Abstract
Purpose
The purpose of our study was to evaluate the underestimation rate of atypical ductal hyperplasia (ADH) on vacuum-assisted breast biopsy (VABB), and to examine the correlation between residual microcalcifications and the underestimation rate of ADH.
Methods
A retrospective study was performed on 27 women (mean age, 49.2±9.2 years) who underwent additional excision for ADH via VABB for microcalcifications observed by using mammography. The mammographic findings, histopathologic diagnosis of all VABB and surgical specimens, and association of malignancy with residual microcalcifications were evaluated. The underestimation rate of ADH was also calculated.
Results
Of the 27 women with microcalcifications, nine were upgraded to ductal carcinoma in situ (DCIS); thus, the underestimation rate was 33.3% (9/27). There was no difference in age (p=0.40) and extent of microcalcifications (p=0.10) when comparing benign and malignant cases. Six of 17 patients (35.3%) with remaining calcifications after VABB were upgraded to DCIS, and three of 10 patients (30%) with no residual calcifications after VABB were upgraded (p=1.00).
Conclusion
The underestimation rate of ADH on VABB was 33.3%. Furthermore, 30% of patients with no remaining calcifications were upgraded to DCIS. Therefore, we conclude that all ADH cases diagnosed via VABB should be excised regardless of the presence of residual microcalcifications.
Keywords: Breast, Calcinosis, Large-core needle biopsy, Mammography, Segmental mastectomy
INTRODUCTION
Atypical ductal hyperplasia (ADH) of the breast is associated with a 4- to 5-fold increased risk of malignancy. Owing to this increased risk, an ADH diagnosis is typically an indication for surgical excision [1]. ADH usually manifests as microcalcifications on mammograms, and it has been diagnosed via stereotactic vacuum-assisted breast biopsy (VABB). If suspicious microcalcifications are diagnosed as ADH via VABB, prompt surgery is necessary because of the potential to upgrade to malignancy. The upgrade rate to invasive cancer or ductal carcinoma in situ (DCIS) in the literature varies from 11.5% to 62% [2,3,4,5]. This range is influenced by either imaging features (i.e., presence of calcifications rather than masses) or needle size [6,7,8,9].
VABB is an accurate and useful method for diagnosing mammographically detected lesions including suspicious microcalcifications [6,10,11]. Compared to 14-gauge core needle biopsy (CNB), VABB has an advantage of lower underestimation rate because of the larger amount of calcification retrieval. Therefore, VABB is preferred for biopsy of suspicious microcalcifications on mammograms [1]. Occasionally, microcalcifications can be completely extracted during the procedure, and whether further excision is necessary is debatable, particularly in cases where residual calcifications are absent [1,8,12,13,14].
The purpose of our study was to evaluate the underestimation rate of ADH on VABB, and to examine the correlation between residual microcalcifications and the underestimation rate of ADH.
METHODS
Between June 2008 and January 2013, 570 cases of 528 patients in our institution underwent VABB for the presence of microcalcifications on mammograms. Thirty-five of these patients were diagnosed with ADH (6.6%, 35/528). Of the 35 ADH cases, additional excision was not performed in eight patients (7, owing to follow-up loss; 1, owing to treatment with chemotherapy for contralateral breast cancer), all of whom were categorized as 4A (low suspicion of malignancy) of Breast Imaging-Reporting and Data System (BI-RADS). The remaining 27 patients who underwent additional surgery (49.2±9.2 years; age range, 31-68 years; median age, 49 years) were enrolled in this retrospective study. The Institutional Review Board approved this retrospective study, and informed consent was waived for the review of images and records (Ethics Committee reference number: 2013-0799-001).
