Abstract
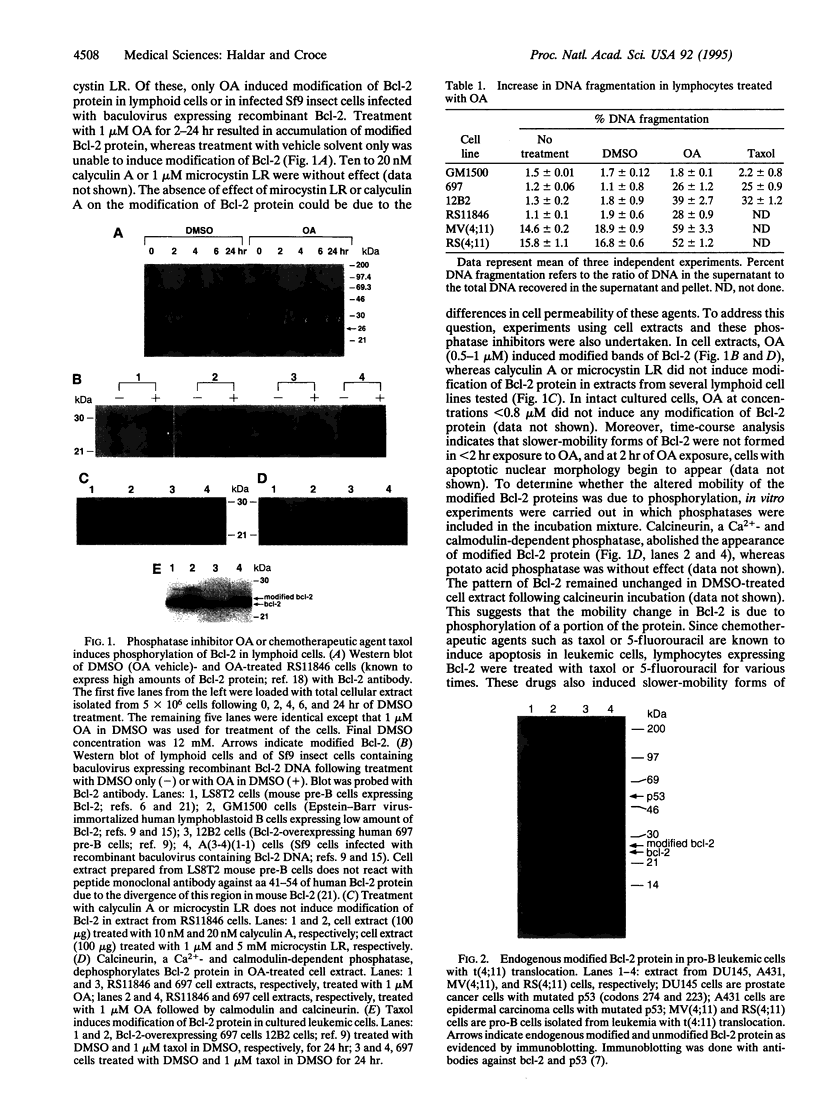
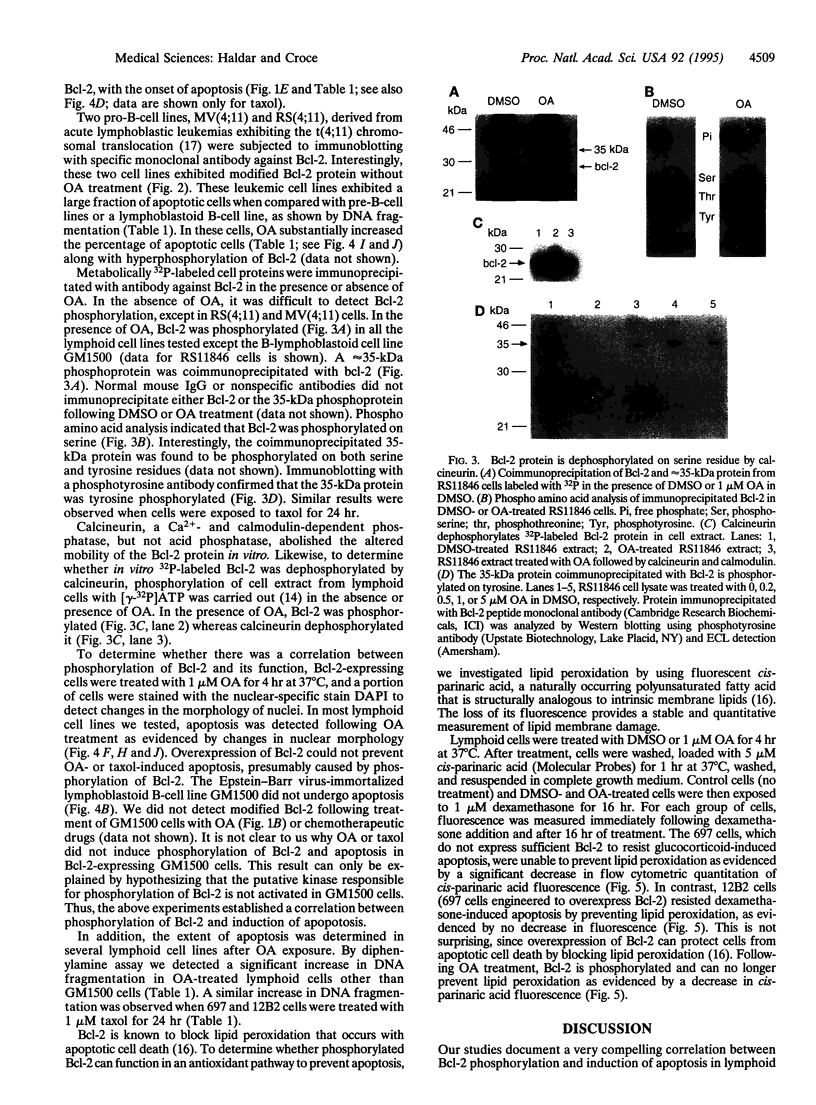
The antiapoptosis potential of Bcl-2 protein is well established, but the mechanism of Bcl-2 action is still poorly understood. Using the phosphatase inhibitor okadaic acid or the chemotherapeutic drug taxol, we found that Bcl-2 was phosphorylated in lymphoid cells. Phospho amino acid analysis revealed that Bcl-2 was phosphorylated on serine. Under similar conditions, okadaic acid or taxol treatment led to the induction of apoptosis in these cells. Thus, phosphorylation of Bcl-2 seems to inhibit its ability to interfere with apoptosis. In addition, phosphorylated Bcl-2 can no longer prevent lipid peroxidation as required to protect cells from apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Fernandes T. F., Haldar S., Croce C. M., Litwack G. Involvement of BCL-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992 Jan 15;52(2):491–495. [PubMed] [Google Scholar]
- Alnemri E. S., Robertson N. M., Fernandes T. F., Croce C. M., Litwack G. Overexpressed full-length human BCL2 extends the survival of baculovirus-infected Sf9 insect cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøe R., Gjertsen B. T., Vintermyr O. K., Houge G., Lanotte M., Døskeland S. O. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991 Jul;195(1):237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- Carson W. E., Haldar S., Baiocchi R. A., Croce C. M., Caligiuri M. A. The c-kit ligand suppresses apoptosis of human natural killer cells through the upregulation of bcl-2. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7553–7557. doi: 10.1073/pnas.91.16.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Bischoff J. R. Bcl-2 associates with the ras-related protein R-ras p23. Nature. 