Abstract
Background
The trend of extended-spectrum beta-lactamases producing Escherichia coli (ESBL-EC) is increasing in Nepal. Limited studies have been reported investigating ESBL types and carbapenemases in E. coli.
Methods
A cross sectional study was conducted between June 2012 to January 2013 in Kathmandu Medical College and Teaching Hospital, Nepal. Non-repetitive clinical samples from out-patient department (OPD) and Intensive Care Units (ICU) were processed for bacteriological culture and identification of E. coli. Antibiotic susceptibility test, screening and phenotypic confirmation for ESBLs and carbapenemases and PCR (blaCTX-M, blaSHV and blaTEM-type ESBLs, blaVIM, blaIMP and blaNDM-1-type carbapenemases, and class 1 integron element integrase gene) were performed. Clones were resolved by PCR-Randomly Amplified Polymorphic DNA.
Results
Out of 332 non-repetitive clinical specimens processed for culture and identification 160 (48.2%) were culture positive. Of which, 93 (58.1%) were E. coli. Of these, 24 (25.8%) were phenotypically confirmed as ESBL-EC and 3 (12.50%) of 24 ESBL-EC were carbapenemase producers. blaCTX-M-type ESBL was most common (23, 95.8%) followed by blaTEM (7, 29.2%) and blaSHV (3, 12.5%). blaVIM, blaIMP and blaNDM-1 were present in 3, 2 and 2 ESBL-EC, respectively. Class 1 integron element was present in 18 (75.0%) ESBL-EC. Nine isolates possessed more than one type of beta-lactamases. Interestingly, all carbapenemase producers were isolated form ICU and co-existence of blaCTX-M, blaSHV, blaTEM, blaIMP, blaVIM and blaNDM-1 beta-lactamases was documented in one ESBL-EC (EC104). All most all isolates had different RAPD patterns.
Conclusions
For the first time in Nepal, high prevalence of blaCTX-M-type ESBL and co-existence of ESBLs and carbapenemases has been described. Continuous monitoring and surveillance and proper infection control and prevention practices will limit the further spread of these super-bugs within this hospital and beyond.
Keywords: ESBL producing Escherichia coli, Carbapenemases, Clinical isolates, Integron element
Background
Escherichia coli is associated with numerous infections such as urinary tract infection, surgical site infection, skin and soft tissues infection, bacteraemia, pneumonia etc [1–3]. These infections are common among out-patient and Intensive Care Unit (ICU) admitted patients [4]. ICU patients are subjected to numerous invasive procedures and are susceptible to ICU acquired infections (IAI) and Escherichia coli is a common pathogen [5]. Extended-spectrum beta-lactam antibiotics-third generation cephalosporins- are commonly used for treating infections caused by Escherichia coli in Kathmandu Medical College and Teaching Hospital (KMCTH), Nepal. These antibiotics are less effective as Extended-spectrum beta-lactamase producing isolates of E. coli (ESBL-EC) is increasing in this institution [6]. Carbapenems are current choice for treating the infection caused by ESBL-EC however, emergence of carbapenem-resistant isolates has also been noticed [7]. Hence, the successful treatment outcome of E. coli infections is seriously tempered by these ESBL- and carbapenem-resistance.
Increase trends of ESBL and carbapenem-resistance over the past two decades has been noticed globally and also in Nepal [6, 8, 9]. Several variants of ESBLs; TEM, SHV and CTX-M have been described however; there is paucity in studies of ESBL and carbapenemases types from this institution and Nepal. This study aims to detect common ESBLs (blaTEM, blaSHV, and blaCTX-M) and carbapenemases (blaIMP, blaVIM, and blaNDM) in ESBL-EC isolated from Kathmandu Medical College Teaching Hospital, Nepal. Here, we describe high prevalence of blaCTX-M type ESBL and carbapenemases producing E. coli and co-existence of ESBLs and carbapenemases in these isolates.
Methods
Specimens, inclusion criteria and identification of E. coliisolates
Non-repetitive clinical specimens (mid-stream urine, pus, discharge from surgical wound, endotracheal secretions, sputum, catheter tips etc.) received as part of standard patient care investigation from Intensive Care Unit (ICU) and out-patient department (OPD) in Kathmandu Medical College and Teaching Hospital between June 2012 to January 2013 were processed for culture and antibiotic susceptibility testing from patients attending OPD and admitted in ICU were included in the study. The patients already on antibiotics were excluded based on the history of antibiotics mentioned in the culture investigation form. E. coli isolates were isolated and identified using standard microbiological technique [5].
