules produced by Sertoli cells cultured in vitro: biological effects on lymphocytes. Biochem Biophys Res Commun. 1992;186:1639–1646. doi: 10.1016/s0006-291x(05)81596-7. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- Verhoeven G, Hoeben E, de Gendt K. Peritubular cell–Sertoli cell interactions: factors involved in PmodS activity. Andrologia. 2000;32:42–45. [PubMed] [Google Scholar]
- Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-α. Endocrinology. 2008;149:1678–1686. doi: 10.1210/en.2007-1064. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A, Dejucq-Rainsford N. HIV infection of the male genital tract–consequences for sexual transmission and reproduction. Int J Androl. 2010;33:e98–e108. doi: 10.1111/j.1365-2605.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raburn DJ, Coquelin A, Reinhart AJ, Hutson JC. Regulation of the macrophage population in postnatal rat testis. J Reprod Immunol. 1993;24:139–151. doi: 10.1016/0165-0378(93)90016-b. [DOI] [PubMed] [Google Scholar]
- Hedger M, Meinhardt A. Local regulation of T cell numbers and lymphocyte-inhibiting activity in the interstitial tissue of the adult rat testis. J Reprod Immunol. 2000;48:69–80. doi: 10.1016/s0165-0378(00)00071-1. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Mihara T, Okazaki T, Shitanaka M, Kushino R, Ikeda C. Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum Reprod. 2011;26:2799–2806. doi: 10.1093/humrep/der234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SH, Hamil KG, French FS. Host defense proteins of the review male reproductive tract. J Androl. 2001;23:585–597. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human Toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- Pudney J, Anderson DJ. Expression of Toll-like receptors in genital tract tissues from normal and HIV-infected men. Am J Reprod Immunol. 2011;65:28–43. doi: 10.1111/j.1600-0897.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J, Anderson DJ. Immunobiology of the human penile urethra. Am J Pathol. 1995;147:155–165. [PMC free article] [PubMed] [Google Scholar]
- Girling JE, Hedger MP. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol Cell Biol. 2007;85:481–489. doi: 10.1038/sj.icb.7100086. [DOI] [PubMed] [Google Scholar]
- Hakovirta H, Syed V, Jégou B, Parvinen M. Function of interleukin-6 as an inhibitor of meiotic DNA synthesis in the rat seminiferous epithelium. Mol Cell Endocrinol. 1995;108:193–198. doi: 10.1016/0303-7207(95)03475-m. [DOI] [PubMed] [Google Scholar]
- Lee NP, Yan Cheng C. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′, 5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- Parvinen M, Soder O, Mali P, Froysa B, Ritzen EM. In vitro stimulation of stage-specific deoxyribonucleic acid synthesis in rat seminiferous tubule segments by interleukin-l α. Endocrinology. 1991;129:1614–1620. doi: 10.1210/endo-129-3-1614. [DOI] [PubMed] [Google Scholar]
- Russo CL, Spurr-Michaud S, Tisdale A, Pudney J, Anderson D, Gipson IK. Mucin gene expression in human male urogenital tract epithelia. Hum Reprod. 2006;21:2783–2793. doi: 10.1093/humrep/del164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Ho SB, Spurr-Michaud SJ, Tisdale AS, Zhan Q, Torlakovic E. Mucin genes expressed by human female reproductive tract epithelia. Biol Reprod. 1997;56:999–1011. doi: 10.1095/biolreprod56.4.999. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cellular Biochem. 2007;102:1103–1116. doi: 10.1002/jcb.21539. [DOI] [PubMed] [Google Scholar]
- Porter E, et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell–neutrophil interactions. Infect Immun. 2005;73:4823–4833. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- García JR, Krause A, Schulz S, Rodríguez-Jiménez FJ, Klüver E, Adermann K. Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–1821. [PubMed] [Google Scholar]
- Gropp R, Frye M, Wagner TO, Bargon J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther. 1999;10:957–964. doi: 10.1089/10430349950018355. [DOI] [PubMed] [Google Scholar]
- Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- Schröder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- Tauber PF, Zaneveld LJ, Propping D, Schumacher GF. Components of human split ejaculates. II. Enzymes and proteinase inhibitors. J Reprod Fertil. 1976;46:165–171. doi: 10.1530/jrf.0.0460165. [DOI] [PubMed] [Google Scholar]
- Ohlsson K, Bjartell A, Lilja H. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J Androl. 1995;16:64–74. [PubMed] [Google Scholar]
