Abstract
Background
It is poorly understood why many transforming proteins reportedly enhance both cell growth (transformation) and cell death (apoptosis). At first glance, the ability to transform and the ability to engender apoptosis seem to be contradictory. Interestingly, both abilities have been widely reported in the literature for the HTLV-I Tax protein.
Results
To reconcile these apparently divergent findings, we sought to understand how Tax might cause apoptosis in a Jurkat T-cell line, JPX-9. Tax expression can be induced equally by either cadmium (Cd) or zinc (Zn) in JPX-9 cells. Surprisingly, when induced by Zn, but not when induced by Cd, Tax-expression produced significant apoptosis. Under our experimental conditions, Zn but not Cd, induced SAPK (stress activated protein kinase)/JNK (Jun kinase) activation in cells. We further showed that transient over-expression of Tax-alone or Jun-alone did not induce cell death. On the other hand, co-expression of Tax plus Jun did effectively result in apoptosis.
Conclusion
We propose that Tax-expression alone in a T-cell background insufficiently accounts for apoptosis. On the other hand, Tax plus activation of a stress kinase can induce cell death. Thus, HTLV-I infection/transformation of cells requires two discrete events (i.e. oncoprotein expression and stress) to produce apoptosis.
Background
Human T-lymphotropic virus type I (HTLV-I) causes adult T-cell leukemia (ATL; reviewed in [1-3]). ATL develops in a minority of HTLV-I infected individuals with a long latent period. This pathological course suggests a multistage process of immortalization and transformation of T- lymphocyte. HTLV-I encodes a 40 kDa phosphoprotein, Tax. Tax immortalizes T- lymphocytes [4-6] and transforms rat fibroblasts [7,8]. Tax is also a transcriptional activator of the HTLV-I LTR [9-11]; reviewed in [12]. While the exact events leading to transformation are incompletely understood, several important cellular processes are dysregulated by Tax in parallel (reviewed in [1,13,14]. This is likely explained by the fact that Tax can activate NF-κB, SRF-, and CREB/ATF-responsive genes and can markedly accelerate cell cycle progression [15]; reviewed in [16].
The ability to transform and the ability to engender apoptosis seem to be contradictory functions. Interestingly, both abilities have been widely reported in the literature for the HTLV-I Tax protein. Tax has been shown to inhibit apoptosis [4,5,17-22]. On the other hand, Tax has also been shown to induce apoptosis [23-32]. Indeed Kao et al. showed that Tax sensitized cells to apoptotic cell death induced by DNA damaging agents [33]. It remains puzzling why Tax like many other oncoproteins seemingly enhances both cell growth (transformation) and cell death (apoptosis) ([34]).
To dissect the cell growth/death paradox relevant to HTLV-I, we sought to examine the requirements for Tax to cause apoptosis in a T-cell line, Jurkat. We used JPX-9, a stable transfectant of Jurkat that has incorporated a Tax-gene under the inducible control of a metallothionein promoter [35]. In JPX-9, Tax expression can be induced equally-well using either Cd or Zn. Intriguingly, we found that under our induction conditions the latter (Zn) but not the former (Cd) represented a stress stimulus. Thus, we observed marked activation of SAPK/JNK in our JPX-9 cells exposed to Zn, but not Cd. We propose that the combined effects of Tax expression and stress kinase activation perturb the cell growth/cell death equilibrium to favour the latter.
Results
Zinc, but not cadmium, treatment induces apoptosis in JPX-9 cells
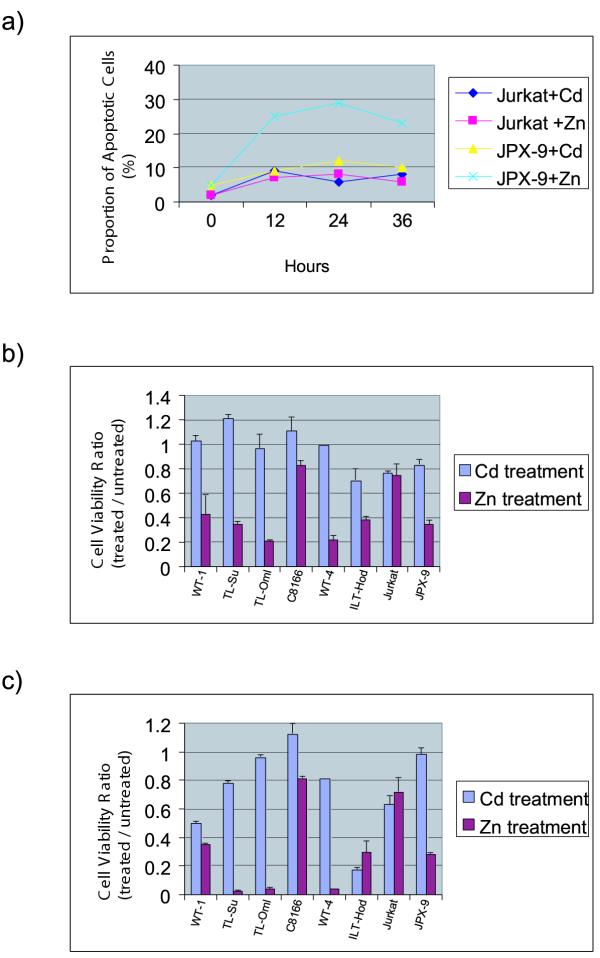
To examine the effect of Tax on the growth/death of Jurkat cells, we studied its induction by Zn or Cd in the JPX-9 cell line. As mentioned above, JPX-9 is a Jurkat derived cell line in which Tax expression is dictated by a metallothionein promoter. We treated JPX-9 or parental control Jurkat cells with either Zn or Cd. Interestingly, when nuclear morphologies were examined by staining with Hoechst 33258, we saw that JPX-9 cells treated with Zn showed significant apoptosis, while JPX-9 cells treated with Cd or parental Jurkat cells treated with either Zn or Cd were minimally affected (Figure 1A).