In all included cases, standard mediolateral oblique views, craniocaudal views, and additional magnification views were obtained with dedicated equipment (Senographe DS, GE Medical Systems, Milwaukee, USA; Lorad Selenia, Hologic, Danbury, USA) before VABB. We performed additional breast ultrasonography for lesion localization, but there was no evidence of sonographic abnormalities that correlated with microcalcifications. For the biopsy, the patients lay on a stereotactic VABB table (Digital Stereotaxy with Senographe DS Interventional; GE Medical Systems) in the lateral decubitus position with the breast lesion side up. Digital scout images and 15° paired stereotactic images were obtained to localize the microcalcifications. A short skin incision was made using local anesthesia under aseptic conditions. The lesion was localized, and the VABB device (Mammotome; Biopsys/Ethicon Endo-Surgery, Cincinnati, USA) was inserted into the appropriate depth by using a single 8- or 11-gauge needle with gauge size determined on a case-by-case basis. A localizing clip (MicroMark; Biopsys/Ethicon Endo-Surgery) was placed at the site of biopsy, and specimen mammography was performed to confirm calcification retrieval. A follow-up unilateral mammography or magnification view was also performed to evaluate residual microcalcifications and the location of the clip. After VABB, all patients underwent additional surgery by using the mammography-guided wire-localization technique. Specimen mammography was also performed to check including clip and residual microcalcifications after surgery.
Prebiopsy and postbiopsy mammograms were reviewed retrospectively by a breast radiologist (I.Y.). All mammograms were interpreted according to the BI-RADS lexicon, and the morphological features of microcalcifications (shape, distribution, numbers, and extent) were evaluated with a final category assessment to determine the characteristics of the lesions. The extent of all microcalcifications was expressed as mean±SD (mm). We also evaluated whether residual microcalcifications were present after the procedure.
We calculated the underestimation rate of ADH, which was defined as the percentage of surgically proven malignant cases among the ADH cases diagnosed via VABB. Furthermore, in lesions of the same BI-RADS category, we analyzed the rate of ADH underestimation between the patients with and without microcalcifications after VABB. We further divided the data based on the needle type (8- or 11-gauge) and calculated the underestimation rate in each group.
The histopathological diagnosis of all VABB and surgical specimens was retrospectively recorded through a pathological report. Categorical data were summarized as frequencies and percentages. We used the two-sample t-test to compare patients with benign or malignancy according to the age and extent of their microcalcifications. We also evaluated the association of malignancy with existing residual microcalcifications by using the Fisher exact test. All statistical analyses were performed by using statistical software (SPSS for Windows, version 20.0; IBM Corp., Armonk, USA). Statistical significance was established considering a p-value of less than 0.05.
RESULTS
Among the 27 ADH patients enrolled in this study (49.2±9.2 years), nine women (51.9±10.0 years) were diagnosed with malignancy after surgery; therefore, the underestimation rate was 33.3% (9/27). All malignancies were DCIS. A subsequent unilateral mammography or magnification view after VABB was performed (mean duration time, 15±11 days; range, 6-46 days); the characteristics of microcalcifications with final category assessments in the benign and malignant groups are presented in Table 1. There was no significant difference in the extent of microcalcifications when comparing the benign and malignant groups (p=0.10; benign, 11.3±9.7 mm; malignant, 16.7±16.4 mm; total, 13.1±12.3 mm). Regarding the shape, the majority of calcifications were composed of amorphous (n=11) or coarse (n=4) calcifications in the benign group, and amorphous (n=3), fine pleomorphic (n=2), or linear branching (n=2) calcifications in the malignant group. Regarding distribution, the number of clustered microcalcifications was the highest in both the benign (n=15) and malignant (n=5) groups. BI-RADS category 4C cases were observed only in the malignant group (n=3). Table 2 summarizes the comparison of pathologic results of VABB and surgical excision. Although there was no significant difference with regard to the needle size (p=0.22), the underestimation rate was 16.7% (2/12) on 8-gauge and 46.7% (7/15) on 11-gauge needles.
Table 1.
