Abstract
Heart failure is common in older people and its prevalence is increasing. The Heart ‘omics’ in AGEing (HOMAGE) project aims to provide a biomarker approach that will improve the early diagnosis of heart failure. A large clinical database, based on (1) prospective population studies or (2) cross-sectional, prospective studies or randomized controlled trials (RCTs) of patients at risk for or with overt cardiovascular disease will be constructed to determine most promising ‘omics’-based biomarkers to identify the risk of developing heart failure and/or comorbidities. Population studies, patient cohorts and RCTs are eligible for inclusion in the common database, if they received ethical approval to obtain and share data and have baseline information on cardiovascular risk factors. Currently, the HOMAGE database includes 43,065 subjects, from 20 studies in eight European countries, including healthy subjects from three population studies in France, Belgium and Italy (n = 7,124), patients with heart failure (n = 4,312) from four cohorts in the UK, Spain and Switzerland and patients at high risk for cardiovascular disease (n = 31,629) in 13 cohorts. It is anticipated that more partners will join the consortium and enlarge the pooled data. This large merged database will be a useful resource with which to identify candidate biomarkers that play a role in the mechanism underlying the onset and progression of heart failure.
Keywords: left ventricle, heart failure, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction, population science, morbidity, mortality
INTRODUCTION
Heart failure is a progressive disease and is generally preceded by asymptomatic structural and functional changes in the heart. It further evolves into clinically overt heart failure, disability and death[1],[2]. The prognosis of heart failure remains poor[3] and the 5-year mortality rate of symptomatic heart failure is about 60%[4]-[6].
Heart failure is a major public health concern with a prevalence of about 1–2% of the adult population in developed countries. It is a common disease in older people (≥ 10% among persons ≥ 75 years) and its prevalence is increasing as more people survive into old age[4],[7]. Heart failure is the single most common cause for hospital admission (5% of all medical and geriatric admissions) in people aged over 65 years[4],[8]. Furthermore, it is complicated by many other medical problems common in older people, such as renal dysfunction, pulmonary disease, depression, cognitive decline, cerebrovascular disease and diabetes mellitus[4],[7].
The diagnosis of heart failure can be difficult, especially in the early stages, since many of the symptoms and signs of heart failure are non-specific and thus do not discriminate between heart failure and other problems[9]-[11]. Echocardiography is the most useful test in patients with suspected heart failure[12]. However, echocardiography is poorly suited for large scale routine screening. Plasma concentrations of natriuretic peptides are better suited as screening tool than echocardiography but their diagnostic accuracy is insufficient in detecting early cardiac dysfunction and they provide little information on etiology[13].
The availability of effective large-scale screening tools for the identification of patients at risk of heart failure (predictive markers) or at an early stage of heart failure (diagnostic markers) is a major unmet clinical need. The concept of the Heart ‘omics’ in AGEing (HOMAGE) project, an EU FP7 program led by Faiez Zannad (University of Lorraine, Nancy, France, www.homage-hf.eu) is to identify ‘omics’-based biomarkers that can detect pathological processes predictive of the development of heart failure to allow early therapeutic interventions for preventing heart failure. The first step in this process is to develop a large database of existing cohorts, including healthy individuals, persons at risk of developing heart failure and patients with heart failure. This database will be used to identify and validate the predictive value of ‘omics’-based biomarkers in the progression of heart failure.
MATERIALS AND METHODS
Selection of studies
Eligible studies include population studies, patient cohorts and randomized controlled trials (RCTs). The studies should have received ethical approval to obtain and share data, have baseline information on cardiovascular risk factors and subsequent follow-up reports of fatal and non-fatal outcomes, including heart failure. Fourteen partners (Supplementary Table 1, available online) involved in the HOMAGE-consortium contributed data from completed and ongoing studies. Supplementary table 2 (available online) gives a description of the work and work packages' leaders involved in HOMAGE.
For database management and statistical analyses, SAS software version 9.3 (SAS Institute, Cary, NC, USA) will be used.
Data collection
The HOMAGE database is constructed and maintained at the Studies Coordinating Centre in Leuven, Belgium. By sending their data, the contributing partner confirmed that their study complies with good clinical practice (Helsinki Declaration), all participants provided written informed consent and the study conformed to national regulations on clinical research in humans and privacy at the time of its conduct. Each investigator received summary statistics of his cohort, after integration of the data into the common database, to ensure that correct information is incorporated. The database will be held in strict confidence and will not be used in any publication without the permission of the investigators who have contributed their data.
