Abstract
TgCRND8 mice represent a transgenic mouse model of Alzheimer's disease, with onset of cognitive impairment and increasing amyloid-β plaques in their brains at 12 weeks of age. In this study, the spatial memory in 25- to 30-week-old TgCRND8 mice was analyzed in two reference and one working memory Morris water maze (MWM) tests. In reference memory tests, the mice were trained to escape to a hidden platform, which in one version of the test was marked by a visual cue. In the working memory test, the hidden platform was moved daily to different locations. The TgCRND8 mice were impaired in reference memory when trained in a hidden platform test. However, the mice developed spatial memory comparable to non-Tg littermates in a cued reference memory test. The mice showed also an impairment in spatial working memory. Analysis of search paths revealed that in contrast to non-Tg littermates, TgCRND8 mice did not use spatial strategies during their navigation. Instead, they learned to locate an escape platform using a nonspatial, chaining strategy. The study showed that (1) the impairment in the reference memory of TgCRND8 mice was reduced when a hidden platform was cued, and that (2) both working and reference memory systems of TgCRND8 mice, but not (3) the plasticity of choice between search strategies, are compromised by the transgene-induced pathology.
Alzheimer's disease (AD) is a neurodegenerative disorder, characterized by a progressive loss of cognitive, language, and behavioral functions (Albert 1996). The pathological hallmarks of AD include parenchymal and cerebrovascular amyloid-β peptide (Aβ) deposits, intracellular neurofibrillary tangles of hyperphosphorylated tau protein (NFT), and neuronal cell loss (Selkoe 1997, 2002; Hardy and Selkoe 2002). Genetic studies support the hypothesis that mutations altering proteolytic processing of the amyloid precursor protein (APP) lead to increased production of the 42-residue Aβ, a highly fibrillogenic peptide, which predominantly aggregates in the plaques (Price and Sisodia 1998; Hardy and Selkoe 2002; Golde 2003). In an effort to address the role of genetic factors implicated in AD in vivo, transgenic (Tg) mice expressing mutated human genes implicated in AD such as amyloid precursor protein (APP), presenilins (PS1 and PS2), tau, and their normal wild-type forms, or genes representing a risk factor for developing the disease, such as apoliprotein E (Apo E4), have been created (Price and Sisodia 1998; van Leuven 2000; Janus et al. 2001).
In the present study, the development of spatial memory in transgenic APP mice (TgCRND8) with an early-onset related AD pathology was characterized in a series of cognitive tests addressing spatial reference and working memory. The mice encode a double mutation of familial Alzheimer's disease in the APP695 (Swedish; KM670/671NL plus Indiana; V717F) genes under the control of the hamster PrP gene promoter (Chishti et al. 2001), and exhibit increasing numbers of Aβ42 deposits and levels of SDS-soluble Aβ by 12 weeks of age. We showed previously that at this age the mice exhibit significant deficits in spatial reference memory (Chishti et al. 2001), which could be offset by early immunization against Aβ42 (Janus et al. 2000b). The significantly improved spatial learning observed in Tg mice immunized against Aβ42 implicated strongly this form of amyloid peptide as a causal factor underlying the compromised cognitive abilities of these mice. Additionally, the immunization study showed that longitudinal testing of younger mice in a conventional reference memory MWM test resulted in a strong carrying over (savings) effect that partly masked cognitive impairment present in experimentally naive TgCRND8 mice immunized with control amyloidogenic peptide. However, at the age of 23 wk, these Tg mice showed a significant spatial learning impairment despite previous experience with the testing situation (Janus et al. 2000b). Therefore, to avoid similar carrying over effects that could mask phenotyping characteristics of Tg mice during repeated testing, older, 25-30-week-old TgCRND8 mice were used in the present studies. At this age, the Tg mice show a profound burden of Aβ40 and Aβ42 comparable to AD cases in regard to (pmoles Aβ)/(mg total protein) (Chishti et al. 2001). In most studies, the evaluation of cognitive deficits in APP transgenic mice focused mainly on spatial reference memory using a conventional Morris water maze (MWM; for reviews, see Hsiao Ashe 2001; Janus and Westaway 2001). However, spatial working memory is often characterized using the radial arm water maze (RAWM; but see Chen et al. 2000 for a notable exception) in which mice are handled more intensively than during a conventional MWM training (Morgan et al. 2000; Arendash et al. 2001). Thus, to avoid procedural differences between MWM and RAWM tests, in the present experiment the behavior of the same cohort of mice was evaluated using reference and working memory versions of the MWM test.
The first experiment of this study addressed the severity of previously documented impairment in spatial reference memory of TgCRND8 mice (Chishti et al. 2001). Because navigation in environment may involve praxis (learning a sequence of movements), taxon (approach or avoidance of a prominent cue), or spatial mapping strategies (O'Keefe and Nadel 1978), which are not mutually exclusive, it is possible that mice trained to navigate to a stationary escape hidden platform always marked by a visible cue (cued reference memory of MWM test) could also develop a reference memory for the location of the platform based on a spatial map of the environment. This hypothesis was supported by parallel findings described in rats (Whishaw 1985a; Whishaw and Mittleman 1986), where the presence of a prominent cue on a platform did not preclude learning of the relationships between the spatial location of the platform and extramaze spatial cues. Accordingly, the hypothesis of this experiment assumed that impaired-in-reference-memory TgCRND8 mice should readily swim to a visible platform during training but should not be able to develop a spatial map, and consequently they should show a random search for the platform in probe trials (memory test) administered later.
Because the clinical studies of AD patients strongly indicate that the deficits in working and episodic memory dominate both preclinical and clinical phases of AD (Backman and Small 1998; Small et al. 2000; Fratiglioni et al. 2001), the second hypothesis of the study assumed that spatial working memory of TgCRND8 mice is compromised, which provides better validation of this mouse model as a model of AD. In this experiment, I used a series-of-learning-reversals version of the MWM test in which mice had to repeatedly learn a new spatial location of an escape platform within four consecutive training trials of a daily session.
The last issue addressed in the study focused on the analysis of search behavior of non-Tg and TgCRND8 mice during their navigation in the MWM test. It is generally hypothesized that formation of toxic assemblies of Aβ peptide disrupts the cognitive function of APP Tg mice, which is reflected by their longer escape latencies caused by an inability to develop and use spatial strategy (Westerman et al. 2002). Because a single measure such as an escape latency or swim path cannot reflect complexities of search behavior or indicate causes of variance, I performed an analysis of search strategies adopted by mice during navigation in a water maze to describe better the behavioral differences between Tg and non-Tg mice. Accordingly, the analysis of search strategies during navigation in conventional and working memory MWM tests (see Fig. 1 for examples) can explain behavioral causes underlying longer escape latencies of impaired TgCRND8 mice. Longer escape latencies shown by mice during spatial navigation may be caused by their constant random search of the entire surface area of the pool, which would indicate a complete lack of spatial learning abilities, or by persistent performance of a less efficient than spatial search strategy. For example, a persistent performance of initial thigmotaxic swims may indicate a disturbance in mice behavioral plasticity (Gass et al. 1998), but adoption of less efficient, albeit systematic search strategy, which would result in a successful location of an escape platform, may indicate that impaired mice were able to learn an alternative solution of the test.