- Levay PF, Viljoen M. Lactoferrin: a general review. Haematologica. 1995;80:252–267. [PubMed] [Google Scholar]
- Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Tack BF, McCray PB, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Sun B, Qi N, Shang T, Wu H, Deng T, Han D. Sertoli cell-initiated testicular innate immune response through Toll-like receptor-3 activation is negatively regulated by Tyro3, Axl, and mer receptors. Endocrinology. 2010;151:2886–2897. doi: 10.1210/en.2009-1498. [DOI] [PubMed] [Google Scholar]
- Wu H, Wang H, Xiong W, Chen S, Tang H, Han D. Expression patterns and functions of Toll-like receptors in mouse sertoli cells. Endocrinology. 2008;149:4402–4412. doi: 10.1210/en.2007-1776. [DOI] [PubMed] [Google Scholar]
- Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol. 2002;57:19–34. doi: 10.1016/s0165-0378(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Kern S, Maddocks S. Indomethacin blocks the immunosuppressive activity of rat testicular macrophages cultured in vitro. J Reprod Immunol. 1995;28:189–201. doi: 10.1016/0165-0378(95)91391-q. [DOI] [PubMed] [Google Scholar]
- Kern S, Robertson SA, Mau VJ, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biol Reprod. 1995;53:1407–1416. doi: 10.1095/biolreprod53.6.1407. [DOI] [PubMed] [Google Scholar]
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–117. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- Gerdprasert O, O'Bryan MK, Nikolic-Paterson DJ, Sebire K, de Kretser DM, Hedger MP. Expression of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor in normal and inflamed rat testis. Mol Hum Reprod. 2002;8:518–524. doi: 10.1093/molehr/8.6.518. [DOI] [PubMed] [Google Scholar]
- Breucker H. Macrophages, a normal component in seasonally involuting testes of the swan, Cygnus olor. Cell Tissue Res. 1978;193:463–471. doi: 10.1007/BF00225344. [DOI] [PubMed] [Google Scholar]
- Gerdprasert O, et al. The response of testicular leukocytes to lipopolysaccharide-induced inflammation: further evidence for heterogeneity of the testicular macrophage population. Cell Tissue Res. 2002;308:277–285. doi: 10.1007/s00441-002-0547-6. [DOI] [PubMed] [Google Scholar]
- Sanchez JF, et al. GABAergic lineage differentiation of AF5 neural progenitor cells in vitro. Cell Tissue Res. 2006;324:1–8. doi: 10.1007/s00441-005-0094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar R, Anton F, Bellido C, Aguilar E, Gaytan F. Testicular serotonin is related to mast cells but not to Leydig cells in the rat. J Endocrinol. 1995;146:15–21. doi: 10.1677/joe.0.1460015. [DOI] [PubMed] [Google Scholar]
- Abe M, Kurosawa M, Ishikawa I, Miyachi Y, Kido H. Mast cell tryptase stimulates both human dermal fibroblast proliferation and type 1 collagen production. Clin Exp Allergy. 1998;28:1509–1517. doi: 10.1046/j.1365-2222.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Jacobo P, Guazzone V, Jarazo-Dietrich S, Theas M, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol. 2009;81:44–54. doi: 10.1016/j.jri.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol. 1991;139:149–160. [PMC free article] [PubMed] [Google Scholar]
- Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod. 2007;22:2928–2935. doi: 10.1093/humrep/dem281. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Kelly RW, Harrison CM, McMillan LA, James K. Relative immunosuppressive activity of human seminal prostaglandins. J Reprod Immunol. 1992;22:185–195. doi: 10.1016/0165-0378(92)90015-v. [DOI] [PubMed] [Google Scholar]
- Kelly RW, Critchley HO. A T-helper-2 bias in decidua: the prostaglandin contribution of the macrophage and trophoblast. J Reprod Immunol. 1997;33:181–187. doi: 10.1016/s0165-0378(97)00021-1. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Prostaglandins in primate semen: biasing the immune system to benefit spermatozoa and virus. Prostaglandins Leukot Essent Fatty Acids. 1997;57:113–118. doi: 10.1016/s0952-3278(97)90000-4. [DOI] [PubMed] [Google Scholar]
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor β—a mediator of immune deviation in seminal plasma. J Reprod Immunol. 2002;57:109–128. doi: 10.1016/s0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- Gutsche S, von Wolff M, Strowitzki T, Thaler C. Seminal plasma induces mRNA expression of IL-1β, IL-6 and LIF in endometrial epithelial cells in vitro. Mol Hum Reprod. 2003;9:785–791. doi: 10.1093/molehr/gag095. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol. 2013;69:315–330. doi: 10.1111/aji.12107. [DOI] [PubMed] [Google Scholar]