Comparison of excisional biopsy results of atypical ductal hyperplasia diagnosed with vacuum-assisted breast biopsy
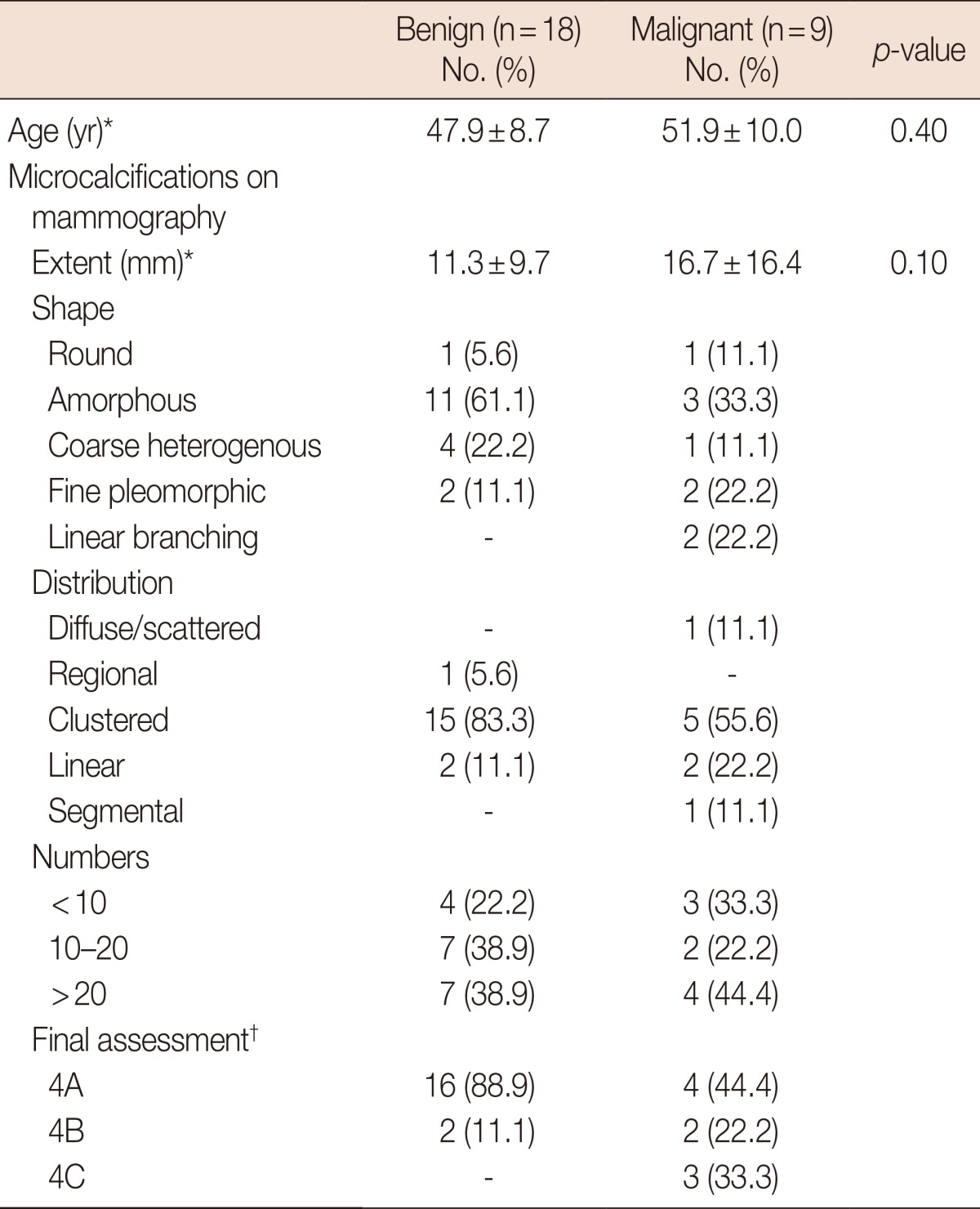
*Mean±SD; †Category of Breast Imaging-Reporting and Data System.
Table 2.