Figure 1.
Quantitation of apoptosis and viability in Jurkat and JPX-9 cells treated with ZnCl2 or CdCl2. A) JPX-9 cell treated with Zn show higher levels of apoptosis, while JPX-9 cell treated with Cd and Jurkat cell treated with either Zn or Cd showed lower levels. Y-axis is % apoptosis, and X-axis is hours after treatment. B) and C) Cell viability was quantified using a modified MTT colorimetric assay. Quantification of viability in HTLV-I transformed cell lines, as indicated, was after treatment with ZnCl2 or CdCl2 for 24 hours (B) or 48 hours (C). HTLV-I transformed cell lines treated with Zn showed lower cell viability compared to cells treated with Cd. Y-axes are % viability with 100% set as 1; X-axes indicate the name of the cell line.
We sought to independently confirm the finding of cell death in JPX-9 cells using a colorimetric MTT assay (see Materials and Methods) which measures cellular viability (Figure 1B,1C). To broaden the generality of our experiment, we also examined 6 HTLV-I transformed T-cells (WT-1, TL-Su, TL-Omi, C8166, WT-4, and ILT-Hod; Figure 1A,1B). Using MTT, we checked cellular viability of these HTLV-I transformed cells as well as Jurkat and JPX-9 treated with either Zn or Cd. Although there were some cell to cell variations, the overall trend from the HTLV-I and JPX-9 cells treated with Zn for 24 (Figure 1B) or 48 (Figure 1C) hours was one of lower viability as compared to counterparts treated with Cd. We noted that Jurkat was an exception in exhibiting no difference in viability between Cd or Zn treatment (Figure 1B,1C).
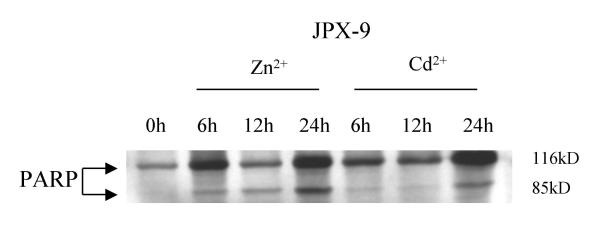
During apoptosis, the cellular 116 kDa nuclear enzyme poly(ADP-ribose) polymerase (PARP) is cleaved by caspase-3 to a smaller 85 kDa moiety [36]. To further characterize and confirm that cell death in JPX-9 was due to apoptosis, we investigated PARP cleavage by Western blotting using anti-PARP (Figure 2). Consistent with apoptotic death, we observed a higher degree of PARP processing in JPX-9 cells treated with Zn than cells treated with Cd (Figure 2).
Figure 2.
PARP cleavage analysis in JPX-9 by Western blotting with anti-PARP. PARP proenzyme (116 kD) and cleaved subunit (85 kD) are indicated on the left by arrows. JPX-9 cell treated with Zn showed higher cleaved/uncleaved PARP.
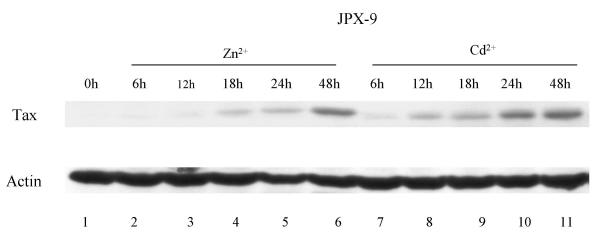
A factor common to all the HTLV-I transformed cells and JPX-9 (Figure 1B,1C) is the expression of Tax. Because JPX-9 cells treated by Zn or Cd should, in principle, induce Tax equally, we were puzzled by the divergent apoptotic phenotypes. To rule out that the variance in Cd- and Zn- apoptotic profiles in JPX-9 was trivially due to different efficiencies of Tax induction by the two cations, we directly examined the kinetics of Tax protein expression after Zn- or Cd- treatment. The results indicated essentially no difference in Tax induction by either Zn or Cd over the 24 to 48 hours treatment period (Figure 3). Thus, the Zn- vs. Cd- variance in JPX-9 apoptosis is unlikely explained simply by differences in Tax expression.
Figure 3.
Western blot analysis of Tax expression in the JPX-9 cells. Tax expression was equally induced by either Zn or Cd. Tax was detected with polyclonal anti-Tax [63]. Equal sample loading was verified with anti-actin (bottom).