Data requested from each contributing partner includes baseline characteristics of each participant, anthropometrics, previous medical history and medication use, routine hematological and biochemical measurements, ECG data and echocardiographic variables. If available, information on already measured biomarkers related to left ventricular dysfunction and heart failure is included. For cohorts where follow-up is still ongoing and that do not have outcome information available at this moment, the database will be updated at regular time points.
At the start of the project, each contributing partner provided a detailed data dictionary that lists all variables made available, describing the variables' names, formats, labels and coding systems used.
Definition of events
Within the HOMAGE consortium an endpoint adjudication committee is established to agree on definitions and criteria to classify clinical events and phenotypes, in particular heart failure events, left ventricular dysfunction, hypertension, diabetes, atrial fibrillation, chronic obstructive pulmonary disease, coronary, cerebrovascular and chronic kidney disease. This committee will retrospectively assess the quality of the endpoint adjudication of the available studies incorporated in the common database
Research objectives
The main goal of the HOMAGE project is to provide a novel set of ‘omics’ based biomarkers that will greatly improve the detection of patients at risk of developing heart failure, improve the accuracy of diagnosis and identify patient subsets based on mechanistic phenotypes with a greater likelihood of response to targeted preventive therapies. We therefore collected data from well-phenotyped cohorts, that include both healthy persons and patients at risk of developing heart failure, or with overt heart failure. The diversity of the populations in terms of age, geography and presence or absence of underlying diseases will provide unique information on the determinants and utility of candidate biomarkers.
First, we will validate the ability of distinct ‘omics’ biomarkers to predict the development of heart failure. Therefore, cases, persons who developed heart failure, and corresponding controls will be defined within the available cohorts.
Other analyses related to information available in the common database can be proposed by each investigator of the HOMAGE consortium and will first be reviewed by the HOMAGE steering committee in terms of the research question and statistical soundness.
RESULTS
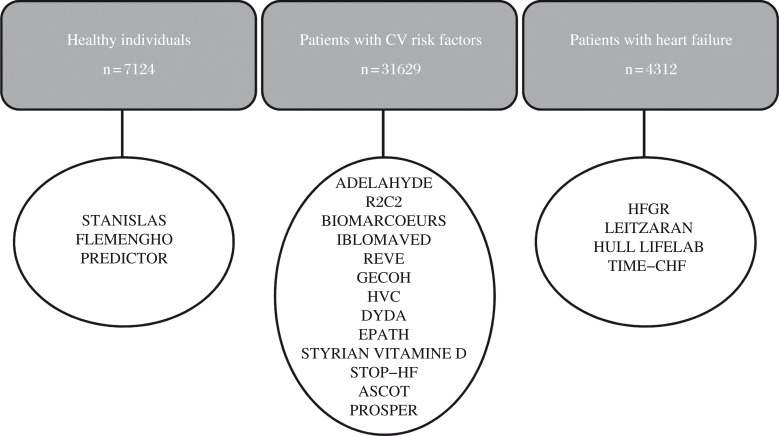
Currently, the HOMAGE database includes baseline information of 20 existing cohorts consisting of a total of 43065 subjects from eight European countries (Fig. 1). Of these 20 cohorts, three studies are population studies (Table 1). Thirteen cohorts (Table 2 and 3) consist of data from patients with cardiovascular risk and four studies include patients with (suspected) heart failure (Table 4). Information on outcome, if currently available, is given in Table 5. Inclusion of new studies and regular updates of existing cohorts on follow-up of the subjects and on information of morbidity and mortality is planned.
Fig. 1. Overview of studies included in the HOMAGE database.
Table 1. Baseline characteristics of population cohorts.