Identification of E. coliisolates
All specimens were cultured on MacConkey and blood agar and incubated overnight at 37°C in the department of microbiology, KMCTH using standard microbiological technique [10]. On grown lactose fermenting colonies biochemical tests was performed to identify E. coli.
Anti-microbial susceptibility technique
Kirby-Bauer disk diffusion test was performed on identified E. coli on Mueller-Hinton agar according to Clinical and Laboratory Standards Institute guidelines (CLSI) [11]. The following antibiotic disks procured from Hi Media Pvt. Ltd., India were used; ampicillin (20 μg) nalidixic acid (30 μg), amoxycillin (30 μg), amoxycillin-clavulinic acid (30 μg/10 μg), cefepime (30 μg), cefotaxime (30 μg), ceftriaxone (30 μg) and ceftazidime (30 μg), amikacin (30 μg), tobramycin (30 μg), gentamycin (10 μg) and imipenem (10 μg).
Screening and phenotypic confirmation of ESBL and carbapenemase producers
The screening for ESBLs producers were performed using cefotaxime (30 μg), ceftazidime (30 μg) and ceftriaxone (30 μg) and interpreted based on CLSI guidelines [11]. Phenotypic confirmation of ESBL producers were confirmed using ceftazidime disc (30 μg) alone and in combination with clavulanic acid (10 μg). Similarly, imipenem resistant E. coli were confirmed for carbapenemase production by modified Hodge test [11].
Genotype confirmation of ESBLs and carbapenemases
Crude DNA was extracted from pure culture of E. coli. Briefly, fresh culture of the test organism was suspended in 500 μl of saline, vortexed, boiled for 10 minutes, cellular debris was removed by centrifugation at 11,000 rpm for 5 min and supernatant was used as DNA template for PCR analysis. PCR amplification of the drug resistance genes like blaTEM, blaSHV, blaCTX-M,blaIMP, blaVIM and blaNDM-1 was performed using gene specific primers (Table 1) and amplification profiles described earlier [12–14]. The PCR was performed in Genesis Laboratory and Research, Kathmandu, Nepal.
Table 1.
Primers used in this study
| Name of the primer | Target | Amplicon (bp) | Sequence 5′-3′ |
|---|---|---|---|
| TEM1F | bla TEM | 864 | ATGAGTATTCAACATTTCCG |
| TEM1R | CTGACAGTTACCAATGCTTA | ||
| SHVF | bla SHV | 865 | GGTTATGCGTTATATTCGCC |
| SHVR | TTAGCGTTGCCAGTGCTC | ||
| CTX-MA1 | bla CTX-M | 544 | *SCSATGTGCAG≠YACCAGTAA |
| CTX-MA2 | CCGC¥RATATGRTTGGTGGTG | ||
| IMP F | bla IMP | 569 | TTGCCAGATTTAAAAAT |
| IMP 003 | ACCAGTTTTGCCTTACCATA | ||
| VIM F | bla VIM | 551 | GTCTACCCGTCCAATGGTCTCA |
| VIM R | AGCAAGTCTAGACCGCCCG | ||
| NDM-1 F | bla NDM-1 | 593 | GGTTTGGCGATCTGGTTTTC |
| NDM-1 R | CGGAATGGCTCATCACGATC | ||
| IntI1F | intI1 | 471 | AAGGATCGGGCCTTGATGTT |
| IntI1R | CAGCGCATCAAGCGGTGAGC |
Note: *S = G or C, ≠Y = C or T, ¥R = A or T.
Controls
ESBL negative E. coli (ATCC 25922), ESBL positive K. pneumoniae (ATCC 700603) and Imipenemase producing Pseudomonas aeruginosa were used as controls in disk diffusion test, screening and confirmation tests. Multiple strains of P. aeruginosa genetically characterized to produce TEM, CTX-M, SHV, IMP, VIM and NDM-1 were used as positive controls for PCR.