Zn and Cd treatments activated SAPK/JNK differently
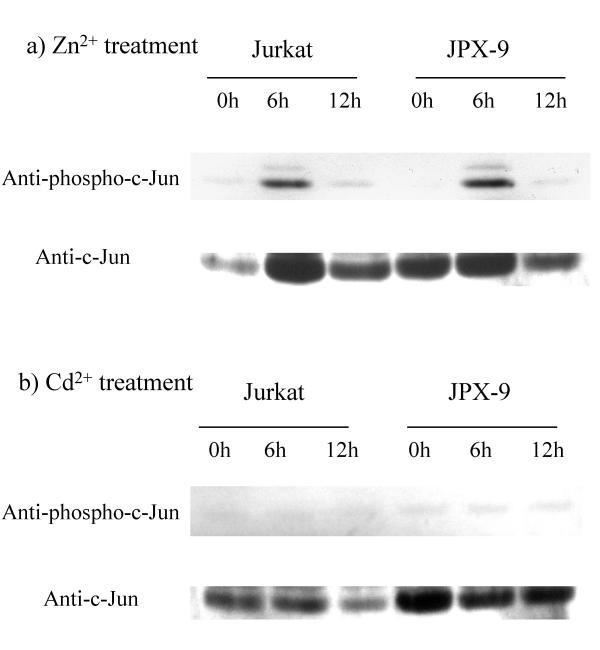
Activation of SAPK/JNK has been reported to play a role in the stress induction of cellular apoptosis [14,37,38]. We next investigated whether Zn and Cd may have different thresholds for activation of SAPK/JNK. Figure 4 shows sequential SAPK/JNK activity in Jurkat and JPX-9 cells upon treatment with Zn or Cd. We monitored the activation of JNK using anti-phospho-c-Jun specific antibody. Based on Western blotting results, SAPK/JNK was activated by phosphorylation in both Jurkat and JPX-9 cells after Zn, but not Cd, treatment. Hence, phospho-c-Jun was detected by 6 hours after treatment with Zn in JPX-9 and Jurkat cells (Figure 4A), but no such phosphorylation was seen with Cd treatment (Figure 4B). These findings suggest that JPX-9 cells treated with Zn would contain both activated SAPK/c-Jun and Tax, while the same cells treated with Cd would have only Tax.
Figure 4.
Zn activated phosphorylated SAPK/JNK in Jurkat and JPX-9 cells. Western blotting detected phosphorylated c-Jun within 6 hours after Zn treatment (A), but was not seen after Cd treatment (B). Anti-phospho-c-Jun was used to detect phosphorylated c-Jun while anti-c-Jun detected total c-Jun protein.
Different caspase profiles after Zn and Cd treatments
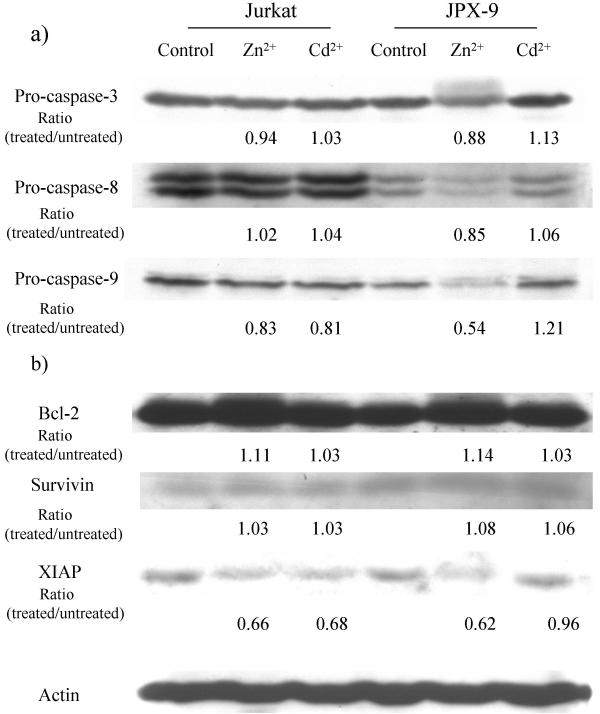
Because caspases are the effector proteases for apoptosis, we next investigated caspase profiles in Jurkat and JPX-9 cells after Zn and Cd treatments (Figure 5A). Activation of caspases 3, 8, and 9 requires the processing of pro-protein precursors to smaller active forms. To monitor the effects of Zn and Cd, we examined the integrity of pro-caspase 3, 8 and 9 in control and cation-treated Jurkat and JPX-9 cells. When compared to control, there was little reduction in the three pro-capases upon Cd treatment (Figure 5A). On the other hand, the levels of pro-caspase-3, -8 and -9 in JPX-9 cells treated with Zn were all decreased. Of particular note, pro-caspase 9 was reduced by approximately 50% in JPX-9 cells treated with Zn (i.e. 0.54; Figure 5A).
Figure 5.
Western blotting analyses of caspase-3, -8, -9, Bcl-2, survivin and XIAP in Jurkat and JPX-9 cells after Zn or Cd treatment. A) Enhanced processing of pro-caspase 9 in JPX-9 cells after Zn treatment. Expression of pro-caspase-3, -8, and -9 in Jurkat and JPX-9 cells were checked by Western blotting. Jurkat and JPX-9 cells were treated with ZnCl2 or CdCl2 for 24 hours, and the indicated proteins were detected using specific anti-sera. Ratio is the band intensity in treated sample versus untreated control. B) Expression of Bcl-2, survivin and XIAP in Jurkat and JPX-9 cells. Jurkat and JPX-9 cells were treated with ZnCl2 or CdCl2 for 24 hours. Note that XIAP expression in ZnCl2 treated JPX-9 cells was reduced while its expression in CdCl2-treated JPX-9 cells was maintained.