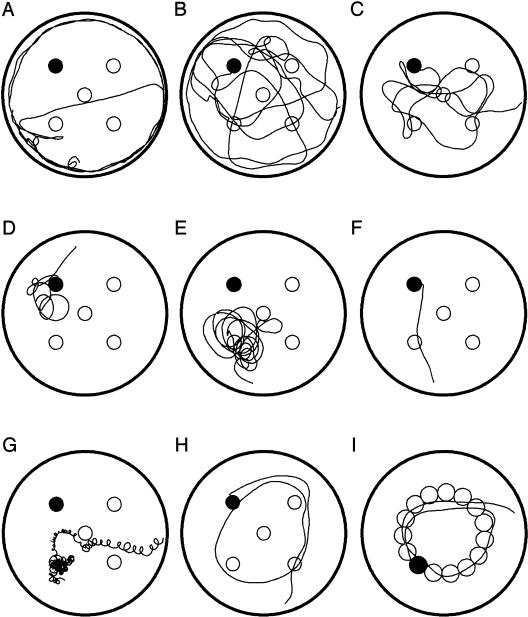
Figure 1.
A training trial in a Morris water maze (MWM) consists of an unconstrained search by a mouse of the entire area of the pool within a limited period of time. A submerged underwater platform, conventionally located in the center of one of the pool's quadrants (a target quadrant), provides an escape from water. When a mouse finds and climbs a platform, a training trial is terminated. (A) A mouse naive to the water maze initially tends to swim along the wall of the pool (thigmotaxic or wall-hugging swim). In later training trials, a mouse finds an escape platform by chance while making occasional searches of the inner area of the pool. As training progresses, a mouse begins to search the whole surface area of the pool, first randomly (B), and later, selectively scanning the inner area of the pool (C) containing the escape platform. The development of a spatial memory for the platform location is reflected by a focal search of a target quadrant (D), or by the direct swim to the platform (F). Occasionally, a mouse may perform a focal search in an incorrect area of the pool (E), or it may intermittently perform a circling behavior (G). In the present study, a search of an incorrect quadrant of the pool was observed in TgCRND8 mice mainly during the working memory version of MWM. Also, only the Tg mice performed occasionally circling swims. In the case of a chaining response strategy (H), a mouse systematically searches the area of the pool at the constant distance of the platform location from the wall, thus crossing all possible platform locations (I).
RESULTS
Reference Memory in TgCRND8 Mice
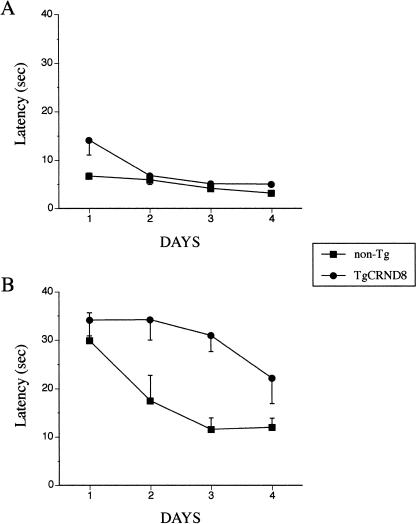
TgCRND8 mice and non-Tg littermates that were naive to the water maze showed no deficiencies in swimming abilities, directional swimming toward the cued platform, or climbing onto a hidden platform during nonspatial pretraining (see Materials and Methods). The average latencies of the mice to reach an escape platform in cued and conventional MWM tests are shown in Figure 2. Global analysis revealed that in both tests all mice significantly reduced their escape latency over the training period (F(3, 57) = 10.7, p < 0.001, days factor). As expected, mice trained in the cued conditions showed significantly shorter escape latencies than mice trained in conventional MWM conditions (F(1, 19) = 138.1, p < 0.001, test factor). Overall, the TgCRND8 mice differed significantly from non-Tg mice (F(1, 19) = 34.1, p < 0.001, genotype factor), and their escape latencies were significantly affected by the type of test (F(1, 19) = 15.4, p < 0.01, test × genotype interaction). To elucidate the nature of this interaction, escape latencies were analyzed separately for mice trained in cued and in conventional MWM tests. In cued conditions, both TgCRND8 and non-Tg mice navigated readily to the visible platform and significantly improved their performance over sessions (F(2, 33) = 11.1, p < 0.01, days factor; Fig. 2A). The Tg mice, however, showed a trend of longer latency (F(1, 11) = 3.5, p = 0.09, genotype factor), which changed differently during training than latencies of non-Tg mice (F(2, 33) = 3.2, p = 0.07, genotype × days interaction; Fig. 2A). Subsequent analysis, excluding scores of the first day, showed comparable performance between the genotypes in the cued MWM test. Therefore, it is likely, and the initial difference in performance of TgCRND8 mice during the first day of training could be caused by subtle differences in their reactivity to altered conditions after the curtain present during nonspatial pretraining was removed. A similar, transient initial difference in escape latencies to a cued platform was observed in the Tg65Dn mouse model of Down syndrome (Bimonte-Nelson et al. 2003). In a conventional reference memory test, all mice improved their performance over training (F(3, 24) = 4.5, p < 0.05, days factor), but TgCRND8 mice showed significantly longer escape latency than non-Tg littermates (F(1, 8) = 27.2, p < 0.01, genotype factor; Fig. 2B), which confirmed our previous findings (Janus et al. 2000b; Chishti et al. 2001). Because the swim speed of Tg and non-Tg mice in both cued and conventional MWM training conditions was comparable, increasing significantly as training progressed (F(3, 57) = 3.4, p < 0.05, days factor), it is unlikely that the observed differences in escape latencies were caused by differences in mice locomotor abilities. In conclusion, the presence of the cue that indicated the location of a hidden escape platform significantly reduced escape latencies of all mice with no differences in performance between genotypes. In a conventional (hidden platform) MWM test, not only were the escape latencies of all mice longer, indicating increased difficulty of this spatial learning task, but also the TgCRND8 mice showed a significant impairment in the acquisition of spatial information as compared with non-Tg littermates.
Figure 2.
The performance of TgCRND8 mice and non-Tg littermates in a cued (A) and a conventional (B) reference memory version of the Morris water maze (MWM) test. In the cued MWM test, a hidden escape platform was always marked with a visible cue (black and white post) and placed in a fixed location (NE quadrant of the pool) throughout the experiment. With the exception of Day 1 of training, the TgCRND8 mice showed escape latencies comparable to their non-Tg littermates (A). In the conventional MWM test, the TgCRND8 mice showed a significant impairment in their latencies to find a hidden escape platform (p < 0.01) as compared with their non-Tg littermates (B).
The development of spatial memory for the platform location during training in cued and conventional reference memory MWM tests was evaluated in two probe trials administered 1 h and 24 h at the end of each test. The overall analysis revealed that mice trained in the cued MWM test spent a higher proportion of time searching a quadrant containing an escape platform during training (target quadrant, TQ) than the mice trained in a conventional reference MWM test (F(1, 19) = 6.3, p < 0.05). Both Tg and non-Tg mice trained in cued MWM conditions spent a comparable amount of time (∼50%) searching the TQ in both probe trials. However, after training in conventional MWM conditions, TgCRND8 mice spent less time searching the TQ (36% and 40% for probes 1 and 2, respectively) as compared with non-Tg mice (∼50% for both probe trials; F(1, 8) = 10.1, p < 0.02). However, this decrease in time of TQ exploration by Tg mice was not large enough to produce a significant genotype × test condition interaction effect.