1993 Nov 18;366(6452):274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- Gaestel M., Benndorf R., Hayess K., Priemer E., Engel K. Dephosphorylation of the small heat shock protein hsp25 by calcium/calmodulin-dependent (type 2B) protein phosphatase. J Biol Chem. 1992 Oct 25;267(30):21607–21611. [PubMed] [Google Scholar]
- Gu Y., Nakamura T., Alder H., Prasad R., Canaani O., Cimino G., Croce C. M., Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992 Nov 13;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Haldar S., Beatty C., Tsujimoto Y., Croce C. M. The bcl-2 gene encodes a novel G protein. Nature. 1989 Nov 9;342(6246):195–198. doi: 10.1038/342195a0. [DOI] [PubMed] [Google Scholar]
- Haldar S., Jena N., Coss R., Sedar A. W., Wachsberger P. R., Beatty C., Croce C. M. Cellular localization of the bcl-2 protein and response to glucocorticoid stress. Cell Death Differ. 1994;1(2):109–115. [PubMed] [Google Scholar]
- Haldar S., Jena N., DuBois G. C., Takayama S., Reed J. C., Fu S. S., Croce C. M. Purification and characterization of the bcl-2 protein. Arch Biochem Biophys. 1994 Dec;315(2):483–488. doi: 10.1006/abbi.1994.1529. [DOI] [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Negrini M., Silini E., Kozak C., Tsujimoto Y., Croce C. M. Molecular analysis of mbcl-2: structure and expression of the murine gene homologous to the human gene involved in follicular lymphoma. Cell. 1987 May 22;49(4):455–463. doi: 10.1016/0092-8674(87)90448-x. [DOI] [PubMed] [Google Scholar]
- Somers J. P., DeFranco D. B. Effects of okadaic acid, a protein phosphatase inhibitor, on glucocorticoid receptor-mediated enhancement. Mol Endocrinol. 1992 Jan;6(1):26–34. doi: 10.1210/mend.6.1.1310797. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994 May 26;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]