Bcl-2 [39], survivin [40], and XIAP [41] are three cellular anti-apoptotic factors. We also asked whether these three factors contribute to Zn-induced apoptosis of JPX-9 cells. Using specific antisera, we compared the levels of these three factors in untreated Jurkat/JPX-9 to their Zn or Cd-treated counterparts (Figure 5B). We saw no difference in Bcl-2 and survivin levels between JPX-9/Zn and JPX-9/Cd cells. However, XIAP level was more significantly reduced in JPX-9/Zn (0.62) than in JPX-9/Cd (0.96) cells, suggesting that this factor may contribute to Zn-induced apoptotic outcome (Figure 5B, right).
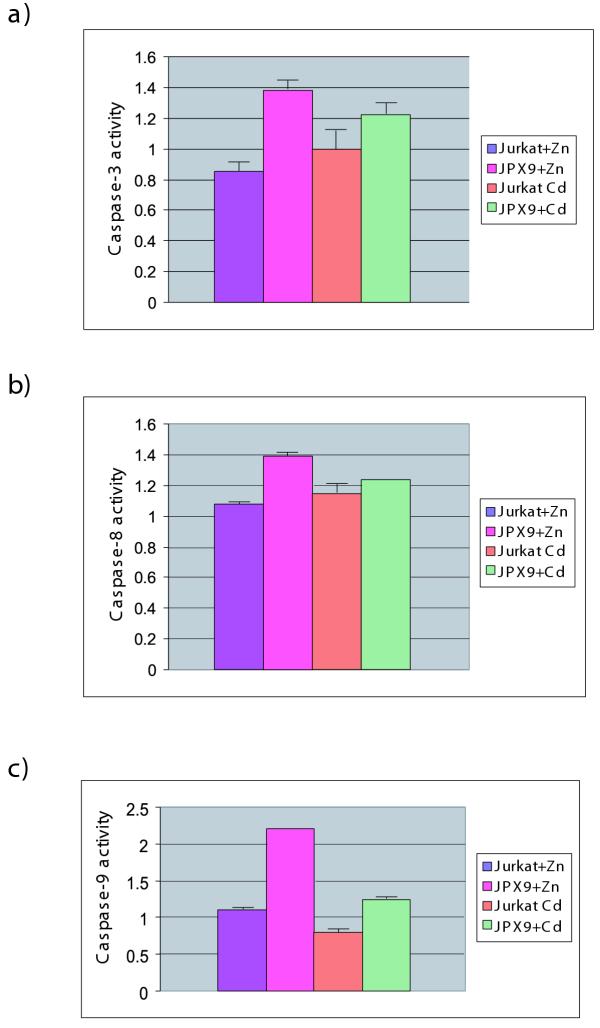
To confirm the Western blot results in figure 5A, we further quantified the enzymatic profiles of caspase 3, 8 and 9 using a spectrophotometric peptide cleavage assay. Compared to controls, we observed that capase 8 activity was mildly enhanced in JPX-9/Zn cells (Figure 6B) while caspase 3 (Figure 6A) and caspase 9 (Figure 6C) activities were more significantly increased.
Figure 6.
Enzymatic assays of caspases in JPX-9 cell treated with Zn or Cd. Spectrophotometric assays of caspase activities are as described in Methods. Caspase 3 (A), caspase 8 (B), and caspase 9 activities were measured in cells 24 hours after treatment. Caspase 9 activity was especially enhanced in JPX-9 cells treated with Zn (C).
Over-expression of Jun cooperates with Tax to induce apoptosis
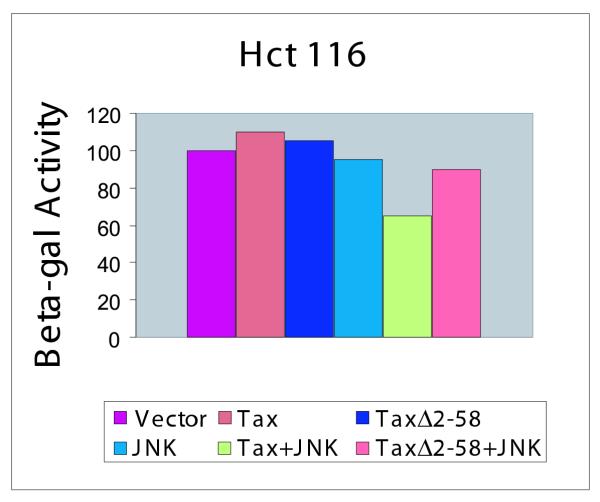
In other settings, SAPK/Jun activation has been shown to be involved in cellular apoptosis. Above data suggest that Tax expression alone is insufficient to cause cell death. On the other hand, our findings are compatible with Tax expression plus Jun activation cooperating to induce apoptosis in JPX-9 cells. We next performed transient transfection experiments in order to address the cooperativity between Tax and Jun. Because suspension cells are notoriously difficult to transfect with efficiency, in figure 7, we transiently transfected diploid Hct116 colon carcinoma cell line separately with vector control, Tax, inactive Tax mutant Δ2-58, pCMV-HA-JNK, Tax + pCMV-HA-JNK or TaxΔ2-58 + pCMV-HA-JNK. 48 hours after transfection, cells were examined morphologically for signs of apoptosis. At transfection efficiency where 50% of cells received DNA (data not shown), we observed that approximately 40% of Tax + JNK cells became apoptotic (Figure 7). Normalized to transfection efficiency, this suggested that 80% of all cells that received Tax + JNK succumbed to apoptosis. By contrast, no significant apoptosis was observed for either Tax-alone or JNK-alone suggesting that under the conditions employed neither is sufficient to elicit significant cell death (Figure 7).