Underestimation rate of atypical ductal hyperplasia with comparison of residual pathology on excisional biopsy samples
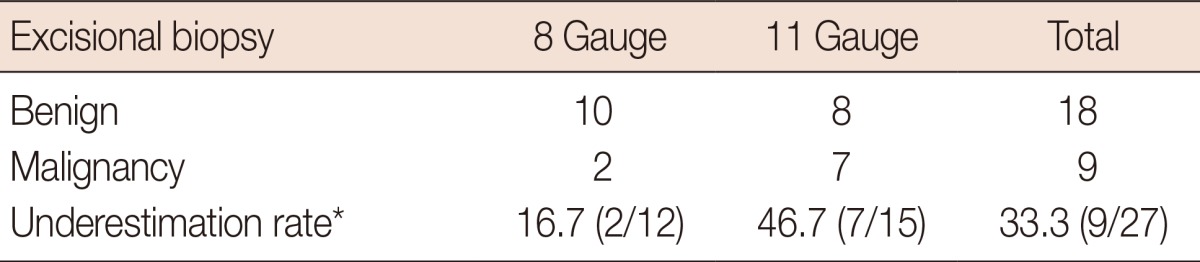
*Percent (number of proven malignancytotal number).
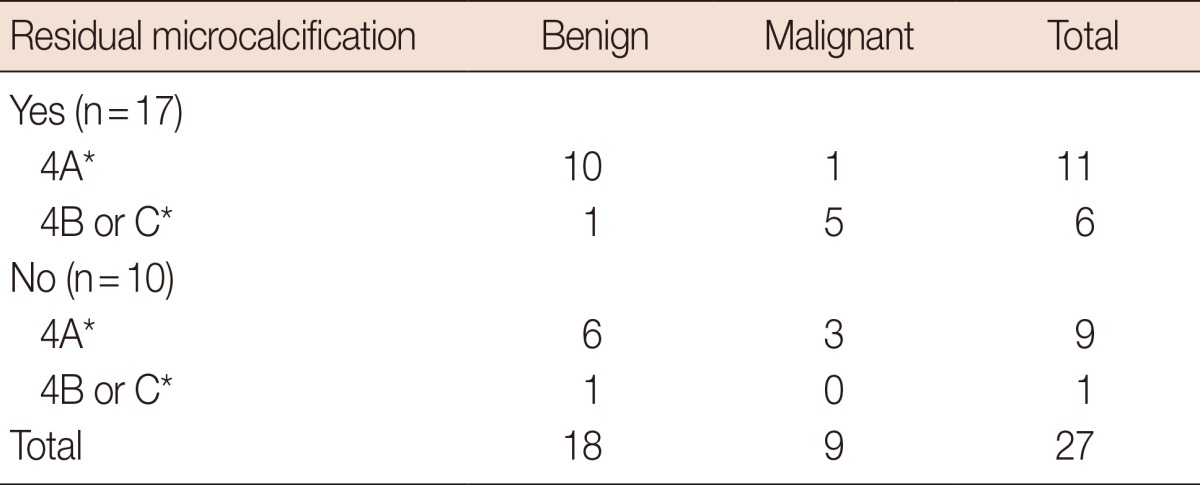
The residual calcifications after VABB in the benign and malignant groups are presented in Table 3. On the basis of the results on mammograms after VABB, three patients (30%) were upgraded to DCIS among the patients without residual microcalcifications (Figure 1), whereas six of 17 patients with remaining microcalcifications (35.3%) were upgraded to DCIS (p=1.00) (Figure 2). Of the three patients with underestimation and in whom no remaining microcalcifications were observed on the mammogram after VABB, an 11-gauge needle was used in two patients and an 8-gauge needle in one patient. Among the patients classified as having BI-RADS category 4A lesions, three out of nine patients without residual calcifications (33.3%) were upgraded, whereas only one out of 11 patients with residual calcifications (9.1%) was upgraded. Among the patients classified with category 4B or 4C lesions, there were no upgraded patients without residual calcifications (0/1), whereas five out of six patients with residual calcifications (83.3%, 5/6) were upgraded.
Table 3.