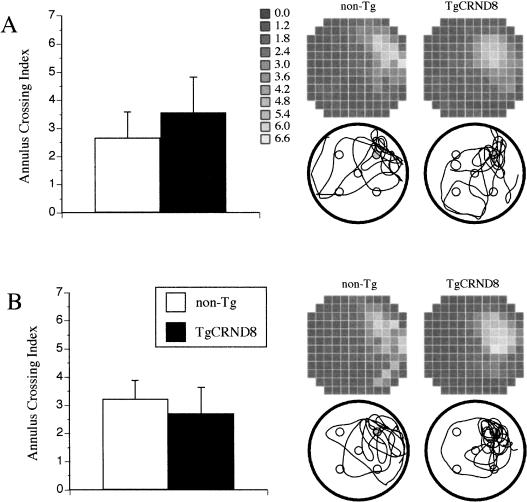
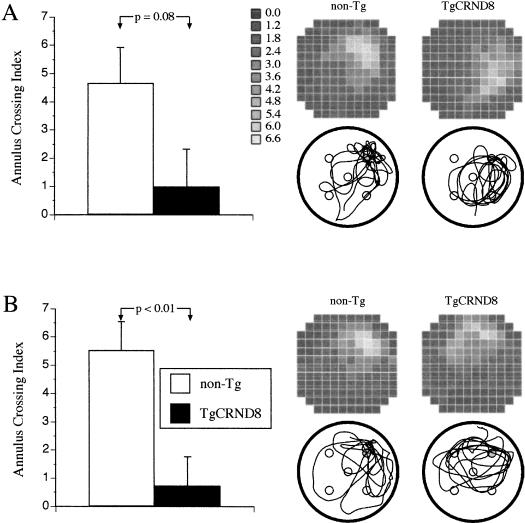
The evaluation of spatial memory for exact location of the escape platform was done computing an annulus crossing index (ACI). ACI represents several swims over the platform site in the TQ adjusted for swims over corresponding sites in other quadrants (see Materials and Methods). The global analysis of ACI scores between testing conditions and genotypes did not reveal any significant main effect owing to testing conditions, but showed that spatial memory was significantly affected by the genotype of the mice and the training conditions (F(1, 19) = 5.0, p < 0.05, genotype factor; and F(1, 19) = 6.0, p < 0.05, genotype × test interaction). Following separate analyses for each testing condition revealed that both TgCRND8 and non-Tg mice showed a comparable spatial memory for the platform site in both probe trials at the end of a cued reference memory training (Fig. 3A,B, left panels). In contrast, the TgCRND8 mice showed a significantly weaker spatial memory than non-Tg littermates in both probe trials administered after training in conventional reference memory MWM conditions (F(1, 8) = 8.9, p < 0.02). Their inferior performance bordered the significance level in the first probe trial [t(8) = 2.0, p = 0.08; Fig. 4A, left panel], whereas in the second probe trial, their memory for the platform site was significantly worse than non-Tg littermates [t(8) = 3.3, p < 0.01; Fig. 4B, left panel]. In a similar manner to the learning acquisition phase, TgCRND8 mice did not differ during the probe trials from non-Tg littermates in their locomotor activity and motivation to swim as evaluated by swim speed, latency to start swimming, or by floating rate.
Figure 3.
The development of spatial memory for a platform location during the probe trials administered 1 h (A) and 24 h (B) after training in a cued reference memory version of the Morris water maze test. An annulus crossing index represents the average frequency of swims over the platform site in a target quadrant adjusted for swims over sites in other quadrants of the pool. Both TgCRND8 and non-Tg littermates showed a comparable spatial memory for the platform site (left panels), and a comparable search preference of the (NE) target quadrant (top right panels of A and B) in both probe trials. Search preference was calculated by dividing the surface area of the pool into 144 (12 × 12) tiles (Wintrack program), and calculating for each tile the ratio of the time spent by a mouse in this tile to the time expected by chance. Values >1 (lighter color) indicate a preference for the respective area of the pool. Representative search paths for mice of each genotype are provided below each surface preference panel. Vertical bars represent SEM.
Figure 4.
The development of spatial memory for a platform location during the probe trials after training in a conventional reference memory version of the Morris water maze test. TgCRND8 mice were significantly impaired in their spatial memory of the platform location as compared with non-Tg littermates (left panels of A and B showing the scores of the annulus crossing index) The analysis of search preference revealed that TgCRND8 mice showed less focused search of the pool during the first (A) and the second (B) probe trials (top right panels of A and B). The examination of search paths (representative paths are shown below each search preference panel) revealed that the lower annulus crossing index and less intense search of the target quadrant by the Tg mice was caused by their increased search of the quadrants adjacent to the TQ. Vertical bars represent SEM.
A distribution of search for the platform location during probe trials by TgCRND8 and non-Tg littermates was also presented as a surface-area analysis. In this analysis, the whole surface area of the pool was divided into 144 (12 × 12 cm) tiles, and the search in each tile was expressed as the ratio of the time spent by a mouse in this tile to the time expected by chance. Thus, values >1 represent frequent visits to a particular area of the pool. The surface analysis revealed that after being trained in a cued reference MWM test, both Tg and non-Tg mice spent about four to five times more time in the close vicinity of the platform site than in other areas of the pool (Fig. 3A,B, right upper panels, for probes 1 and 2, respectively). Also, all mice trained in cued conditions had positive ACI scores that significantly differed from the 0 value representing a random score (p < 0.05 for Tg and non-Tg mice in both probe trials). Examples of representative swim paths during probe trials show that both Tg and non-Tg mice exhibited a focal search for a platform site after being trained in cued reference memory conditions (Fig. 3A,B, lower right panels).
In contrast, analysis of surface area in probe trials administered after a conventional MWM training revealed that only non-Tg mice showed a focal search in the vicinity of the platform site, spending up to 6.6 times more time in that area (Fig. 4A,B, right upper panels, non-Tg) than in other areas of the pool, and their ACI scores in both probe trials were positive and significantly larger than chance values (p < 0.05). TgCRND8 mice, on the other hand, searched for the platform site in a less precise manner, showing shifts in their searches either to the right or to the left of TQ (Fig. 4A,B, right upper panels, TgCRND8). Their ACI scores, although positive, did not differ from a chance level. The examination of search paths (representative examples are given in Fig. 4A,B, right lower panels) confirmed focal search in the area of the platform site by non-Tg mice, but revealed that TgCRND8 mice performed more circular, wider swims that often covered the neighboring quadrant areas. These search patterns were also different from focal search patterns of their counterparts trained in a cued reference memory test (see, for comparison, Fig. 3A,B, right lower panels, TgCRND8 mice).
It should be stressed that the direct comparison of spatial memory for the platform location after training in cued and conventional reference memory tests should be made cautiously. The experimental designs of both tests put different emphasis on the use of spatial mapping strategy, with the cued reference memory MWM test being less demanding in this respect. This was confirmed by the fact that first, mice trained in the cued MWM test had a lower total number of swims over all platform sites than mice trained in conventional MWM conditions (F(1, 19) = 13.4, p < 0.01), and second, that control, non-Tg mice showed a trend of stronger spatial memory after conventional training than after cued training (F(1, 9) = 3.4, p = 0.10). Conversely, TgCRND8 mice showed a trend of stronger spatial memory for the platform site after training in cued than in conventional conditions (F(1, 10) = 2.8, p = 0.12), which indicates a facilitating role of a cue in the development of spatial memory in these mice. This opposite direction of change in ACI scores observed in mice of both genotypes was likely responsible for a significant overall genotype × test interaction. Therefore, the conclusion that the presence of a visible cue facilitates the development of spatial memory in TgCRND8 mice is based on the comparison with the ceiling performance of non-Tg littermates trained in the same cued reference memory conditions.