| Healthy subjects | STANISLAS | FLEMENGHO | PREDICTOR |
| Number of patients | 4295 | 828 | 2001 |
| Women, No. (%) | 2138 (50) | 421 (51) | 967 (48) |
| Age, years | 27±14 | 51±16 | 73±5 |
| Body mass index, kg/m2 | 21.9±4.4 | 26.6±4.4 | 26.5±4.2 |
| Weight, kg | 58.6±17.7 | 75.8±14.5 | 72.9±13.5 |
| Height, cm | 162±14 | 169±9 | 166±9 |
| Smokers, No. (%) | 793 (19) | 169 (20) | 259 (13) |
| Consume alcohol, No. (%) | 1501 (35) | 571 (69) | 1177 (59) |
| Hypertension, No. (%) | NA | 333 (43) | 1157 (58) |
| Diabetes mellitus, No. (%) | NA | 10 (1.2) | 332 (17) |
| Heart failure, No. (%) | NA | 6 (0.72) | 128 (6) |
| Systolic blood pressure, mmHg | 119±13 | 129±17 | 139±17 |
| Diastolic blood pressure, mmHg | 67±13 | 80±10 | 81±9 |
| Heart rate, beats per minute | 70±12 | 61±10 | 71±12 |
| Cholesterol, mmol/L | 5.2±1.1 | 5.3±0.97 | 5.3±1.0 |
| Glucose, mmol/L | 5.0±0.55 | 4.9±0.81 | 5.8±1.6 |
| Hemoglobin, g/dL | 14.2±1.2 | 13.8±1.3 | 14.0±1.3 |
| Red blood cell count, 1012 cells/L | 4.8±0.38 | 4.6±0.41 | 4.7±0.47 |
| White blood cell count, 109 cells/L | 7.0±1.8 | 6.4±1.8 | 6.4±2.8 |
| Platelets, 109 cells/L | 284±62 | 233±59 | 232±68 |
| Creatinine, μmol/L | 73.3±15.3 | 84.2±16.2 | 84.9±23.7 |
| BNP, pg/mL | NA | NA | NA |
| NT-proBNP, pg/mL | NA | NA | 92 (47–186) |
| Left ventricular mass, g | NA | 172±49 | 166±48 |
| Left ventricular ejection fraction, % | NA | 69±7 | 66±7 |
| Proportion with LVEF <40% | NA | 2 (0.28) | 28 (1.5) |
| Proportion with LVEF 40–50% | NA | 7 (0.98) | 33 (1.8) |
| Proportion with LVEF >50% | NA | 702 (98.7) | 1797 (96.7) |
| E/A ratio | NA | 1.3±0.50 | 0.80±0.25 |
Data are reported as number (%), mean±SD or median (25th–75th percentile) for non-normally distributed variables.
NA: data not available within the cohort
Table 2. Baseline characteristics of CV-risk patient cohorts.
| CV-risk patients | ADEDLAHYDE | R2C2 | BIOMARCOEURS | IBLOMAVED | REVE | GECOH | HVC |
| Number of patients | 378 | 169 | 1117 | 502 | 512 | 266 | 1880 |
| Women, No. (%) | 205 (54) | 87 (51.5) | 473 (42.3) | 156 (31.1) | 113 (22.1) | 162 (60.9) | 961 (51.1) |
| Age, years | 70±6 | 55±6 | 71±16 | 60±13 | 58±14 | 50±17 | 58±15 |
| Body mass index, kg/m2 | 28.0±4.3 | 28.8±5.3 | 26.6±6.2 | 27.0±5.4 | 27.1±4.7 | 28.7±6.3 | 27.3±5.4 |
| Weight, kg | 76.1±14.0 | 80.7±16.4 | 74.4±18.3 | 77.2±18.1 | 78.5±15.1 | 82.9±19.0 | 79.4±17.4 |
| Height, cm | 165±9 | 167±9 | 167±9 | 169±9 | 170±8 | 170±9 | 170±10 |
| Smokers, No. (%) | 30 (7.9) | 24 (14) | 261 (23) | 67 (14) | 238 (47) | 29 (11) | 362 (19) |
| Consume alcohol, No. (%) | 258 (68) | 125 (74) | 112 (10) | NA | NA | 114 (43) | NA |
| Hypertension, No. (%) | 378 (100) | NA | 614 (55) | 227 (45) | 209 (41) | 269 (100) | 784 (42) |
| Diabetes mellitus, No. (%) | 40 (11) | NA | 291 (26) | 100 (20) | 111 (22) | 25 (9) | 211 (11) |
| Heart failure, No. (%) | NA | NA | 361 (32) | 332 (66) | NA | NA | 53 (3) |
| Systolic blood pressure, mmHg | 139±18 | 125±14 | 138±30 | 124±23 | 111±16 | 154±23 | 143±24 |
| Diastolic blood pressure, mmHg | 74±10 | 77±9 | 79±17 | 76±14 | 64±12 | 94±14 | 83±12 |
| Heart rate, beats per minute | 64±10 | 63±9 | 94±25 | 72±16 | 70±13 | 71±11.9 | 74±14 |
| Cholesterol, mmol/L | 5.6±0.89 | 5.7±0.96 | NA | 4.4±1.3 | NA | 5.2±0.98 | 5.4±2.0 |
| Glucose, mmol/L | 5.7±1.5 | 5.1±0.89 | 8.5±4.8 | NA | NA | 5.3±1.4 | 6.0±4.0 |
| Hemoglobin, g/dL | NA | NA | 12.6±1.9 | 13.5±1.8 | NA | 14.1±1.2 | 14.2±1.4 |
| Red blood cell count, 1012 cells/L | NA | NA | NA | NA | NA | 4.8±0.42 | NA |