Residual calcifications with comparison of residual pathology on excisional biopsy of atypical ductal hyperplasia diagnosed with vacuum-assisted breast biopsy
*Category of Breast Imaging-Reporting and Data System.
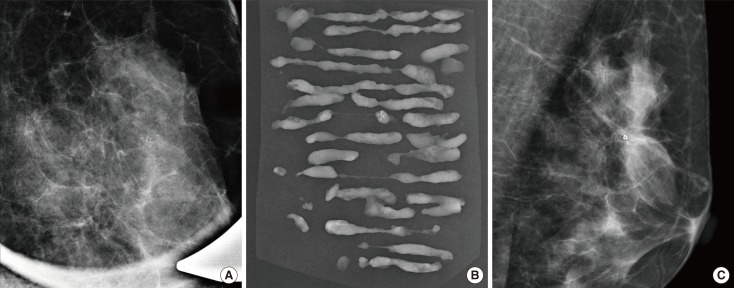
Figure 1.
A 45-year-old woman with ductal carcinoma in situ. (A) Magnification view of mediolateral mammography reveals clustered pleomorphic calcifications measuring 11 mm at the longest dimension in left upper central breast. Vacuum-assisted breast biopsy was performed with 11-gauge needle and the localizing clip was placed. (B) Radiography of the vacuum-assisted breast biopsy specimens revealed calcification and the diagnosis was atypical ductal hyperplasia. (C) Mediolateral mammography of the left breast obtained after 1 week shows localizing clip without evidence of residual calcifications. After surgery, the pathologic diagnosis was ductal carcinoma in situ.
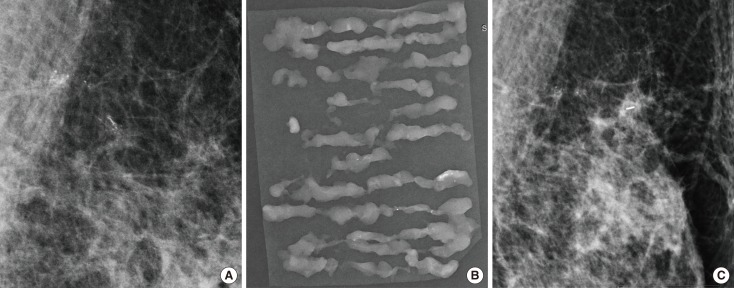
Figure 2.
A 55-year-old woman with ductal carcinoma in situ. (A) Magnification view of mediolateral mammography reveals linear distributed linear branching calcifications measuring 18 mm at the longest dimension in left upper medial breast. Vacuum-assisted breast biopsy was performed with 11-gauge needle and the localizing clip was placed. (B) Radiography of the vacuum-assisted breast biopsy specimens revealed calcification and the diagnosis was atypical ductal hyperplasia. (C) Mediolateral mammography of the left breast obtained after 1 week shows localizing clip with remaining calcifications. After surgery, the pathologic diagnosis was ductal carcinoma in situ.
DISCUSSION
ADH is a disease entity of the proliferative breast lesion characterized as a ductal lesion with cytoarchitectural atypia beyond the normal range, yet insufficient for a DCIS diagnosis [15]. It has been found in approximately 4% to 8% of all breast biopsies, which is similar to the 6.7% (35/528) of the VABB biopsies in our study [12,14,15]. Several attempts have been made to identify mammographic characteristics that could differentiate malignant from benign cases in diagnosing ADH on VABB, but none have been established yet [13,14,16].
The 14-gauge CNB is an accurate and easy method to diagnose breast pathology, but its application is limited to diagnosing nonmass lesions with microcalcifications only. VABB is the preferred method to diagnose ADH, which usually manifests as microcalcifications rather than a mass on mammograms [17]. McGhan et al. [2] and Arora et al. [8] reported that using a larger needle would harvest many more tissue cores, which would be necessary for the accurate diagnosis of ADH using VABB. In comparison, Nguyen et al. [1] and Lourenco et al. [9] reported that there was no statistical significance considering the probe size. However, most of these studies were performed by using 11-gauge VABB needles [1,14]. In our study, we examined the underestimation rates of ADH with VABB by comparing the needle size and found that the rates were 46.7% for 11-gauge samples and 16.7% for 8-gauge samples. As has been reported by others, our findings show that the underestimation rate of 8-gauge VABB was much lower than that of 11-gauge VABB, although these differences did not reach statistical significance.