Clonal analysis
Polymerase Chain Reaction-Randomly Amplified Polymorphic DNA (PCR-RAPD) was to study the clonal nature of these isolates as described previously [13].
Data analysis and ethical approval
The data is presented in frequency and percentages. The study was approved by the Institutional Ethical Review Board of Kathmandu Medical College and Teaching Hospital, Kathmandu, Nepal.
Results
During the study period, 332 non-repetitive clinical samples [n = 292 (OPD) and n = 40 (ICU)] were processed and 160 samples (48.2%) [n = 130 (OPD) and n = 30 (ICU)] were culture positive. These specimens were received from urinary tract infections (110, 68.8%), respiratory tract infections (19, 11.9%), surgical site infection (16, 10.0%), skin and soft tissue infection (12, 7.5%) and blood stream infection (3, 1.9%) and E. coli was predominant pathogen (93, 58.1%). E. coli were derived mostly from urinary tract infection (80, 86.0%). Of which, 24 (25.8%) were phenotypically confirmed as ESBL-EC among which 10 ESBL producing isolates were from ICU. All ESBL-EC were found to be resistant to nalidixic acid, amoxycillin, cefepime, cefotaxime, ceftriaxone and ceftazidime. ESBL-EC were also resistant to amoxicillin-clavulinic acid (23, 95.8%), cotrimoxazole (21, 87.5%), imipenem (3, 12.5%), gentamicin (2, 8.3%), amikacin (1, 4.2%) and tobramycin (1, 4.2%).
blaCTX-M was the most prevalent ESBLs (23, 95.8%) followed by blaTEM (7, 29.2%) and blaSHV (3, 12.5%) (Table 2). Among ESBL-EC, 3 (12.5%) (EC100, EC104 and EC107) isolates were screened and confirmed as cabapenemase producers. All these 3 isolates were from ICU. blaVIM was present in all of these isolates , blaNDM-1 was present in 2 isolates (EC100 and EC104) and blaIMP was present in 2 isolates (EC104 and EC 107). Strikingly, carbapenemase harboring isolates were found to contain more than one resistant gene under the study. Co-existence of ESBLs and carbapenemases among ESBL-EC was variable (Table 2). An EC104 harboured all resistant genes investigated. Class 1 integron element was prevalent among the ESBL producers (18, 75.0%).
Table 2.
Distribution of ESBL, carbapenemase and IntI1 in ESBL producing E. coli
| ESBL producing E. coli | bla CTX-M | bla SHV | bla TEM | bla NDM-1 | bla IMP | bla VIM | IntI 1 |
|---|---|---|---|---|---|---|---|
| EC17, -23, -42, -103, -105, -106, -108, -205, -208,-209 | + | - | - | - | - | - | + |
| EC206, -207, -210, -211 | + | - | - | - | - | - | - |
| EC98, -102 | + | + | - | - | - | - | + |
| EC202, -204 | + | - | + | - | - | - | + |
| EC203, -212 | + | - | + | - | - | - | - |
| EC100 | + | - | + | + | - | + | + |
| EC104 | + | + | + | + | + | + | + |
| EC107 | + | - | + | - | + | + | + |
| EC201 | - | - | - | - | - | - | + |
All isolates of E. coli were subjected for RAPD. The isolates showed different RAPD patterns. Isolates EC106 & EC107 possessed similar RAPD patterns and rest of the isolates had individual RAPD patters (Figure 1).
Figure 1.
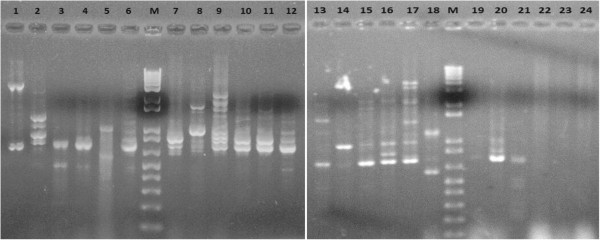
Gel electrophoresis of PCR amplicons of RAPD patterns. Lane M: Molecular weight marker (1 kb+ Invitrogen). Lane 1-24: E.coli isolates, EC17, EC23, EC42, EC98, EC100, EC102, EC103, EC104, EC105, EC106, EC107, EC108, EC201, EC202, EC203, EC204, EC205, EC206, EC207, EC208, EC209, EC210, EC211 and EC212.