Spatial Working Memory in TgCRND8 Mice
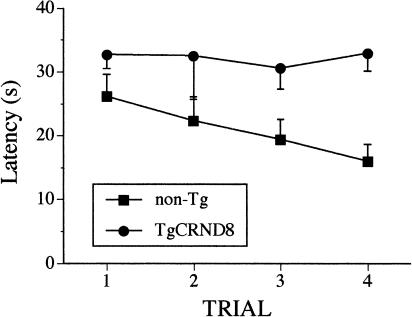
Spatial working memory was evaluated in a series-of-learning-reversals tests in which the mice had to learn a new location of a hidden platform in four consecutive trials administered daily. Latencies of each training trial were averaged for the last 6 d of a 12-d training for the analysis. TgCRND8 mice showed significantly impaired spatial working memory as compared with non-Tg littermates (F(1, 10) = 8.23, p < 0.02; Fig. 5). The mice of both genotypes did not differ in their swim speed nor in thigmotaxic swimming during training. Tg mice showed, on average, a significantly lower percentage of floating than non-Tg mice (F(1, 10) = 6.2, p < 0.05), which was not, however, significantly correlated with escape latency, swim path, or thigmotaxic swimming. Non-Tg mice, on the other hand, showed a tendency of making more frequent stops (periods of inactivity lasting up to 5 sec) during their navigation than the TgCRND8 mice; F(1, 10) = 2.5, p = 0.2). Trend analysis performed separately for each genotype revealed a significant linear improvement in the latencies for the non-Tg mice (F(3, 15) = 4.7, p < 0.02) but not for TgCRND8 mice, which did not change their escape latency over the four training trials (none of the polynomial components were significant).
Figure 5.
The average (±SEM) escape latency (in seconds) in a working memory version of the Morris water maze test in which the mice had to learn a new location of submerged platform within four daily training trials. TgCRND8 mice showed escape latencies significantly longer than the non-Tg mice (p < 0.02). The scores represent the average over the last 6 d of a 12-d training period.
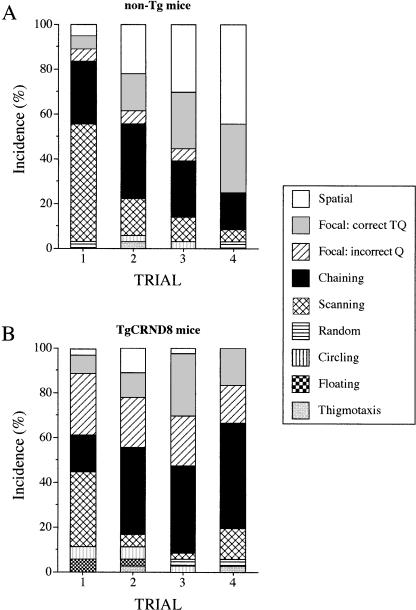
The distributions of search strategies performed during training in a working memory and reference memory versions of MWM test are shown in Figures 6 and 7, respectively. During the working memory training, in the first trial of the day when mice had to find a new location of the escape platform, non-Tg mice predominantly scanned the pool (53%) or to a lesser extent, used a nonspatial, chaining strategy (28%; Fig. 6A). In subsequent trials, both scanning and chaining strategies decreased (to 6% and to 17%, respectively, in the last, fourth trial), while the mice increasingly used spatial focal search and spatial strategies during their navigation (75% of incidences for both strategies combined in the last, fourth trial of training; Fig. 6A). TgCRND8 mice, on the other hand, did not use spatial strategies when searching for the platform (Fig. 6B). Although, similar to non-Tg mice, they showed a considerable amount of pool scanning during the first trial of the day (33%), they also performed frequent focal searches in quadrants that did not contain the platform. These focal searches of incorrect quadrants were observed in Tg mice throughout all trials, at a rate of ∼20% (Fig. 6B). Most importantly, in later trails, the Tg mice were not able to develop and use focal or direct spatial strategies during their navigation. Instead, the mice appeared to adopt a nonspatial, chaining strategy, which predominated in trials 2 and 3 at a rate of 39%, and increased to 47% in trial 4 (Fig. 6B).
Figure 6.
The distribution of search strategies used by mice in a working memory version of MWM test. In the first trial of the day, non-Tg mice predominantly scanned the pool, and in later trials they used predominantly spatial strategies (focal and mapping) to navigate to the platform (A). In contrast, the TgCRND8 mice did not use spatial strategies reliably. Instead, they used predominantly a chaining strategy to locate the platform (B), or occasionally searched the pool in incorrect quadrants, not containing the platform. These data represent an average percentage of each strategy performed in a trial over the last 6 d of the 12-d training period. See Materials and Methods for strategies definitions and Figure 1 for representative examples.
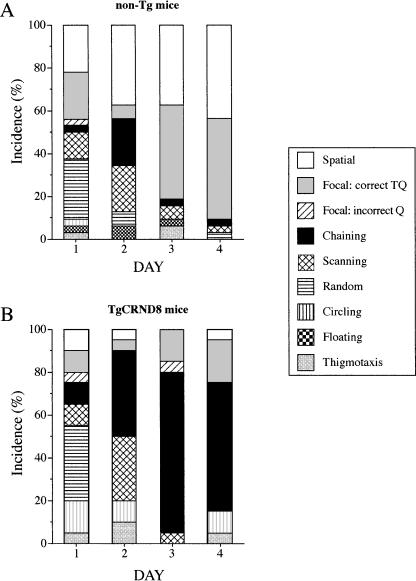
Figure 7.
The distribution of search strategies used by mice during learning acquisition in a conventional reference memory Morris water maze test. Although both non-Tg and TgCRND8 mice searched randomly or scanned the pool during the first day of the test, by the end of training (days 3 and 4), control, non-Tg mice used predominantly spatial strategies (A), and TgCRND8 mice used chaining strategy during their navigation to the platform. These data represent an average percentage of each strategy performed during day of the four-day learning acquisition test.
The distribution of search strategies during each day of training in the reference memory MWM test is shown in Figure 7. At the beginning of training, both non-Tg and TgCRND8 mice searched the pool using a random strategy (28% and 35%, Fig. 7A and 7B, respectively). As training progressed, in the second day of training, the non-Tg mice switched to scanning (22%) and chaining (22%) strategies, but also increasing the proportion of direct swims to the platform. Later, during days 3 and 4, random and chaining strategies were virtually absent and were replaced by spatial strategies (Fig. 7A). In contrast, TgCRND8 mice continued to use random and chaining strategies during the second day of the test, with chaining strategy becoming a dominant search strategy on later days (75% and 60% for days 3 and 4, respectively; Fig. 7B).