| White blood cell count, 109 cells/L | NA | NA | 10.0±6.4 | 7.5±2.4 | NA | 6.3±2.2 | NA |
| Platelets, 109 cells/L | NA | NA | NA | NA | NA | 253±65 | NA |
| Creatinine, μmol/L | NA | 84.6±11.9 | 116.2±90.4 | 108.9±66.8 | NA | 83.9±53.2 | 88.7±44.4 |
| BNP, pg/mL | NA | NA | 713 (211–1583) | 493 (315–1750) | NA | NA | NA |
| NT-proBNP, pg/mL | NA | NA | NA | NA | NA | 78 (40–167) | 32 (10–130) |
| Left ventricular mass, g | NA | 162±50 | NA | NA | NA | NA | NA |
| Left ventricular ejection fraction, % | NA | 68±7 | 45±17 | 46±20 | 49±9 | NA | 61±8 |
| Proportion with LVEF <40% | NA | 0 | 198 (37) | 217 (45) | 70 (14) | NA | 43 (3) |
| Proportion with LVEF 40–50% | NA | 0 | 91 (17) | 60 (12) | 193 (38) | NA | 54 (4) |
| Proportion with LVEF >50% | NA | 154 (100) | 246 (46) | 209 (43) | 244 (48) | NA | 1167 (92) |
| E/A ratio | NA | 1.2±0.33 | NA | NA | NA | NA | 1.2±3.3 |
Data are reported as number (%), mean±SD or median (25th–75th) for non-normally distributed variables.
NA: data not available within the cohort
Table 3. Baseline characteristics of CV-risk patient cohorts (continuation of Table 2 ).
| CV-risk patients | DYDA | EPATH | STYRIAN VITD | STOP-HF | ASCOT | PROSPER |
| No. of patients | 937 | 40 | 286 | 481 | 19257 | 5804 |
| Women, No. (%) | 358 (38) | 32 (80.0) | 136 (48) | 264 (55) | 4515 (24) | 3000 (52) |
| Age, years | 62±8 | 69±10 | 60±11 | 67±10 | 63±9 | 75±3 |
| Body mass index, kg/m2 | 28.6±4.4 | 28.7±6.4 | 29.5±4.8 | 30.3±4.8 | 28.7±4.6 | 26.8±4.2 |
| Weight, kg | 79.5±14.6 | 75.0±16.9 | 84.7±17.6 | 85.4±16.3 | 84.6±15.5 | 73.4±13.4 |
| Height, cm | 167±10 | 162±9 | 169±9 | 168±10 | 172±9 | 165±9 |
| Smokers, No. (%) | 179 (19) | 4 (10) | 42 (14) | 36 (8) | 6277 (33) | 1558 (27) |
| Consume alcohol, No. (%) | NA | 19 (48) | 239 (84) | 298 (62) | 14294 (74) | 3228 (56) |
| Hypertension, No. (%) | 602 (64) | 24 (60) | 286 (100) | NA | 19257 (100) | 3592 (62) |
| Diabetes mellitus, No. (%) | 937 (100) | 3 (8) | 58 (20) | 145 (30) | 5145 (27) | 623 (11) |
| Heart failure, No. (%) | 0 | 4 (10) | NA | 0 | 0 | 0 |
| Systolic blood pressure, mmHg | 137±15 | 143±20 | 141±18 | 132±17 | 164±18 | 155±22 |
| Diastolic blood pressure, mmHg | 80±8 | 87±11 | 87±12 | 82±11 | 95±10 | 84±12 |
| Heart rate, beats per minute | 73±10 | 66±11 | 63±10 | 70±12 | 72±13 | NA |
| Cholesterol, mmol/L | 5.0±0.95 | 5.1±1.1 | 5.2±1.2 | 4.7±0.94 | 5.9±1.1 | 5.7±0.91 |
| Glucose, mmol/L | 8.3±2.3 | 5.5±1.2 | 6.0±1.9 | 7.1±2.7 | 6.2±2.1 | NA |
| Hemoglobin, g/dL | NA | 13.7±1.4 | 14.2±1.3 | NA | NA | NA |
| Red blood cell count, 1012 cells/L | NA | 4.6±0.44 | 4.8±0.40 | NA | NA | NA |
| White blood cell count, 109 cells/L | NA | 6.1±1.8 | 5.9±1.6 | NA | NA | NA |
| Platelets, 109 cells/L | NA | 225±64 | 234±61 | NA | NA | NA |
| Creatinine, μmol/L | 80.3±23.7 | 73.5±15.8 | 82.7±23.6 | NA | 98.7±16.8 | NA |
| BNP, pg/mL | NA | NA | NA | 24 (12–52) | NA | NA |
| NT-proBNP, pg/mL | 36 (16–69) | 112 (51–186) | 85 (45–159) | NA | NA | NA |
| Left ventricular mass, g | 172±42 | NA | NA | 178±49 | NA | NA |
| Left ventricular ejection fraction, % | 61±5 | 64±9 | NA | 67±8 | NA | NA |
| Proportion with LVEF <40% | 3 (0.44) | 1 (3) | NA | 2 (0.42) | NA | NA |
| Proportion with LVEF 40–50% | 16 (2) | 1 (3) | NA | 9 (2) | NA | NA |
| Proportion with LVEF >50% | 669 (97) | 34 (94) | NA | 465 (98) | NA | NA |
| E/A ratio | 0.89±0.24 | 0.95±0.38 | NA | 0.85±0.27 | NA | NA |
Data are reported as number (%), mean±SD or median (25th–75th) for non-normally distributed variables.