Figure 7.
Induction of cell death in Hct116 cells by Tax + Jun kinase. Hct116 cells were transfected with pCMV-beta-gal and control plasmid pUC19 or the indicated plasmids. Reduction in beta-gal values reflects cell death. Cells transfected with CMV IE-driven JNK-expression plasmid (pCMV-HA-JNK) + CMV-Tax showed significantly lower level of beta-gal values, while cells with other combinations of transfected DNA showed higher levels. TaxΔ2-58 is an inactive Tax mutant. Transfection efficiencies achieved in the experiments were approximately 50%. Values represent averages from three independent experiments.
Discussion
Why oncoproteins seemingly enhance both cell growth (tranformation) and cell death (apoptosis) remain incompletely elucidated. Here, using HTLV-I Tax as a model we asked whether expression of this oncoprotein alone is sufficient to damage/stress the cell such as to provoke demise. Our findings suggest that Tax cannot singularly induce apoptosis efficiently in a T-cell line.
In an attempt to better understand HTLV-I biology, we sought to define the requirements for Tax to cause apoptosis in a Jurkat T-cell line. We used JPX-9, a stable transfectant of Jurkat in which Tax expression is controlled by a metallothionein promoter which can be equally activated by Zn or Cd. In this experimental background, we found that Tax-expression when induced by Zn, but not when induced by Cd, provoked highly significant apoptotic death at otherwise non-cytotoxic concentrations for each divalent cation-alone (Figure 1). Tax + Zn-induced apoptosis was most strongly associated with enhanced caspase 9 activity, although smaller increases in caspase 3 and caspase 8 were also observed (Figures 5A, 6). Currently, we do not know whether the caspase 9 findings reflect yet characterized mitochondrial toxicity of Tax.
How can one explain the different presentations for Zn and Cd in Tax-induced apoptosis? First, using phospho-specific antibody, we observed increased activation SAPK/JNK in cells exposed to Zn, while Cd exposure conducted in parallel did not activate SAPK/JNK. At the low dose (20 μM) used in our study, Cd has been shown not be perturb SAPK/JNK [42]. However, we caution that higher doses of Cd (i.e. >30 μM) can also activate SAPK/JNK. On the other hand, consistent with our results, acute exposure to Zn, as performed here, has also been reported to enhance SAPK/JNK activity in human bronchial epithelial cells [43]. While we used a higher concentration of Zn than Cd to induce JPX-9 cells, the salient point is that under conditions of equal induction of Tax, the former activated SAPK/JNK while the latter did not. With higher concentrations of Cd which did induce SAPK/JNK, Tax expression plus Cd treatment also produced apoptosis (data not shown). Hence the critical apoptosis requirement is Tax plus SAPK/JNK activation; and it matters not whether this occurs via Tax plus Zn or Tax plus Cd. We also noted with interest that similar to our findings (Figure 6A,6C), Zn activation of a death pathway in a human Burkitt lymphoma B cell line was associated with activation of caspase-9 and caspase-3 [44].
Consistent with our observations, several studies support that SAPK/JNK plays an important role in apoptosis [37,45-48]. A requirement for SAPK/JNK in apoptotic induction by UV irradiation was demonstrated using embryonic fibroblasts derived from a double-knockout mouse which lacked expression of both JNK1 and JNK2 [49]. Moreover, it was shown that ionizing radiation induced the translocation of JNK/SAPK to the mitochondria and the association of JNK/SAPK with Bcl-xL protein [50]. Additional factors required for UV and SAPK/JNK induced apoptosis include the cytochrome C effectors Apaf-1, caspase-9, and caspase-3 [51,52].
In a parallel oncoprotein system, Evan et al. had previously demonstrated that expression of c-Myc engendered apoptosis in serum-deprived rodent fibroblasts [53,54]. Related to these findings, Yu et al. found that Myc-dependent apoptosis was also associated with activation of JNK/SAPK [55]. Accordingly, Tax resembles Myc in that both proteins are transforming entities which share conditional apoptotic properties when expressed in the context of activated SAPK/JNK. One interpretation which emerges plausibly from our current work is that Tax primarily enforces changes in cellular metabolism for accelerated growth and transformation; however, these driving impulses may unwittingly dysregulate normal physiological balance to an extent that sensitizes cells to various pro-apoptotic insults. A similar interpretation has also been suggested for c-Myc [56].