Statistical analysis of chaining strategy using scores extracted by the Wintrack program confirmed the qualitative analysis of data obtained in working and reference memory MWM tests. In the case of the working memory test, TgCRND8 mice showed a tendency to use chaining at higher than non-Tg mice rates during navigation (F(1, 10) = 3.05, p = 0.1, genotype factor). Their average chaining scores did not change during training trials (9.4 ± 2.2, 7.8 ± 1.8, 9.5 ± 2.6, 8.3 ± 2.0 for trials 1 to 4, respectively). In contrast, non-Tg mice showed fewer chaining responses, especially in later trials (7.9 ± 1.3, 5.3 ± 0.9, 5.2 ± 1.8, 3.0 ± 0.8, for trials 1 to 4, respectively). Analysis of chaining in trials 2 to 4 confirmed that TgCRND8 mice showed a tendency to rely on a chaining strategy more often than the non-Tg littermates (F(1, 10) = 4.1, p = 0.07). The chaining response of non-Tg mice was reduced as the training trial progressed (F(3, 30) = 2.3, p = 0.1, genotype × trial interaction). Similarly, during the reference memory test, TgCRND8 mice relied on chaining strategy significantly more often than non-Tg littermates (F(1, 11) = 14.5, p < 0.01, genotype factor). Their average chaining scores were within a range of 8-13 on days 2 to 4, whereas non-Tg mice performed, on average, 3 incidences of chaining (F(3, 33) = 5.2, p < 0.01, genotype × days interaction). In summary, although the criteria for evaluation of chaining strategy using the computer program were more stringent (see Materials and Methods) than evaluation of swim paths by the experimenter, both methods led to the similar conclusion that the TgCRND8 mice showed deficiencies in the use of spatial strategies, but were able to solve the task adopting the alternative, nonspatial strategy of chaining during working and reference memory versions of MWM test.
DISCUSSION
The present study demonstrated that 25- to 30-week-old TgCRND8 APP mice showed a significant impairment in spatial learning and memory in a conventional (hidden platform) reference memory Morris water maze test. In a cued version of the test, the TgCRND8 mice showed latencies comparable to non-Tg littermates' of reaching the platform when swimming to a stationary hidden platform marked by a visible cue. Moreover, when tested in probe trials after training in cued MWM tests, they showed a positive spatial memory comparable to non-Tg littermates' for a platform location. However, because the cued reference memory version of the MWM test does not require the development of spatial mapping strategy for its solution, it is not surprising that non-Tg, control mice developed a weaker spatial bias for the platform site than their counterparts trained in the conventional MWM conditions (Figs. 3 and 4, non-Tg). Therefore, the parsimonious explanation of the present results could be that in cued MWM conditions, both TgCRND8 and non-Tg mice were able to develop spatial memory for the platform site at the ceiling level for these training conditions. On the other hand, when trained under more demanding conventional (unmarked hidden platform) conditions, the Tg mice demonstrated a significant spatial memory impairment.
The present study also demonstrated for the first time that APP TgCRND8 mice were significantly impaired in spatial working memory. The cause of this impairment could not be attributed to consistent thigmotaxic swims, which would indicate the lack of behavioral flexibility observed in mice with CREB mutations (Gass et al. 1998), because the TgCRND8 mice did not differ from non-Tg littermates in thigmotaxic swims, swim speed, or floating rate. The impairment in working memory in TgCRND8 mice is in agreement with the documented impairment in this memory system in another APP transgenic mouse model, Tg2576, and in double transgenic Tg2576(APP) × PS1 mice tested in the six-arm radial-arm water maze (Morgan et al. 2000). The memory impairment in AD patients is often difficult to specify precisely because of the heterogeneity of psychopathology, confounding impairments in other faculties, or difficulties in determining the duration of illness, but the general consensus is that the working memory system is compromised first at early stages of the disease development (Morris and Baddeley 1988; Hulme et al. 1993; Hodges and Patterson 1995; Desgranges et al. 1996; Backman et al. 2001). In this respect, the impairment in working memory and task-dependent impairment in reference memory observed in the APP TgCRND8 transgenic mouse model of AD may likely correspond to early clinical stages of the disease.
Analysis of search strategies used by mice during navigation helps to explain further the behavioral nature of cognitive impairment of TgCRND8 mice in the MWM test. It revealed that whereas non-Tg mice used predominantly spatial strategies, swimming either directly to the platform or performing a focal search, the TgCRND8 mice searched the pool using a chaining strategy. During working memory training, they additionally performed a focal search strategy in incorrect quadrants of the pool (e.g., Figs. 6B and 1E), which was likely caused by daily displacement of the platform location. The search in incorrect quadrants of the pool was virtually absent in Tg mice during a conventional reference memory MWM training. The use of a chaining strategy by TgCRND8 mice indicates that they were able to learn the distance between locations of the escape platform and the wall of the pool (which, in the present experiment, was always constant), and they used this information as a reliable cue in a search for the platform. However, because chaining strategy was less efficient than spatial strategy in locating an escape platform, it resulted in longer escape latencies of Tg mice in both tests. The comparison of the distribution of search strategies in working and reference memory MWM test also explains the differences in learning speed observed in each test. The working memory training paradigm used in the present study required that mice should learn the platform location within four consecutive daily trials. This paradigm proved to be difficult for both non-Tg and Tg mice, which during the first days of training persistently searched the pool using random strategy, and their escape latencies were on average 30-35 sec (comparable to escape latencies in the first day of a conventional reference memory training) with no significant improvement over trials (data not shown). Mice showed a reliable performance and improved their escape latencies only in the second week of training. By that time, all mice likely learned that the platform was always located within an inner area of the pool at a constant distance from its wall. Therefore, both non-Tg and Tg mice started their search for a new platform location using more efficient scanning or chaining strategies (Trial 1, Fig. 6). Because the platform location was changed daily, even non-Tg mice continued to perform chaining and scanning of the pool at low rates throughout training, which resulted in less rapid, but significant improvement in their escape latencies (Fig. 6A). The predominant use of a chaining strategy and searching in the incorrect quadrants of the pool by TgCRND8 mice (Fig. 6B) resulted in virtually no improvement in their latencies across training trials in the working memory test (see Fig. 5). On the other hand, in a conventional reference memory MWM test, all mice began to search the pool using a random strategy, but the stationary location of the platform virtually eliminated chaining in non-Tg mice, which quickly adopted spatial search strategies, thus rapidly decreasing their escape latency during training (Fig. 7A). On the other hand, the predominant reliance of TgCRND8 mice on chaining during all stages of reference memory training resulted in only slight improvement in their performance (Fig. 7B). Similar results of choosing a nonspatial chaining search strategy or “looping pattern of swimming” were reported in rats during training conditions when the choice of spatial mapping strategy was prevented by occlusion of extra-maze cues (Sutherland and Dyck 1984), or when the function of the septo-hippocampal cholinergic system was compromised (Sutherland et al. 1982).
Although the focus on search strategies extends previously documented complex behavioral analysis of spatial navigation in the Morris water maze (Whishaw 1985b; Whishaw and Mittleman 1986; Bannerman et al. 1995; Saucier and Cain 1995; Cain et al. 1996, 1997; Saucier et al. 1996; Cain 1997; Gass et al. 1998; Janus et al. 2000a), it has to be noted, however, that a post hoc classification of search strategies into mutually exclusive categories can potentially introduce a bias in the interpretation of behavior in the MWM test. Such classification only indicates which strategy predominates during navigation, but, for example, fails to reflect transitions between strategies within a single trial. Also, this type of classification does not take into account random behavioral events, but assumes that an animal follows a specific set of search rules appropriate to learned information about the environment. However, there are instances when a mouse finds an escape platform by chance, for example, in the case of the present experiment, during the first trial of the day during working memory training (when the mice had no previous knowledge of the platform location). These coded paths were classified as spatial strategies, and although they occurred at low frequency (Fig. 6), it is important to distinguish such occurrences.