NA: data not available within the cohort
Table 4. Baseline characteristics of heart failure patient cohorts.
| Heart failure patients | HFGR | LEITZARAN | HULL LIFELAB | TIME-CHF |
| Number of patients | 218 | 233 | 3239 | 622 |
| Women, No. (%) | 59 (27) | 109 (46) | 1068 (33) | 253 (40.7) |
| Age, years | 67±13 | 74±9 | 71±11 | 77±8 |
| Body mass index, kg/m2 | 28.7±5.6 | 31.3±5.3 | 29.2±6.0 | 25.6±4.4 |
| Weight, kg | 82.6±19.2 | 79.4±16.3 | 82.4±19.3 | 72.0±14.2 |
| Height, cm | 171±10 | 159±10 | 168±10 | 167±9 |
| Smokers, No. (%) | NA | NA | 300 (9) | 74 (12) |
| Consume alcohol, No. (%) | NA | 18 (8) | 1179 (36) | NA |
| Hypertension, No. (%) | 92 (42) | 233 (100) | 1115 (35) | 462 (74) |
| Diabetes mellitus, No. (%) | 75 (34) | 0 (0) | 784 (24) | 222 (36) |
| Heart failure, No. (%) | 218 (100) | 233 (100) | 2306 (71) | 622 (100) |
| Systolic blood pressure, mmHg | 121±22 | 148±24 | 137±25 | 122±20 |
| Diastolic blood pressure, mmHg | 68±11 | 85±13 | 79±14 | 72±12 |
| Heart rate, beats per minute | 72±14 | 79±20 | 74±17 | 76±14 |
| Cholesterol, mmol/L | 4.0±1.2 | 5.2±1.8 | 4.4±1.3 | 4.2±1.1 |
| Glucose, mmol/L | 8.0±3.3 | 6.0±1.7 | 6.8±2.9 | 6.7±2.4 |
| Hemoglobin, g/dL | 13.0±1.9 | 14.0±1.5 | 13.3±3.7 | 13.1±1.8 |
| Red blood cell count, 1012 cells/L | NA | 4.6±0.47 | NA | 4.3±0.60 |
| White blood cell count, 109 cells/L | 7.7±2.7 | 7.0±1.8 | 7.6±2.8 | 7.8±4.9 |
| Platelets, 109 cells/L | 223±71 | 223±58 | 242±95 | NA |
| Creatinine, μmol/L | 111.2±34.8 | 96.4±33.8 | 105.7±50.6 | 115.9±38.4 |
| BNP, pg/mL | NA | NA | NA | NA |
| NT-proBNP, pg/mL | NA | NA | 880 (279–2088) | 3836 (1919–6895) |
| Left ventricular mass, g | NA | 270.6±115.8 | 271.6±116.0 | NA |
| Left ventricular ejection fraction, % | 35±12 | 55±16 | 41±14 | 35±13 |
| Proportion with LVEF <40% | 70 (65) | 24 (17) | 880 (51) | 402 (65) |
| Proportion with LVEF 40–50% | 20 (19) | 21 (15) | 360 (21) | 108 (17) |
| Proportion with LVEF >50% | 17 (16) | 96 (68) | 481 (28) | 112 (18) |
| E/A ratio | NA | 0.90±0.46 | 1.1±0.85 | NA |
Data are reported as number (%), mean±SD or median (25th–75th) for non-normally distributed data.