Recent studies indicate that the extraction of all calcifications (not just some), results in lower underestimation rates of ADH with VABB, supporting the hypothesis that retrieving a larger volume of tissue lowers the underestimation rate for ADH [6,7,12,16]. In line with this hypothesis, the diagnosis of ADH would be more reliable and subsequent surgery would be unnecessary, if all calcifications were extracted after VABB. However, the necessity of further surgery is still highly debated, and it remains controversial [1,8,12,13,14]. Some authors reported that if microcalcifications that have been removed completely via VABB/CNB show ADH, the lesion may be considered to be adequately represented with no risk of ADH underestimation, and subsequent excision may not be necessary [1,2,12,13,16,18]. Most of these reports were based on the results of 11-gauge/14-gauge VABB samples except for one study that examined samples via 9-gauge/11-gauge/14-gauge VABB. Other authors reported that patients with no residual calcifications on post-VABB mammograms still require subsequent surgery owing to the possibility of underestimation [5,8,14]. These studies were conducted by using results found with either an 11-gauge or 8-gauge needle. Most previous studies were performed by using 11-gauge or smaller needles except for the studies performed by Arora et al. and Nguyen et al. [1,5,6,8,12,13,14,16,18]. Our data showed that the underestimation rate was 30% even though the calcifications were completely extracted after VABB, and that underestimation was also present in the 8-gauge VABB needle group (n=1). Moreover, in category 4A lesions, the group with complete extraction of microcalcifications (33.3%) showed a higher upgrade rate than that with incomplete extraction (9.1%) after VABB; in category 4B and 4C lesions, there were no upgraded cases with complete extraction. It is worth noting, however, that the sample size was too small to evaluate statistical significance between complete extraction of microcalcifications and BI-RADS category. A larger sample size would be needed for future studies assessing this correlation.
This study has several limitations. First, it was a retrospective study and patients underwent mammography-guided VABB with subsequent surgery because of an ADH diagnosis. Therefore, a selection bias might exist. Moreover, eight ADH patients who did not undergo additional surgery and who were excluded from this study were classified as 'category 4A,' which could further influence the selection bias. Second, as the samples we collected were only from patients with ADH on VABB, we had a small sample size (27 patients), which is not sufficient to provide an overall picture.
In conclusion, the underestimation rate of ADH was 33.3% on VABB. There was no statistically significant difference based on the needle size. Even if all microcalcifications were completely extracted via VABB, the underestimation rate of ADH was 30%. Therefore, subsequent excision should be recommended regardless of the presence of residual microcalcifications.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Nguyen CV, Albarracin CT, Whitman GJ, Lopez A, Sneige N. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol. 2011;18:752–761. doi: 10.1245/s10434-010-1127-8. [DOI] [PubMed] [Google Scholar]
- 2.McGhan LJ, Pockaj BA, Wasif N, Giurescu ME, McCullough AE, Gray RJ. Atypical ductal hyperplasia on core biopsy: an automatic trigger for excisional biopsy? Ann Surg Oncol. 2012;19:3264–3269. doi: 10.1245/s10434-012-2575-0. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK, Mehta R, Dimitrov R, Larsson LG, Musto PM, Hodges KB, et al. Atypical ductal hyperplasia: interobserver and intraobserver variability. Mod Pathol. 2011;24:917–923. doi: 10.1038/modpathol.2011.66. [DOI] [PubMed] [Google Scholar]