Our work provides added insight into the various reports that Tax is both pro- and anti-apoptotic. We believe that Tax can provoke a pro-apoptotic phenotype in a setting when the cell is faced with an additional stress stimulus manifested through the JNK/SAPK cascade. On the other hand absent additional stress, Tax is primarily pro-survival through its effects on the NF-κB cascade [12]. Indeed, NF-κB has been clearly shown to serve a protective pro-survival role through its upregulation of anti-apoptotic genes [57-59]. Finally, the clinical presentation of ATL does argue that in contesting opposing effects the pro-transforming/pro-survival function of Tax ultimately prevails. Nevertheless, the extremely long latency (20 to 30 years) after HTLV-I infection required for ATL emergence suggests that most virally infected cells suffer apoptotic fates and that clonal escape from apoptosis to transformation is an exceedingly rare event.
Conclusions
Because Tax is a transforming protein, it seems unlikely that this oncoprotein's primary function is to induce apoptosis. Here, we show that Tax-alone, consistent with its oncogenic role, is insufficient to induce cell death in a Jurkat T-cell line. On the other hand, Tax plus a stress stimulus which activates SAPK/JNK can collectively cause apoptosis. Our work helps to reconcile the divergent reports that Tax is both apoptosis inducing and anti-apoptotic (i.e. transforming).
Methods
Cell culture
Jurkat cells (ATCC), and Tax-inducible JPX-9 and control JPX/M cells [35] were cultured in RPMI 1640 supplemented with 10% fetal calf serum (RPMI-FCS). Expression of Tax was induced by addition of ZnCl2 to 120 μM or CdCl2 to 20 μM, respectively. MT-I, TL-OmI, TL-Su, C8166, MT-4, and ILT-Hod are human HTLV-1-transformed T-cell lines (MT-I,TL-OmI, TL-Su, C8166, and MT-4 are IL-2 independent. ILT-Hod is IL-2 dependent.). ILT cell line was cultured in RPMI-FCS with 10 U/ml IL-2.
Apoptosis assay
Analysis of apoptotic cells was by Hoechst dye staining to characterize nuclear morphology. Cells were harvested at designated intervals up to 48 h. After harvesting, the cells were pelleted by centrifugation (1500 rpm, 5 minutes) and washed with PBS. The cell pellets were resuspended into 50 μl of 1% formaldehyde-0.2% glutaraldehyde. 20 μl of the cell suspension was dried on a poly-L-lysine coated slide. After wash with PBS, slides were stained with PBS containing 10 μg/ml of Hoechst 33258 (Sigma) for 10 minutes at room temperature. Fluorescence microscopy was used to assess the percentage of apoptotic cells. To measure the proportion of apoptotic cells, at least 300 cells were counted.
Cell survival assay
T-cells (5 × 104 cells/ml) in 96-well flat-bottom plates were preincubated for 24 h and then treated with ZnCl2 (120 μM) or CdCl2 (20 μM) at 37°C for 48 hours. Cells were harvested at 12 hour time intervals up to 48 hours. The number of viable cells in each clone was measured by a dye-reduction assay using WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) (Dojindo Molecular Technologies, Gaithersburg, MD, USA). Cell viability represented the ratio of WST-8 activity of cells treated with these drugs relative to that of untreated cells.
Western blotting
Cells were collected by centrifugation at 1,500 rpm, after washing in PBS. Then cells were lysed by the addition of extraction buffer and sonicated. Protein concentrations were determined using the Bio-Rad protein assay system (Bio-Rad, Richmond, CA, USA). Polyclonal anti- caspase-3, polyclonal anti-caspase-9, and monoclonal anti-caspase-8 were purchased from Pharmingen. Monoclonal anti-XIAP was purchased from Panvera. Polyclonal anti-survivin, -cIAP-1, and -cIAP-2, and monoclonal anti-PARP and anti-Bcl-2 were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti-actin (clone AC-15) was purchased from Sigma. Cell lysates were fractionated in 10% SDS-polyacrylamide gels prior to transfer to membrane (Immobilon-P; Millipore, Bedford, MA, USA) by standard protocol. Blots were visualized by chemiluminescence (Tropix, Bedford, MA, USA). c-Jun phosphorylation was selectively measured using a phospho-c-Jun antibody.
Caspase assays
Cells were grown in RPMI 1640 supplemented with 10% fetal calf serum (RPMI-FCS) and treated with ZnCl2 or CdCl2 for 24 hours. Cells (2 × 106) were collected by centrifugation at 200 × g for 10 minutes. Pellets were resuspended into 50 μl of cold cell lysis buffer provided in ApoAlert caspase colorimetric assay kits (Clontech, Palo Alto, CA) or caspase-9 colorimetric protease assay kit (Panvera/Takara). Cell lysates were microcentrifuged at 12,000 rpm for 3 min at 4°C and the supernatants were transferred to 96-well plates for detection of caspase-3 or caspase-8 activities. Caspase-3 and caspase-8 activities were measured using spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA and IETD-pNA, respectively. Caspase-9 activity was measured using spectrophotometric detection of the chromophore pNA after cleavage from the labeled substrate LEHD-pNA.
Transfection
For assay of cooperativity between JNK and Tax in the induction of apoptosis, we used colon cancer cell lines (Hct116) [60]. CMV IE-driven JNK-expression plasmid (pcDNA-HA-JNK) [61] and CMV-Tax and CMV-Tax mutant (Δ2-58) plasmids have been previously described [62]. Cells were transfected with CMV-beta-gal and either control plasmid pUC19 or the indicated combination of plasmids. Beta-gal activities were measured 24 hours after transfection. Individual beta-gal values are expressed relative to the value from cells transfected with CMV-beta-gal and control pUC19 plasmid. Reduction in beta-gal values was quantitated as a reflection of cell death.