NA: data not available within the cohort
Table 5. Outcome information.
| STANISLAS | FLEMENGHO | PREDICTOR | HULL LIFELAB | BIOMARCOEURS | REVE | HVC | DYDA | STOP-HF | TIME-CHF | ASCOT | PROSPER | |
| Number of patients | 2979 | 828 | 1851 | 3239 | 934 | 470 | 732 | 933 | 481 | 622 | 19257 | 5804 |
| Median FU, years(5th–95th percentile) | 6.9(4.2–18.3) | 6.1(3.7–7.4) | 3.9(2.7–5.0) | 4.7(1.8–8.9) | 1.1(0.23–2.4) | 1.0(0.91–1.3) | 0.9(0.05–4.8) | 2.0(1.8–2.3) | 2.0(1.0–2.5) | 2.2(0.1–4.7) | 5.5(3.9–6.6) | 3.3(1.7–3.8) |
| Total mortality | 18 (0.6) | 27 (3.3) | 135 (7.3) | 1007 (31) | 142 (15) | 19 (4.0) | 121 (17) | 15 (1.6) | 1 (0.2) | 247 (40) | 1558 (8.1) | 604 (10) |
| CV mortality | NA | 9 (1.1) | 42 (2.3) | 525 (16) | 57 (6.1) | 12 (1.3) | 18 (2.5) | 3 (0.3) | NA | 183 (29) | 605 (3.1) | 544 (9.4) |
| Fatal heart failure | NA | 2 (0.2) | 0 (0) | 141 (4.4) | NA | NA | 6 (0.8) | 0 (0) | NA | 97 (16) | 65 (0.3) | NA |
| Non-fatal heart failure | NA | 14 (1.7) | 36 (1.9) | 907 (28) | NA | NA | 25 (3.4) | 2 (0.2) | 1 (0.2) | 202 (32) | 244 (1.3) | 234 (4.0) |
Data are reported as median (5th–95th percentile) for follow-up (FU) or as number (%) for total mortality, cardiovascular (CV) mortality and fatal and non-fatal heart failure
NA: data not available within the cohort
Population cohorts
Description
The STANISLAS cohort is a family cohort consisting of 4,295 healthy participants (1,006 families). The participants were identified from the files of the State Health Insurance Fund and invited to the Center for Preventive Medicine of Vandoeuvre-lès-Nancy (eastern France). The first visit was scheduled between 1993-1995. After the initial examination, participants have been followed up 3 times every 5 years[14],[15].
The second population cohort is a large-scale family-based study on the genetic epidemiology of cardiovascular phenotypes, the FLEmish study on Environment, Genes and Health Outcome (FLEMENGHO). Recruitment started in 1985 through 1999, in a random sample of households living in a geographically defined area of Northern Belgium[16],[17]. From 2005 until 2010, 1,208 former participants were invited for a follow-up examination at the field center, including echocardiography. 828 participants renewed their informed consent and are included in the HOMAGE database. Follow-up of these participants is still ongoing.
A third population-based study is the PREDICTOR cohort. From 2007 to 2010, a sample of 2001 elderly (aged 65–84 years) residents of the Lazio region in central Italy, was randomly selected from the Regional Health Registry[18].
Characteristics
Baseline characteristics of these population cohorts, including 7124 subjects, are given in Table 1. Overall 3526 (49%) are women and 1221 are smokers (17%), with a mean (standard deviation [SD]) age of 43 (24) years and a mean (SD) body mass index of 24 (5) kg/m2.
Cardiovascular risk patient cohorts and RCTs
Description
ADELAHYDE is a cross-sectional study of 378 hypertensive, elderly (> 65 years) patients, treated with at least one antihypertensive agent and presenting with subjective memory complaints. Patients were recruited between 2003 and 2005 by local press advertisements or referred by general practitioners from an investigator network of the Inserm Clinical Investigation Center, France[19].
The R2C2 cohort on obesity and heart failure risk factors aims to investigate the transition from obesity to heart failure and consists of 169 subjects with abdominal obesity, born or living in France for at least 10 years, that had been recruited between 2006 and 2009[20]. No outcome information is available.
BIOMARCOEURS recruited 1,117 French patients with a primary complaint of acute dyspnea at the emergency department. Follow-up, by phone contact, was performed after one year[21].
The IBLOMAVED cohort includes 502 subjects, born or living in France for at least 10 years, and was designed to investigate biomarkers of asymptomatic left ventricular dysfunction. The selected subjects are both patients at risk of developing heart failure as well as patients with heart failure. IBLOMAVED is a cross-sectional study, with no follow-up data[22].
Together, REVE-1 (n = 266) and REVE-2 (n = 246) include 512 patients with anterior acute myocardial infarction and were designed to study left ventricular remodelling after myocardial infarction. Recruitment took place in Region Nord-Pas-de-Calais, France between 2002 and 2004 for REVE-1[23] and between 2006 and 2008 for REVE-2[24]. At this moment, all patients have been followed-up two times at 3 months and at 12 months after the baseline examination, during hospitalization.