The present study demonstrates that the impairment in spatial working and reference memory of TgCRND8 mice was caused by inefficient use of a spatial mapping strategy. However, the TgCRND8 APP mice showed intact behavioral flexibility in the choice of alternative, nonspatial strategies while solving a spatial cognitive task. This may have practical implications when testing potential AD therapeutics. In longitudinal experimental paradigms that focus on the time course of a drug effect, negative behavioral results may lead to false conclusions regarding treatment efficacy, if the treated mice learn to use a less efficient strategy for solving a problem before the positive effect of the drug engages. For these types of experiments, groups of identically treated mice, but tested in cross-sectional manner at different time points from the onset of the treatment, should be used as a control for drug efficacy. The broader implication of the present study highlights the complexity of mouse behavior in the water maze, and points out that detailed analyses of search strategies may better explain which aspects of search behavior contribute to measures of compromised performance.
MATERIALS AND METHODS
Mice
The transgenic APP mice (TgCRND8) used in this study encoded a double mutation of familial Alzheimer's disease in the amyloid precursor protein 695 (Swedish; KM670/671NL plus Indiana; V717F) under the control of the hamster PrP gene promoter (Chishti et al. 2001). The mice were maintained on a hybrid genetic background (C57BL/6 × C3H) and were derived from crosses of TgCRND8 males (C57BL/6 × C3H) to C57BL/6 wild-type females. I used 24 mice (12 TgCRND8 and 12 non-Tg litter-mates), between 25 and 30 weeks old, with gender and weight balanced. Mice were genotyped by analysis of tail DNA with a human APP hybridization probe, as described previously (Chishti et al. 2001).
Apparatus and Testing Room
The water maze apparatus consisted of a circular pool (1.2 m in diameter and 0.47 m high) made of white plastic. The pool was filled to a depth of 20 cm with water (24°-25°C) that was made opaque by the addition of nontoxic white paint. During a conventional reference memory MWM training, an escape platform (10 cm in diameter), made of white plastic with a grooved surface for a better grip, was submerged 0.5 cm under the water level. During cued reference memory MWM training, the location of a hidden escape platform was marked with a centrally mounted post (10 cm high, 1 cm in diameter), which was painted with black-and-white horizontal stripes and fitted with a 2.5-cm white ball on the top.
All tests of the study were carried out in the same experimental room (3.6 × 4.6 m) containing three cage racks. One of the racks, which was fitted with an opaque back wall, divided the room into a small area for recording equipment and a testing area (3 × 3 m) in the middle of which the water maze pool was placed. Dark posters, different in shape (one per wall), and a small (40W) light in one of the room's corners provided additional distant landmarks in the testing area of the room. The swim path of a mouse during each trial was recorded by a video camera suspended 2.5 m above the center of the pool and connected to a video tracking system (HVS Image Advanced Tracker VP200, HVS Image) and a PC computer running HVS software.
Nonspatial Pretraining
Two days before the spatial training commenced, all mice underwent a nonspatial pretraining (NSP) to assess their swimming abilities and familiarize them with the requirements of the test. During the NSP, the pool was surrounded by a white curtain, and each mouse was carried to the pool in a covered opaque holding cage using a disorientation procedure (Dudchenko et al. 1997; Martin et al. 1997). This involved a complete horizontal, clockwise or anticlockwise rotation of the cage before entering the curtain at semirandomly chosen points. During the first day of NSP, each mouse was first placed on a cued platform located in the center of the pool and allowed to remain there for 20 sec. In the following three trials, a mouse was released into water about 10 cm away from the cued platform. If a mouse failed to swim to the platform or stay on it for 20 sec, it was placed on the platform by an experimenter. During the second day of NSP, four 60-sec trials of swimming to the cued platform were administered. In these trials, mice were released into the water facing the wall of the pool from semirandomly chosen cardinal compass points (N, E, S, W).
Cued and Conventional Reference Memory MWM Paradigms
A total of 14 mice (NTg = 7; Nnon-Tg = 7) were allocated to cued reference memory, and 10 mice (NTg = 5; Nnon-Tg = 5) were allocated to a conventional reference memory training paradigm. One day after the end of NSP, the curtains surrounding the pool were removed and the mice were given 4 d of training with four 60-sec training trials (ITI = 20-30 min) per day in the cued or conventional versions of the MWM test. The platform was always placed in the same spatial location of the pool (NE quadrant) throughout the training period in both paradigms. During each trial, a mouse was released into water facing the wall of the pool from semirandomly chosen cardinal compass points. After climbing a platform, the mouse was allowed to stay on it for 20 sec.
On day 4, 1 h after the last training trial, all mice were given a probe trial to evaluate their spatial memory for the platform position. A second probe trial was administered the following day (24 h after training). During both probe trials, the escape platform was removed from the pool and the mice were allowed to search the pool uninterrupted for 60 sec.
Series of Spatial-Learning-Reversals Test (Working Memory Version of MWM)
Six weeks after the end of the first series of experiments, the cohort of mice trained in the cued reference memory MWM test (NTg = 6, Nnon-Tg = 6; one non-Tg mouse was removed as a non-learner because it continuously performed thigmotaxic swim, and one Tg mouse died) was trained in a series of spatial-learning-reversals tests. In this test, each mouse was given four consecutive 60-sec training trials (ITI = 10-15 sec) every day for 12 d. The location of a hidden escape platform was in the center of one of the pool's quadrants for all four trials each day, but was changed semirandomly between days according to the following pattern (repeated twice): SE, NW, NE, SW, SE, NW. To prevent the use of a nonspatial strategy involving learning in consecutive training trials a sequence of movements or turns (O'Keefe and Nadel 1978; Whishaw 1985a; Whishaw and Tome 1987), the release points of mice into the pool were counterbalanced for each trial to control for their spatial reference to the platform location. For example, the release points in two consecutive trials were never in the same relation (to the right or to the left) from the platform location. Also, the distance of a release point from the platform location was randomized and counterbalanced to prevent the development of search strategy within close proximity to the release point. Upon finding the platform, the mice were allowed a 20-sec post-trial period on the platform.
Data Analysis
The search paths of each mouse were extracted and plotted by HVS Image software developed by Richard Baker (HVS Image), and the Wintrack program developed by David Wolfer (Wolfer et al. 2001). The following variables characterizing the performance of mice in the water maze were chosen for analysis: latency—time (in seconds) it took a mouse to reach and climb the platform, and a length of a swim path (in centimeters). In most cases, the latency and the path length were highly positively correlated; therefore, only the latency was reported. The locomotor activity of the mice was analyzed using an average swim speed (in meters per second, excluding bouts of inactivity or floating); floating (percent of time with swim speed below the 0.06-m/sec threshold), the latency of search (time in seconds) to start active search after being released into water), and thigmotaxic swim (percent of path parallel to the pool's wall within a 12-cm distance from the wall). The spatial memory for the platform location during probe trials was evaluated by the analysis of the dwelling time in each of the pool's quadrants, and the analysis of an annulus crossing index (ACI). The ACI represents the number of crosses over the platform site in a quadrant that contained the escape platform (target quadrant, TQ) adjusted for crosses over platform sites in alternative quadrants; that is, ACI = the number of site crosses in TQ minus an average of crosses of sites in the three other quadrants of the pool. ACI represents an unbiased evaluation of spatial memory in the MWM test controlling for nonspatial search strategies like chaining (see Fig. 1), where a mouse learns to search for the platform within the area of the pool at constant distance from the wall (Wehner et al. 1990; Gass et al. 1998; Wolfer and Lipp 2000), or persistent scanning of target and adjacent quadrants, which may yield high platform site crossing scores for both scanned quadrants. A strong bias for the spatial location of the platform is expressed by positive ACI values. An ACI that is not different from zero indicates that the mice searched platform sites in all four quadrants with comparable frequency. A high, positive ACI that is significantly different from zero indicates spatial memory for the platform location, whereas negative ACI values indicate that mice tend to search quadrants other than the target quadrant. Because the annulus crossing index transforms the raw data, the total number of platform crosses in all four quadrants of the pool was also analyzed. The search for the platform location during probe trials was also presented graphically using a surface analysis (Wintrack program; Wolfer et al. 2001). In the analysis, the whole surface of the pool was divided into 144 (12 × 12) tiles (squares), and the time spent by a mouse in each of the tiles was expressed as a ratio with reference to the time expected by chance. Positive values, >1, indicate a preference for the respective area of the pool.