- Sharkey DJ, Macpherson AM, Tremellen KP, Robertson SA. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol Hum Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- Kafka JK, Sheth PM, Nazli A, Osborne BJ, Kovacs C, Kaul R. Endometrial epithelial cell response to semen from HIV-infected men during different stages of infection is distinct and can drive HIV-1-long terminal repeat. AIDS. 2012;26:27–36. doi: 10.1097/QAD.0b013e32834e57b2. [DOI] [PubMed] [Google Scholar]
- Alexander R, Mestecky J. Neutralizing antibodies in mucosal secretions: IgG or IgA. Curr HIV Res. 2007;5:588–593. doi: 10.2174/157016207782418452. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–437. doi: 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood–testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett RJ, Hedger MP, McLachlan RI, Wreford N. The effects of gonadotropin-releasing hormone immunization and recombinant follicle-stimulating hormone on the Leydig cell and macrophage populations of the adult rat testis. J Androl. 1997;18:417–423. [PubMed] [Google Scholar]
- Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-γ expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–E863. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- Garolla A, Pizzol D, Bertoldo A, Menegazzo M, Barzon L, Foresta C. Sperm viral infection and male infertility: focus on HBV, HCV, HIV, HPV, HSV, HCMV, and AAV. J Reprod Immunol. 2013;100:20–29. doi: 10.1016/j.jri.2013.03.004. [DOI] [PubMed] [Google Scholar]
- UNAIDS . Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Cardona-Maya W, Velilla PA, Montoya CJ, Cadavid A, Rugeles MT. In vitro human immunodeficiency virus and sperm cell interaction mediated by the mannose receptor. J Reprod Immunol. 2011;92:1–7. doi: 10.1016/j.jri.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Eyre RC, Zheng G, Kiessling AA. Multiple drug resistance mutations in human immunodeficiency virus in semen but not blood of a man on antiretroviral therapy. Urology. 2000;55:591. doi: 10.1016/s0090-4295(99)00592-0. [DOI] [PubMed] [Google Scholar]
- Gupta P, Leroux C, Patterson BK, Kingsley L, Rinaldo C, Ding M. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral Quasi species between blood and semen. J Infect Dis. 2000;182:79–87. doi: 10.1086/315644. [DOI] [PubMed] [Google Scholar]
- Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. J Virol. 2005;79:1734–1742. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping LH, Cohen MS, Hoffman I, Vernazza P, Seillier-Moiseiwitsch F, Chakraborty H. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol. 2000;74:8946–8952. doi: 10.1128/jvi.74.19.8946-8952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- Münch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology. 2013;10:148. doi: 10.1186/1742-4690-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- Bourlet T, Cazorla C, Berthelot P, Grattard F, Cognasse F, Fresard A. Compartmentalization of HIV-1 according to antiretroviral therapy: viral loads are correlated in blood and semen but poorly in blood and saliva. AIDS. 2001;15:284–285. doi: 10.1097/00002030-200101260-00025. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- Sheth PM, et al. Persistent HIV RNA shedding in semen despite effective antiretroviral therapy. AIDS. 2009;23:2050–2054. doi: 10.1097/QAD.0b013e3283303e04. [DOI] [PubMed] [Google Scholar]
- Dejucq N, Jegou B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol Mol Biol Rev. 2001;65:208–231. doi: 10.1128/MMBR.65.2.208-231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmeyer T, Mueller V, von zur Muehlen A, Schmidt R. Endocrine testicular function in HIV-infected outpatients. Eur J Med Res. 1997;2:275–281. [PubMed] [Google Scholar]
- Christeff N, Gharakhanian S, Thobie N, Rozenbaum W, Nunez EA. Evidence for changes in adrenal and testicular steroids during HIV infection. J Acquir Immune Defic Syndr. 1992;5:841–846. [PubMed] [Google Scholar]
- Merenich JA, McDermott MT, Asp AA, Harrison SM, Kidd GS. Evidence of endocrine involvement early in the course of human immunodeficiency virus infection. J Clin Endocrinol Metab. 1990;70:566–571. doi: 10.1210/jcem-70-3-566. [DOI] [PubMed] [Google Scholar]
- CDC Male Circumcision Atlanta, GA: CDC; 2013. Available from: http://www.cdc.gov/hiv/prevention/research/malecircumcision/. [Google Scholar]