The Graz Endocrine Causes of Hypertension (GECOH) study is a single center study in patients referred for endocrine hypertension to the outpatient clinic of a tertiary care center[25]. Currently, baseline data from 266 Austrian patients are available.
The HVC database consists at this moment of 1,880 patients referred to the cardiovascular outpatient clinic of Maastricht University, Netherlands, for a consultation by a cardiologist for the first time. Both baseline and follow-up information is available.
The left ventricular Dysfunction in DIAbetes (DYDA) cohort assessed the prevalence of left ventricular systolic or diastolic dysfunction in 937 patients with diabetes. Patients were recruited from 37 Italian diabetes referral centers from 2006 until 2008. Follow-up data up until 24 months are available[26].
EPATH is a single-center, double-blind, placebo controlled, randomized, parallel group trial in patients with primary hyperparathyroidism, who are randomized to receive eplerenone or placebo[27]. This trial is ongoing and at this moment baseline information of 40 subjects is available.
The Styrian Vitamin D Hypertension trial is a randomized double-blind placebo controlled trial with the main aim to study the effects of vitamin D supplementation on systolic blood pressure in vitamin D deficient hypertensive patients recruited from Austria. The trial is ongoing and currently baseline data from 286 subjects are available.
The St Vincent's Screening to Prevent Heart Failure Study (STOP-HF) is a parallel-group randomized trial involving 1,374 participants with cardiovascular risk factors recruited from 39 primary care practices in Ireland between 2005 and 2009 and followed up until 2011, with a mean follow-up time of 4.2 years[28]. Patients were randomly assigned to receive usual primary care or screening with brain natriuretic peptide (BNP) testing (n = 697). Baseline and follow-up information of 481 of the 1,374 subjects is now included in the common database.
Patients included in the multicenter, prospective, randomized Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) are also included in the common database[29],[30]. The 19,257 hypertensive patients were recruited between 1998 and 2000, and randomized to amlodipine adding perindopril as required to reach blood-pressure targets (amlodipine-based regimen) or atenolol adding bendroflumethiazide and potassium as required (atenolol-based regimen). The median follow-up was 5.5 years.
The PROspective study of pravastatin in the elderly at risk (PROSPER) cohort contributes 5804 elderly subjects (≥ 70 years) with vascular disease or at high risk of developing vascular disease. This double-blind, randomized, placebo-controlled trial was designed to examine the hypothesis that pravastatin, at a dose of 40 mg/day, would reduce the risk of cardiovascular and cerebrovascular events in elderly subjects. Recruitment took place between 1997 and 1999, with a median follow-up of 3.3 years[31],[32].
Characteristics
The baseline characteristics of these 31,629 patients are given in Table 2 and 3. The thirteen cohorts (both observational studies and RCTs) include 10,462 women and 9,107 smokers with a mean (SD) age of 65 (11) years and a mean (SD) body mass index of 28 (5) kg/m2. Twenty-four percent of the patients suffer from diabetes mellitus, while 85 % report a history of hypertension.
Heart failure patient cohorts and RCTs
Description
The Heart Failure Glucose Regulation (HFGR) cohort consists of baseline data from 218 patients with heart failure and left ventricular systolic dysfunction, attending heart failure clinics in Manchester. Patients are followed up every 12 months over a 5 year period. As follow-up is still ongoing, no outcome information is available at this moment.
LEITZARAN contributes data from 233 Spanish patients with heart failure of hypertensive origin. Currently, no follow-up information is available.
The HULL LIFELAB cohort includes 3,239 patients with suspected heart failure. Both baseline and outcome information are available within this cohort.
Data from The Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) were contributed to the database[33]. TIME-CHF is a multicenter, randomized trial conducted in 15 centers in Switzerland and Germany. Patients aged 60 years or older with dyspnea (New York Heart Classification NYHA class ≥ II with current therapy), a history of hospitalization for heart failure within the last year, and an nt-proBNP level of ≥ 400 pg/mL (in patients younger than 75 years) or a level of 800 pg/mL (in patients aged 75 years or older) were eligible for inclusion. The study randomized 499 symptomatic heart failure patients with a left ventricular ejection fraction of ≤ 45% to intensified, NT-proBNP-guided versus a standard, symptom-guided therapy. Baseline data from 622 patients are included in the common database (499 randomized patients + additional 123 patients with a left ventricular ejection fraction of > 45%).
Characteristics
Table 4 summarizes the baseline characteristics of the 4,312 heart failure patients from these four studies, with mean (SD) age of 72 (11) years, and with a proportion of 1,376 (53%) patients with left ventricular ejection fraction below 40%. In HFGR, 25% of patients were classified into NYHA class I, 51% to class II, 23% to class III and 1% to class IV.