Competing interests
None declared.
Authors' contributions
TKperformed most of the experiments. Both TK and KTJ participated in experimental design, data interpretation and writing of manuscript.
Acknowledgments
Acknowledgements
We thank Lan Lin for help with preparation of manuscript and figure, and RK Yedavalli for assistance with reference formatting.
Contributor Information
Takefumi Kasai, Email: tkasai@m.kufm.kagoshima-u.ac.jp.
Kuan-Teh Jeang, Email: kj7e@nih.gov.
References
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19:475–496. doi: 10.1146/annurev.immunol.19.1.475. [DOI] [PubMed] [Google Scholar]
- Poiesz BJ, Poiesz MJ, Choi D. The human T-cell lymphoma/leukemia viruses. Cancer Invest. 2003;21:253–277. doi: 10.1081/CNV-120016422. [DOI] [PubMed] [Google Scholar]
- Matsuoka M. Human T-cell leukemia virus type I and adult T-cell leukemia. Oncogene. 2003;22:5131–5140. doi: 10.1038/sj.onc.1206551. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Berchtold S, Radant I, Alt M, Fleckenstein B, Sodroski JG. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol. 1992;66:4570–4575. doi: 10.1128/jvi.66.7.4570-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar MC. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a Herpesvirus saimiri vector. Proc Natl Acad Sci U S A. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin O, Koch C, Schmitt I, Semmes OJ, Jeang KT, Grassmann R. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J Biol Chem. 1998;273:6698–6703. doi: 10.1074/jbc.273.12.6698. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci U S A. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Shibata H, Fujisawa JI, Inoue H, Hakura A, Tsukahara T, Fujii M. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J Virol. 1997;71:4445–4451. doi: 10.1128/jvi.71.6.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J, Jeang KT, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M, Inoue J, Takeda T, Hikikoshi A, Sato M, Yoshida M. The p40x of human T-cell leukemia virus type I is a trans-acting activator of viral gene transcription. Jpn J Cancer Res. 1985;76:1127–1131. [PubMed] [Google Scholar]
- Jeang KT, Boros I, Brady J, Radonovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62:4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang KT. Functional activities of the human T-cell leukemia virus type I Tax oncoprotein: cellular signaling through NF-kappa B. Cytokine Growth Factor Rev. 2001;12:207–217. doi: 10.1016/S1359-6101(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Marriott SJ, Lemoine FJ, Jeang KT. Damaged DNA and miscounted chromosomes: human T cell leukemia virus type I tax oncoprotein and genetic lesions in transformed cells. J Biomed Sci. 2002;9:292–298. doi: 10.1159/000064998. [DOI] [PubMed] [Google Scholar]
- Jeang KT, Giam CZ, Majone F, Aboud M. Life, Death and Tax: role of HTLV-I oncoprotein in genetic instability and cellular transformation. J Biol Chem. 2004 doi: 10.1074/jbc.R400009200. [DOI] [PubMed] [Google Scholar]
- Neuveut C, Low KG, Maldarelli F, Schmitt I, Majone F, Grassmann R, Jeang KT. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol Cell Biol. 1998;18:3620–3632. doi: 10.1128/mcb.18.6.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuveut C, Jeang KT. HTLV-I Tax and cell cycle progression. Prog Cell Cycle Res. 2000;4:157–162. doi: 10.1007/978-1-4615-4253-7_14. [DOI] [PubMed] [Google Scholar]
- Brauweiler A, Garrus JE, Reed JC, Nyborg JK. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology. 1997;231:135–140. doi: 10.1006/viro.1997.8509. [DOI] [PubMed] [Google Scholar]
- Mulloy JC, Kislyakova T, Cereseto A, Casareto L, LoMonico A, Fullen J. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J Virol. 1998;72:8852–8860. doi: 10.1128/jvi.72.11.8852-8860.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73:7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Nakashima T, Sakai H, Urayama S, Yamasaki S, Hida A. Inhibition of caspase cascade by HTLV-I tax through induction of NF-kappaB nuclear translocation. Blood. 1999;94:3847–3854. [PubMed] [Google Scholar]
- Nakashima K, Kawakami A, Hida A, Yamasaki S, Nakamura H, Kamachi M. Protection of mitochondrial perturbation by human T-lymphotropic virus type 1 tax through induction of Bcl-xL expression. J Lab Clin Med. 2003;142:341–347. doi: 10.1016/S0022-2143(03)00134-3. [DOI] [PubMed] [Google Scholar]
- Saggioro D, Acquasaliente L, Daprai L, Chieco-Bianchi L. Inhibition of apoptosis by human T-lymphotropic virus type-1 tax protein. Ann N Y Acad Sci. 2003;1010:591–597. doi: 10.1196/annals.1299.111. [DOI] [PubMed] [Google Scholar]
- Chen X, Zachar V, Zdravkovic M, Guo M, Ebbesen P, Liu X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J Gen Virol. 1997;78:3277–3285. doi: 10.1099/0022-1317-78-12-3277. [DOI] [PubMed] [Google Scholar]
- Chlichlia K, Los M, Schulze-Osthoff K, Gazzolo L, Schirrmacher V, Khazaie K. Redox events in HTLV-1 Tax-induced apoptotic T-cell death. Antioxid Redox Signal. 2002;4:471–477. doi: 10.1089/15230860260196263. [DOI] [PubMed] [Google Scholar]