- Weiss HA, Quigley MA, Hayes RJ. Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS. 2000;14:2361–2370. doi: 10.1097/00002030-200010200-00018. [DOI] [PubMed] [Google Scholar]
- Siegfried N, Muller M, Deeks J, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men (Review) Cochrane Database Syst Rev. 2009. p. CD003362. [DOI] [PMC free article] [PubMed]
- Hirbod T, Bailey RC, Agot K, Moses S, Ndinya-Achola J, Murugu R. Abundant expression of HIV target cells and C-type lectin receptors in the foreskin tissue of young Kenyan men. Am J Pathol. 2010;176:2798–2805. doi: 10.2353/ajpath.2010.090926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161:867–873. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo R, Short RV. How does male circumcision protect against HIV infection. BMJ. 2000;320:1592–1594. doi: 10.1136/bmj.320.7249.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis. 2012;205:97–105. doi: 10.1093/infdis/jir700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatté J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol. 2007;81:13723–13734. doi: 10.1128/JVI.01079-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatte J, Wolfgang F, Ceballos A, Morelle W, Rodriguez Rodrigues C, Lenicov FR. Semen clusterin is a novel DC-SIGN ligand. J Immunol. 2011;187:5299–5309. doi: 10.4049/jimmunol.1101889. [DOI] [PubMed] [Google Scholar]
- Stax MJ, van Montforta T, Sprengerb RR, Melchersa M, Sandersa RW, van Leeuwen E. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4+ T-lymphocytes. Virology. 2009;391:203–211. doi: 10.1016/j.virol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Martellini JA, Cole AL, Venkataraman N, Quinn GA, Svoboda P, Gangrade BK. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J. 2009;23:3609–3618. doi: 10.1096/fj.09-131961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold F, Schnella J, Zirafia O, Stürzela C, Meierb C, Weil T. Naturally occurring fragments from two distinct regions of the prostatic acid phosphatase form amyloidogenic enhancers of HIV infection. J Virol. 2012;86:1244–1249. doi: 10.1128/JVI.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology. 2010;7:55. doi: 10.1186/1742-4690-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch J, Rücker E, Ständker L, Adermann K, Goffinet C, Schindler M. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Roan NR, Müller JA, Liu H, Chu S, Arnold F, Stürzel CM. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe. 2011;10:541–550. doi: 10.1016/j.chom.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roan NR, Münch J, Arhel N, Mothes W, Neidleman J, Kobayashi A. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol. 2009;83:73–80. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogi A, Presentini R, Moretti E, Strazza M, Piomboni P, Costantino-Ceccarini E. New insights into the interaction between the gp120 and the HIV receptor in human sperm (human. sperm/gp120/galactoglycerolipid/antigalactosylceramide/seminolipid/spermatogonia) J Reprod Immunol. 1998;41:213–231. doi: 10.1016/s0165-0378(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Gadella B, et al. Glycolipids as potential binding sites for HIV: topology in the sperm plasma membrane in relation to the regulation of membrane fusion. J Reprod Immunol. 1998;41:233–253. doi: 10.1016/s0165-0378(98)00061-8. [DOI] [PubMed] [Google Scholar]
- Denny TN, Skurnick JH, Garcia A, Perez G, Passannante MR, Colon J. Lymphocyte immunoregulatory cells present in semen from human immunodeficiency virus (HIV)-infected individuals: a report from the HIV Heterosexual Transmission Study. Cytometry. 1996;26:47–51. doi: 10.1002/(SICI)1097-0320(19960315)26:1<47::AID-CYTO7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A, Le Grand R, Denis H, Satie AP, Mannioui K, Roques P. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS ONE. 2008;3:e1792. doi: 10.1371/journal.pone.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politch JA, Mayer KH, Anderson DJ. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J Acquir Immune Defic Syndr. 1999;50:283. doi: 10.1097/QAI.0b013e3181989870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Stoecklin S, Gommet C, Corneau AB, Guenounou S, Torres C, Dejucq-Rainsford N. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in macaques. PLoS Pathog. 2013;9:e1003810. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]