Discussion
E. coli is responsible for numerous infections and is frequently involved in sepsis and other infections in OPD and critically ill patients in Intensive Care Units (ICU) [5, 15]. The emergence of ESBL producing E. coli (ESBL-EC) is a real challenge for infectious disease medicine as these bugs are increasingly detected worldwide particularly in ICU [15, 16]. ESBL-EC infections ultimately results in unavoidable treatment outcomes and increases the cost in patients. In this study, 58.1% (93/160) of the infections were caused by E. coli. Of these infections, 25.8% (24/93) and 3.2% (3/93) were caused by ESBL-EC and carbapenemase producing E. coli, respectively. Frequency of isolation of E. coli is also common in ICUs elsewhere however infections due to ESBL-EC and carbapenemase producer vary among different geographical regions [5, 17]. Since 2000, the already ubiquitous E. coli has emerged as major ESBL producing organism. In 2007, already 79% of E. coli isolates collected in India were positive for ESBLs, with almost identical prevalence in hospital and community [18], 55% in China and 50.8% in Thailand [17]. ESBL-EC in ICU are increasing than general wards and out patients in this institution which is worrisome [6].
We have detected a variety of beta-lactamases among the isolates of E. coli namely blaCTX-M, blaTEM, blaSHV-type enzymes. The blaCTX-M type was the most prevalent ESBL (n = 23). The incidence of this enzyme surpasses those of blaTEM and blaSHV-type ESBLs in most locales worldwide [19] and also in our study. The wider spread of blaCTX-M is also due to over use of third generation cephalosporins which has selected these strain. Some of the blaCTX-M types are also associated with mobile genetic elements like class 1 integron element which contributes to its wider spread [20]. This was also evident in this study as 18 out of 23 blaCTX-M positive isolates possessed class 1 integron element. However, the location of blaCTX-M in class 1 element was not studied and needs further investigation. One of the isolate was ESBL-EC on screening and phenotypic test but didn’t possessed CTX-M, TEM and SHV enzymes, other ESBLs (AmpC) or other mechanisms could be possible [21].
The prevalence of blaCTX-M, blaTEM and blaSHV- type ESBLs in E. coli is variable across different cities, countries and regions [22]. The prevalence of blaCTX-M and blaSHV genes was reported as 83% and 28%, respectively in ESBL-EC in New York [23] and 22.7% and 9.1%, respectively in ESBL-EC in Turkey [24]. Similarly, prevalence of blaCTX-M and blaTEM was 11% and 50%, respectively in Pakistan [25]. However, the blaCTX-M has displaced other ESBLs in this geographical location as demonstrated in this study and also in Eastern Europe, South America, Japan and India [26].
The important finding in the study was the co-existence of different ESBLs and carbapenemases in the same isolate. Of 24 ESBL-EC, 9 (37.5%) possessed more than one ESBLs. Study in Taiwan reported co-existence of two or more kinds of ESBL in 40.6% of ESBL-EC [27]. Similarly, co-existence of blaCTX-M and blaTEM was found in 52.6% of French ESBL-EC [28]. Co-existence of NDM-1 and OXA-76 has been described in Klebsiella pneumoniae isolates from Nepal [29]. Carbapenemase producers were found to harbor carbapenemases co-existing with ESBLs. Each of EC104, EC100 and EC107 possessed blaCTX-M + blaTEM + blaSHV + blaNDM-1 + blaIMP + blaVIM, blaCTX-M + blaSHV + blaNDM-1+ blaVIM and blaCTX-M + blaTEM + blaIMP + blaVIM, respectively. This co-existence of 6 beta-lactamases in EC104 was confirmed by multiple amplifications which is unique ESBL-EC in Nepal and elsewhere. The presence of carbapenemases like; blaNDM-1, blaIMP and blaVIM and its co-existence with ESBLs like, blaCTX-M, blaTEM and blaSHV in E. coli will seriously limit present and future therapeutic options.