Statistics
The scores of each mouse were averaged across four daily training trials in both cued and conventional reference memory MWM tests. In a working memory MWM test, the scores of each trial were averaged across the last 6 d of the 12-d training period. A factorial model analysis of variance (ANOVA) was used with the genotype (Tg vs. non-Tg littermates) as between subject, and training (days or trials) as within subject (repeated measure) factors. When necessary, degrees of freedom were adjusted by the Greenhouse-Geisser ε-correction for the heterogeneity of variance. For the probe trials, comparisons of ACI between the genotypes were done using the Student's t-test. The critical α level was set to 0.05 for all statistical analyses. Owing to space limitation, only significant results are reported.
Classification and Qualitative Analysis of Search Strategies
The coded swim paths for each mouse in each trial of the reference and working memory versions of MWM test were plotted and categorized into one of the following mutually exclusive search strategies (e.g., see Fig. 1). Thigmotaxis (wall-hugging swim): a persistent swim along the wall of the pool that could include sporadic swims toward the center of the pool. Random search: swimming over the entire area of the pool in straight swims (zig-zag pattern), or in wide circular swims. Scanning: the search path was restricted to a limited, often central, area of the pool. Chaining/serial visits: circular swimming (in anticlockwise or clockwise direction) at a fixed distance from the wall, in which the escape platform was located. Focal search: searching in a restricted area of the pool. The path in a focal search was characterized by a directional, straight swim to a specific area followed by dense concentration of superimposed loops and turns there. Focal search was divided into focal search in a target quadrant, and focal search in an incorrect quadrant. Spatial search was represented by a direct swim path to the location containing the escape platform. Additionally, two other swimming activities were identified and categorized. Floating: a state of inactivity without forward movement. The plotted path is short, often with thick or tight sections caused by nondirectional drift. The categorization of floating using plotted paths was always confirmed by inspection of percent of floating obtained from data extraction program. Circling: swimming in tight circles, often showing a general directional movement. The plotted path was short and wide when tight circles were performed, or had many visible loose anticlockwise or clockwise loops. The use of each search strategy was presented as a percent of incidences during each trial over the whole analyzed experimental period. An experimenter coding the strategies was not aware of the genotype and session and/or trial sequence.
Quantitative Analysis of Chaining Strategy
To substantiate and validate the qualitative evaluation of the strategies, chaining was analyzed statistically using data extracted by the Wintrack computer program. A predefined variable, chaining (chn - Barnes chain [#]), originally developed to evaluate upward and downward serial visits to holes in the Barnes maze test (Barnes 1979), was chosen for this analysis. To extract chaining scores, Wintrack superimposes a series of virtual platforms at the same distance from the wall of the pool as the escape platform on a map of the pool (Fig. 1I). The program then records the number of crosses of the plotted path over every virtual platform. A first chaining event is recorded when a path crosses over three adjacent virtual platforms. Each subsequent cross in a series denotes a successive chaining event. In the present analysis, the virtual platforms were configured to 15 cm in diameter, which resulted in 16 slightly overlapping platform images placed equidistantly from the wall. These chaining scores were analyzed by a factorial (genotype) repeated measures (days of training) ANOVA.
Acknowledgments
I thank D. Westaway, H. Welzl, D. Wolfer, and L. Lovasic for thoughtful comments and critical reading of the manuscript. Work in this laboratory was supported by the Alzheimer Society of Canada, the Institute of Aging (CIHR), and the Alzheimer Society of Sasketchwan.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.70104.
References
- Albert, M.S. 1996. Cognitive and neurobiologic markers of early Alzheimer's disease. Proc. Natl. Acad. Sci. 93: 13547-13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash, G.W., King, D.L., Gordon, M.N., Morgan, D., Hatcher, J.M., Hope, C.E., and Diamond, D.M. 2001. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 891: 42-53. [DOI] [PubMed] [Google Scholar]
- Backman, L. and Small, B.J. 1998. Influences of cognitive support on episodic remembering: Tracing the process of loss from normal aging to Alzheimer's disease. Psychol. Aging 13: 267-276. [DOI] [PubMed] [Google Scholar]
- Backman, L., Small, B.J., and Fratiglioni, L. 2001. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain 124: 96-102. [DOI] [PubMed] [Google Scholar]
- Bannerman, D.M., Good, M.A., Butcher, S.P., Ramsay, M., and Morris, R.G.M. 1995. Prior experience and N-methyl-D-asparate receptor blockade dissociate components of spatial learning in the watermaze. Nature 378: 182-186. [DOI] [PubMed] [Google Scholar]
- Barnes, C.A. 1979. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Compar. Physiol. Psych. 93: 74-104. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson, H.A., Hunter, C.L., Nelson, M.E., and Granholm, A.C. 2003. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 139: 47-57. [DOI] [PubMed] [Google Scholar]
- Cain, D.P. 1997. Prior non-spatial pretraining eliminates sensorimotor disturbances and impairments in water maze learning caused by diazepam. Psychopharmacology 130: 313-319. [DOI] [PubMed] [Google Scholar]
- Cain, D.P., Saucier, D., Hall, J., Hargreaves, E.L., and Boon, F. 1996. Detailed behavioral analysis of water maze acquisition under APV or CNQX: Contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav. Neurosci. 110: 86-102. [DOI] [PubMed] [Google Scholar]
- Cain, D.P., Beiko, J., and Boon, F. 1997. Navigation in the water maze: The role of proximal and visual cues, path integration, and magnetic field information. Psychobiology 25: 286-293. [Google Scholar]
- Chen, G.Q., Chen, K.S., Knox, J., Inglis, J., Bernard, A., Martin, S.J., Justice, A., McConlogue, L., Games, D., Freedman, S.B., et al. 2000. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature 408: 975-979. [DOI] [PubMed] [Google Scholar]
- Chishti, M.A., Yang, D.S., Janus, C., Phinney, A.L., Horne, P., Pearson, J., Strome, R., Zuker, N., Loukides, J., French, J., et al. 2001. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276: 21562-21570. [DOI] [PubMed] [Google Scholar]
- Desgranges, B., Eustache, F., Rioux, P., de La Sayette, V., and Lechevalier, B. 1996. Memory disorders in Alzheimer's disease and the organization of human memory. Cortex 32: 387-412. [DOI] [PubMed] [Google Scholar]
- Dudchenko, P.A., Goodridge, J.P., Seiterle, D.A., and Taube, J.S. 1997. Effects of repeated disorientation on the acquisition of spatial tasks in rats: Dissociation between the appetitive radial arm maze and aversive water maze. J. Exp. Psych. 23: 194-210. [DOI] [PubMed] [Google Scholar]
- Fratiglioni, L., Small, B.J., Winblad, B., and Bäckman, L. 2001. The transition from normal functioning to dementia in the aging population. In Alzheimer's disease: Advances in etiology, pathogenesis and therapeutics (eds. K. Iqbal et al.), pp. 3-10. John Wiley & Sons. Ltd., Chichester, UK.