- 4.Polat AK, Kanbour-Shakir A, Andacoglu O, Polat AV, Johnson R, Bonaventura M, et al. Atypical hyperplasia on core biopsy: is further surgery needed? Am J Med Sci. 2012;344:28–31. doi: 10.1097/MAJ.0b013e318234cc67. [DOI] [PubMed] [Google Scholar]
- 5.Winchester DJ, Bernstein JR, Jeske JM, Nicholson MH, Hahn EA, Goldschmidt RA, et al. Upstaging of atypical ductal hyperplasia after vacuum-assisted 11-gauge stereotactic core needle biopsy. Arch Surg. 2003;138:619–622. doi: 10.1001/archsurg.138.6.619. [DOI] [PubMed] [Google Scholar]
- 6.Liberman L, Smolkin JH, Dershaw DD, Morris EA, Abramson AF, Rosen PP. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998;208:251–260. doi: 10.1148/radiology.208.1.9646821. [DOI] [PubMed] [Google Scholar]
- 7.Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I. Underestimation of breast cancer with II-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000;175:1047–1050. doi: 10.2214/ajr.175.4.1751047. [DOI] [PubMed] [Google Scholar]
- 8.Arora S, Moezzi M, Kim U, Menes TS. Is surgical excision necessary for atypical ductal hyperplasia diagnosed with 8 gauge stereotactic biopsy? Breast J. 2009;15:673–674. doi: 10.1111/j.1524-4741.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 9.Lourenco AP, Mainiero MB, Lazarus E, Giri D, Schepps B. Stereotactic breast biopsy: comparison of histologic underestimation rates with 11- and 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189:W275–W279. doi: 10.2214/AJR.07.2165. [DOI] [PubMed] [Google Scholar]
- 10.Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer. 2011;14:1–7. doi: 10.4048/jbc.2011.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung YJ, Bae YT, Lee JY, Seo HI, Kim JY, Choo KS. Lateral decubitus positioning stereotactic vacuum-assisted breast biopsy with true lateral mammography. J Breast Cancer. 2011;14:64–68. doi: 10.4048/jbc.2011.14.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adrales G, Turk P, Wallace T, Bird R, Norton HJ, Greene F. Is surgical excision necessary for atypical ductal hyperplasia of the breast diagnosed by Mammotome? Am J Surg. 2000;180:313–315. doi: 10.1016/s0002-9610(00)00451-7. [DOI] [PubMed] [Google Scholar]
- 13.Sneige N, Lim SC, Whitman GJ, Krishnamurthy S, Sahin AA, Smith TL, et al. Atypical ductal hyperplasia diagnosis by directional vacuum-assisted stereotactic biopsy of breast microcalcifications. Considerations for surgical excision. Am J Clin Pathol. 2003;119:248–253. doi: 10.1309/0GYV-4F2L-LJAV-4GFN. [DOI] [PubMed] [Google Scholar]
- 14.Teng-Swan Ho J, Tan PH, Hee SW, Su-Lin Wong J. Underestimation of malignancy of atypical ductal hyperplasia diagnosed on 11-gauge stereotactically guided Mammotome breast biopsy: an Asian breast screen experience. Breast. 2008;17:401–406. doi: 10.1016/j.breast.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrell JA, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258–1265. doi: 10.1002/1097-0142(19930215)71:4<1258::aid-cncr2820710415>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548–554. doi: 10.1148/radiol.2242011528. [DOI] [PubMed] [Google Scholar]
- 17.Liberman L, LaTrenta LR, Van Zee KJ, Morris EA, Abramson AF, Dershaw DD. Stereotactic core biopsy of calcifications highly suggestive of malignancy. Radiology. 1997;203:673–677. doi: 10.1148/radiology.203.3.9169687. [DOI] [PubMed] [Google Scholar]
- 18.de Mascarel I, Brouste V, Asad-Syed M, Hurtevent G, Macgrogan G. All atypia diagnosed at stereotactic vacuum-assisted breast biopsy do not need surgical excision. Mod Pathol. 2011;24:1198–1206. doi: 10.1038/modpathol.2011.73. [DOI] [PubMed] [Google Scholar]