The study of variants of ESBL-types, their location in mobile genetic elements (plasmids and integron elements), and clonal analysis of ESBL-EC is required. PCR-RAPD is simple, easy, cost-effective and has short turn-around time to answer the clonal nature of the bacterial isolates. PCR-RAPD was performed to study the clonal nature of these isolates but none of the isolates possessing similar resistance genes were grouped into similar RAPD types. More robust tools like pulse field gel electrophoresis and multi-locus sequence typing would help to know the exact clonal nature of these isolates.
Conclusion
The high prevalence of blaCTX-M-type ESBL and co-existence of ESBLs and carbapenemases were noted in ESBL-EC isolated from Kathmandu Medical College and Teaching Hospital OPD and ICU patients for the first time. Continuous monitoring of this ESBL-EC with nationwide study will shed light in its dissemination and strategy to prevent and control the further spread of these super-bugs.
Acknowledgements
I would like thank Dr. Chanwit Tribuddharat for being so generous to supply the valuable reagents and control for the study and to Ms. Chandrika Devi Shrestha who helped by providing the specimens for our study. I am also grateful to the Department of Microbiology, St. Xavier’s College, Nepal.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RHP collected the samples, conducted the laboratory analysis, analysed the data, and drafted the manuscript; BT designed the study, analysed the data and reviewed the manuscript; RK, PKS and CT were involved in the concept, design and review of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Ram Hari Pokhrel, Email: s4sambuddha@gmail.com.
Badri Thapa, Email: badri_bishal@yahoo.com.
Rajesh Kafle, Email: yours_sathi1030@yahoo.com.
Pradeep Kumar Shah, Email: pkshah21@hotmail.com.
Chanwit Tribuddharat, Email: sictb@mahidol.ac.th.
References
- 1.Jacobsen SM, Stickler DJ, Mobley HLT, Shirtliff ME. Complicated catheter- associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev. 2008;21(1):26–59. doi: 10.1128/CMR.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahane V, Bhawal S, Lele U. Surgical site infections: a one year prospective study in a tertiary care centre. Int J Health Sci. 2012;6(1):79–84. doi: 10.12816/0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JY, Hsueh PR, Wang JT, Lee LN, Yang PC, Luh KT. Recurrent infections and chronic colonization by an Escherichia coli clone in the respiratory tract of a patient with severe cystic bronchiectasis. J Clin Microbiol. 2000;38(7):2766–2767. doi: 10.1128/jcm.38.7.2766-2767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guggenbichler JP, Assadian O, Boeswald M, Kramer A. Incidence and clinical implication of nosocomial infections associated with implantable biomaterials-catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdis Zip. 2011;6(1):Doc 18. doi: 10.3205/dgkh000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meybeck A, Ricard JD, Barnaud G, Eveillard M, Chevrel G, Mounier R, Dreyfuss D. Incidence and impact on clinical outcome of infections with piperacillin/tazobactam resistant Escherichia coli in ICU: a retrospective study. BMC Infect Dis. 2008;8:67. doi: 10.1186/1471-2334-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chander A, Shrestha CD. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res Notes. 2013;6:487. doi: 10.1186/1756-0500-6-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawat D, Nair D. Extended-spectrum beta-lactamases in gram negative bacteria. J Glob Infect Dis. 2010;2(3):263–274. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 9.Hammar DA, Dangol S, Anderson TP, Wong JSJ, Werno AM, Murdoch DR. High prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Nepal. Int J Antimicrob Agents. 2007;30(5):471–472. doi: 10.1016/j.ijantimicag.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Collee JG, Miles RS, Watt B. Tests for identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14. New York: Churchill Livingstone; 1996. pp. 131–149. [Google Scholar]
- 11.Clinical Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Twenty First Informational Supplement (M 100-S21) Wayne PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 12.Pongpech P, Naenna P, Taipobsakul Y, Tribuddharat C, Srifuengfung S. Prevalence of extended-spectrum beta-lactamase and class 1 integron integrase gene intl1 in Escherichia coli from Thai patients and healthy adults. Southeast Asian J Trop Med Public Health. 2008;39(3):425–433. [PubMed] [Google Scholar]