- Gass, P., Wolfer, D.P., Balschun, D., Rudolph, D., Frey, U., Lipp, H.P., and Schütz, G. 1998. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn. Mem. 5: 274-288. [PMC free article] [PubMed] [Google Scholar]
- Golde, T.E. 2003. Alzheimer disease therapy: Can the amyloid cascade be halted? J. Clin. Invest. 111: 11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, J. and Selkoe, D.J. 2002. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297: 353-356. [DOI] [PubMed] [Google Scholar]
- Hodges, J.R. and Patterson, K. 1995. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia 33: 441-459. [DOI] [PubMed] [Google Scholar]
- Hsiao Ashe, K. 2001. Learning and memory in transgenic mice modelling Alzhiemer's disease. Learn. Mem. 8: 301-308. [DOI] [PubMed] [Google Scholar]
- Hulme, C., Lee, G., and Brown, G.D. 1993. Short-term memory impairments in Alzheimer-type dementia: Evidence for separable impairments of articulatory rehearsal and long-term memory. Neuropsychologia 31: 161-172. [DOI] [PubMed] [Google Scholar]
- Janus, C. and Westaway, D. 2001. Transgenic mouse models of Alzheimer's disease. Physiol. Behav. 73: 873-886. [DOI] [PubMed] [Google Scholar]
- Janus, C., D'Amelio, S., Amitay, O., Chishti, M.A., Strome, R., Fraser, P., Carlson, G.A., Roder, J.C., St. George-Hyslop, P., and Westaway, D. 2000a. Spatial learning in transgenic mice expressing human presenilin 1 (PS1) transgenes. Neurobiol. Aging 21: 541-549. [DOI] [PubMed] [Google Scholar]
- Janus, C., Pearson, J., McLaurin, J., Mathews, P.M., Jiang, Y., Schmidt, S.D., Chishti, M.A., Horne, P., Heslin, D., French, J., et al. 2000b. A peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408: 979-982. [DOI] [PubMed] [Google Scholar]
- Janus, C., Phinney, A.L., Chishti, M.A., and Westaway, D. 2001. New developments in animal models of Alzheimer's disease. Curr. Neurology Neurosci. Rep. 1: 451-457. [DOI] [PubMed] [Google Scholar]
- Martin, G.M., Harley, C.W., Smith, A.R., Hoyles, E.S., and Hynes, C.A. 1997. Spatial disorientation blocks goal location on a plus maze but does not prevent goal location in the Morris maze. J. Exp. Psych. 23: 183-193. [DOI] [PubMed] [Google Scholar]
- Morgan, D., Diamond, D.M., Gottschall, P.E., Ugen, K.E., Dickey, C., Hardy, J., Duff, K., Jantzen, P., DiCarlo, G., Wilcock, D., et al. 2000. A β peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408: 982-985. [DOI] [PubMed] [Google Scholar]
- Morris, R.G. and Baddeley, A.D. 1988. Primary and working memory functioning in Alzheimer-type dementia. J. Clin. Exp. Neuropsychol. 10: 279-296. [DOI] [PubMed] [Google Scholar]
- O'Keefe, J. and Nadel, L. 1978. The hippocampus as a cognitive map. Oxford University Press, Oxford.
- Price, D.L. and Sisodia, S.S. 1998. Mutant genes in familial Alzheimer's disease and transgenic models. Annu. Rev. Neurosci. 21: 479-505. [DOI] [PubMed] [Google Scholar]
- Saucier, D. and Cain, D.P. 1995. Spatial learning without NMDA receptor dependent long-term potentiation. Nature 378: 186-189. [DOI] [PubMed] [Google Scholar]
- Saucier, D., Hargreaves, E.L., Boon, F., Vanderwolf, C.H., and Cain, D.P. 1996. Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behav. Neurosci. 110: 103-116. [PubMed] [Google Scholar]
- Selkoe, D.J. 1997. Alzheimer's disease: Genotypes, phenotypes, and treatments. Science 275: 630-631. [DOI] [PubMed] [Google Scholar]
- ____. 2002. Deciphering the genesis and fate of amyloid β-protein yields novel therapies for Alzheimer disease. J. Clin. Invest. 110: 1375-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, B.J., Fratiglioni, L., Viitanen, M., Winblad, B., and Backman, L. 2000. The course of cognitive impairment in preclinical Alzheimer disease: Three- and 6-year follow-up of a population-based sample. Arch. Neurol. 57: 839-844. [DOI] [PubMed] [Google Scholar]
- Sutherland, R.J. and Dyck, R.H. 1984. Place navigation by rats in a swimming pool. Can. J. Psych. 38: 322-347. [Google Scholar]
- Sutherland, R.J., Whishaw, I.Q., and Regehr, J.C. 1982. Cholinergic receptor blockade impairs spatial localization by use of distal cues in the rat. J. Compar. Physiol. Psych. 96: 563-573. [DOI] [PubMed] [Google Scholar]
- van Leuven, F. 2000. Single and multiple transgenic mice as models for Alzheimer's disease. Prog. Neurobiol. 61: 305-312. [DOI] [PubMed] [Google Scholar]
- Wehner, J.M., Sleight, S., and Upchurch, M. 1990. Hippocampal protein kinase C activity is reduced in poor spatial learners. Brain Res. 523: 181-187. [DOI] [PubMed] [Google Scholar]
- Westerman, M.A., Cooper-Blacketer, D., Mariash, A., Kotilinek, L., Kawarabayashi, T., Younkin, L.H., Carlson, G.A., Younkin, S.G., and Ashe, K.H. 2002. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J. Neurosci. 22: 1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw, I.Q. 1985a. Cholinergic receptor blockade in the rat impairs locale but not taxon strategies for place navigation in a swimming pool. Behav. Neurosci. 99: 979-1005. [DOI] [PubMed] [Google Scholar]
- ____. 1985b. Formation of a place learning-set by the rat: A new paradigm for neurobehavioral studies. Physiol. Behav. 35: 139-143. [DOI] [PubMed] [Google Scholar]
- Whishaw, I.Q. and Mittleman, G. 1986. Visits to starts, routes, and places by rats (Rattus norvegicus) in swimming pool navigation tasks. J. Comp. Psychol. 100: 422-431. [PubMed] [Google Scholar]
- Whishaw, I.Q. and Tome, J.-A. 1987. Colinergic receptor blockade produces impairments in a sensorimotor subsystem for place navigation in the rat: Evidence from sensory, motor, and acquisition tests in a swimming pool. Behav. Neurosci. 101: 603-616. [DOI] [PubMed] [Google Scholar]
- Wolfer, D.P. and Lipp, H.P. 2000. Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment. Exp. Physiol. 85: 627-634. [PubMed] [Google Scholar]
- Wolfer, D.P., Madani, R., Valenti, P., and Lipp, H.P. 2001. Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol. Behav. 73: 745-753. [DOI] [PubMed] [Google Scholar]