- Chlichlia K, Busslinger M, Peter ME, Walczak H, Krammer PH, Schirrmacher V. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene. 1997;14:2265–2272. doi: 10.1038/sj.onc.1201070. [DOI] [PubMed] [Google Scholar]
- Fujita M, Shiku H. Differences in sensitivity to induction of apoptosis among rat fibroblast cells transformed by HTLV-I tax gene or cellular nuclear oncogenes. Oncogene. 1995;11:15–20. [PubMed] [Google Scholar]
- Hall AP, Irvine J, Blyth K, Cameron ER, Onions DE, Campbell ME. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J Pathol. 1998;186:209–214. doi: 10.1002/(SICI)1096-9896(1998100)186:2<209::AID-PATH162>3.3.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kitajima I, Nakajima T, Imamura T, Takasaki I, Kawahara K, Okano T. Induction of apoptosis in murine clonal osteoblasts expressed by human T-cell leukemia virus type I tax by NF-kappa B and TNF-alpha. J Bone Miner Res. 1996;11:200–210. doi: 10.1002/jbmr.5650110209. [DOI] [PubMed] [Google Scholar]
- Los M, Khazaie K, Schulze-Osthoff K, Baeuerle PA, Schirrmacher V, Chlichlia K. Human T cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J Immunol. 1998;161:3050–3055. [PubMed] [Google Scholar]
- Kao SY, Lemoine FJ, Mariott SJ. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene. 2000;19:2240–2248. doi: 10.1038/sj.onc.1203559. [DOI] [PubMed] [Google Scholar]
- Nicot C, Harrod R. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol Cell Biol. 2000;20:8580–8589. doi: 10.1128/MCB.20.22.8580-8589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Yamaoka S, Goto T, Nakai M, Tsujimoto Y, Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994;68:3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao SY, Lemoine FJ, Marriott SJ. p53-independent induction of apoptosis by the HTLV-I tax protein following UV irradiation. Virology. 2001;291:292–298. doi: 10.1006/viro.2001.1200. [DOI] [PubMed] [Google Scholar]
- Nilsson JA, Cleveland JL. Myc pathways provoking cell suicide and cancer. Oncogene. 2003;22:9007–9021. doi: 10.1038/sj.onc.1207261. [DOI] [PubMed] [Google Scholar]
- Nagata K, Ohtani K, Nakamura M, Sugamura K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J Virol. 1989;63:3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Chen SL, Tsao YP, Chen YL, Huang SJ, Chang JL, Wu SF. The induction of apoptosis by SV40 T antigen correlates with c-jun overexpression. Virology. 1998;244:521–529. doi: 10.1006/viro.1998.9109. [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez I, Benitah SA, Martinez-Gomariz M, Lacal JC, Perona R. Cell stress and MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell viability. Mol Biol Cell. 2002;13:2933–2945. doi: 10.1091/mbc.E02-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568–8580. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Cohen GM. Death receptors leave a caspase footprint that Smacs of XIAP. Cell Death Differ. 2003;10:4–6. doi: 10.1038/sj.cdd.4401176. [DOI] [PubMed] [Google Scholar]
- Chuang SM, Wang IC, Yang JL. Roles of JNK, p38 and ERK mitogen-activated protein kinases in the growth inhibition and apoptosis induced by cadmium. Carcinogenesis. 2000;21:1423–1432. doi: 10.1093/carcin/21.7.1423. [DOI] [PubMed] [Google Scholar]
- Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am J Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- Schrantz N, Auffredou MT, Bourgeade MF, Besnault L, Leca G, Vazquez A. Zinc-mediated regulation of caspases activity: dose-dependent inhibition or activation of caspase-3 in the human Burkitt lymphoma B cells (Ramos) Cell Death Differ. 2001;8:152–161. doi: 10.1038/sj.cdd.4400772. [DOI] [PubMed] [Google Scholar]
- Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Eilers A, Whitfield J, Babij C, Rubin LL, Ham J. Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J Neurosci. 1998;18:1713–1724. doi: 10.1523/JNEUROSCI.18-05-01713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/S0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/S0092-8674(00)81733-X. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-T. [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bennett MR, Fanidi A, Evan GI. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Ravera CP, Chen YN, McMahon G. Regulation of Myc-dependent apoptosis by p53, c-Jun N-terminal kinases/stress-activated protein kinases, and Mdm-2. Cell Growth Differ. 1997;8:731–742. [PubMed] [Google Scholar]
- Juin P, Hueber AO, Littlewood T, Evan G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–1381. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275:22064–22068. doi: 10.1074/jbc.M910346199. [DOI] [PubMed] [Google Scholar]
- Bunz F, Fauth C, Speicher MR, Dutriaux A, Sedivy JM, Kinzler KW. Targeted inactivation of p53 in human cells does not result in aneuploidy. Cancer Res. 2002;62:1129–1133. [PubMed] [Google Scholar]
- Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem. 1996;271:27225–27228. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- Semmes OJ, Jeang KT. Mutational analysis of human T-cell leukemia virus type I Tax: regions necessary for function determined with 47 mutant proteins. J Virol. 1992;66:7183–7192. doi: 10.1128/jvi.66.12.7183-7192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]