- 13.Thapa B, Tribuddharat C, Srifuengfung S, Dhiraputra C. High prevalence of bla (OXA)-23 in oligoclonal carbapenem-resistant Acinetobacter baumannii from Siriraj Hospital, Mahidol University, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2010;41(3):625–635. [PubMed] [Google Scholar]
- 14.Nordmann P, Poirel L, Carrër A, Toleman MA, Walsh TR. How to detect the NDM-1 producers. J Clin Microbiol. 2011;49(2):718–721. doi: 10.1128/JCM.01773-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47. doi: 10.1186/2110-5820-1-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillon RHP, Clark J. ESBLs: a clear and present danger. Crit Care Res Prac. 2012;2012:625170. doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. Emergence of high levels of extended-spectrum–lactamase-producing gram-negative bacilli in the Asia-Pacific Region: data from the study for monitoring antimicrobial resistance trends (SMART) program, 2007. Antimicrob Agents Chemother. 2009;53(8):3280–3284. doi: 10.1128/AAC.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahan DD, Pitout JD. Infections with extended-spectrum β-lactamase-producing Enterobacteriaceae. Drugs. 2010;70(3):313–333. doi: 10.2165/11533040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen JH, Mc Elmeel ML, Fulcher LC, Zimmer BL. Detection of CTX-M-type extended spectrum beta-lactamase (ESBLs) by testing with microscan overnight and ESBL confirmation panels. J Clin Microbial. 2010;48(1):120–123. doi: 10.1128/JCM.01507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhury PR, Ingold A, Vanegas N, Martinez E, Merlino J, Merkier AK, Castro M, Rocha G, Borthagaray G, Centron D, Toledo HB, Morquez CM, Stokes HW. Dissemination of multiple drug resistance genes by class 1 integrons in Klebsiella pneumonia isolates from four countries: a comparative study. Antimicrob Agents Chemother. 2011;55(7):3140–3149. doi: 10.1128/AAC.01529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doddaiah V, Anjaneya D. Prevalence of ESBL, AmpC and carbapenemase among Gram negative bacilli isolated from clinical specimens. Am J Life Sci. 2014;2(2):76–81. doi: 10.11648/j.ajls.20140202.17. [DOI] [Google Scholar]
- 22.Al-Jasser AM. Extended-spectrum ß-Lactamases (ESBLs): a global problem. Kuwait Med J. 2006;38(3):171–185. [Google Scholar]
- 23.Jones TH, Tuckman M, Keeney D, Ruzin A, Bradford PA. Characterization and sequence analysis of extended-spectrum lactamase-encoding genes from Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates collected during Tigecycline Phase 3 clinical trials. Antimicrob Agents Chemother. 2008;53(2):465–475. doi: 10.1128/AAC.00883-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bali EB, Acik L, Sultan N. Phenotypic and molecular characterization of SHV, TEM, CTX-M and extended-spectrum beta-lactamase produced by Escherichia coli, Acinobacter baumannii and Klebsiella isolates in a Turkish hospital. Afr J Microbiol Res. 2010;4(8):650–654. [Google Scholar]
- 25.Hasim RB, Husin S, Rahman MM. Detection of beta-lactamase producing bacterial genes and their clinical features. Pak J Biol Sci. 2011;14(1):41–46. doi: 10.3923/pjbs.2011.41.46. [DOI] [PubMed] [Google Scholar]
- 26.Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9(5):466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Lin CF, Hsu SK, Chen CH, Huang JR, Lo HH. Genotypic detection and molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a regional hospital in central Taiwan. J Med Microbiol. 2010;59(6):665–671. doi: 10.1099/jmm.0.015818-0. [DOI] [PubMed] [Google Scholar]
- 28.Eckert C, Gautier V. Dissemination of CTX-M-Type β-Lactamases among clinical isolates of Enterobacteriaceae in Paris, France. Antimicrob Agents Chemother. 2004;48(4):1249–1255. doi: 10.1128/AAC.48.4.1249-1255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tada T, Miyoshi-Akiyama T, Dahal RK, Mishra SK, Ohara H, Shimada K, Kirikae T, Pokhrel BM. Dissemination of multidrug-resistant Klebsiella pneumoniae clinical isolates with various combinations of carbapenemases (NDM-1 and OXA-72) and 16S rRNA methylases (ArmA, RmtC and RmtF) in Nepal. Int J Antimicrob Agents. 2013;42(4):372–374. doi: 10.1016/j.ijantimicag.2013.06.014. [DOI] [PubMed] [Google Scholar]