Fifty percent of patients in HULL LifeLab were classified to NYHA class II, 28% to class III and 2% to class IV. For TIME-CHF, 24% of the patients belonged to NYHA class II, 62% to class III and 14% to class IV.
DISCUSSION
Our database already includes 43065 subjects, of which more than half (51.6%) are older than 65 years and about 20% are older than 75 years. The main goal of the HOMAGE project is to provide a novel set of ‘omics’ based biomarkers that will greatly improve the detection of patients at risk of developing heart failure, improve the accuracy of diagnosis and identify patient subsets based on mechanistic phenotypes with a greater likelihood of response to targeted preventive therapies. The first step in this process is the development of the common database consisting of existing cohorts to validate the most promising biomarkers for the prediction of incident heart failure in elderly patients at risk of or with asymptomatic left ventricular dysfunction. Furthermore, the cohort will also provide epidemiological data from a wide number of partner institutions across Europe regarding the prevalence of comorbid conditions, treatments and outcomes in patients with cardiovascular disease and at high risk for developing heart failure as well as in patient with heart failure.
Most of the cohorts do not include patients with overt heart failure stage C and D as defined by the ACC/AHA working group.34 Incident cases of heart failure, either using the definition supplied for the individual population and patient cohorts or using the common criteria developed by the adjudication committee, will be compared with matched controls, also selected from the included ‘healthy’ cohorts. This will be used to identify one or more biomarker ‘footprints’ for new-onset heart failure. Further prospective validation of this biomarker profile will be done, on the one hand, by identifying new cases of heart failure within the available cohorts that continue to follow their patients and, on the other hand, by setting up a new trial in elderly patients at high risk of heart failure investigating the safety and therapeutic response to mineralocorticoid receptor antagonists according to ‘omics’-based biomarker profiles.
Treating hypertension is highly effective at delaying the onset of and perhaps preventing heart failure in older people[35]. However, not all antihypertensive agents are similarly beneficial, but the number needed to treat is rather high[36],[37]. Other preventive therapies targeting glycemic control and cholesterol have been disappointing. Many patients with heart failure have associated comorbidities such as hypertension, diabetes mellitus, ischemic heart disease, atrial fibrillation, chronic lung disease, renal dysfunction and anemia. Stratification beyond such gross phenotypes using ‘omic’ technologies may help target therapeutic intervention more safely and effectively.
Candidate ‘omics’-based biomarkers (Supplementary Table 3, available online) relevant to heart failure, processes of aging, inflammation, fibrosis or myocyte damage and related to main comorbid conditions of heart failure in elderly people have been selected after a systematic search of relevant scientific and patent databases after consulting experts within the present HOMAGE consortium. All candidate biomarkers have passed the discovery and verification phase, are published or patented by SMEs or academic groups, including groups in the HOMAGE consortium and are readily measurable by available techniques. Emphasis is on validation of clinically useful biomarkers that can predict the risk of developing heart failure, help make an early diagnosis or help choose the right treatment, at the right time for the right patient. The consortium is also open for discovery of novel markers, novel insights into pathophysiology and identification of novel therapeutic targets.
Each investigator of the HOMAGE consortium can propose analyses using the information available in the common database that eventually could lead to the discovery of new biomarkers, patents or publications.
In conclusion, HOMAGE has assembled a very large set of international data and biological samples focusing on patients at risk of, or with heart failure, that will be an important platform for bio-marker discovery. This research will increase understanding of the mechanisms underlying the development and progression of heart failure. It will also focus attention on the need to select, more precisely, treatments that are safe and highly effective rather than the traditional cardiovascular approach that often relies on modest average benefits, at a population level, that usually reflects the net impact of a large benefit in a few and no effect or harm in others. We should and can do better than this!
Supplementary Table 1. Contributing HOMAGE investigators.
| Leuven, Belgium | Kei Asayama, Yumei Gu, Asuza Hashimoto, Judita Knez, Yanping Liu, Thibault Petit, Fangfei We, Zhenyu Zhang |
| Inserm Nancy, France | Adriano Burcheri-Curatolo, Daniele Dobre, Nicoloas Girerd, Ludovic Merckle, Anne Pizard, Faiez Zannad |
| Inserm Paris, France | Oksana Kovalska, Damien Logeart, Saïd Laribi, Marie-France Seronde, Ali Tahraoui, Nicolas Deye, Jean-Marie Launay, Philippe Manivet, Remi Bonnet, Béatrice Lemosquet, Nicolas Segal, Patrick Plaisance, Malha Sadoune, Nicolas Vodovar, Alain Cohen Solal |
| Inserm Toulouse, France | Franck Desmoulin, Manon Barutaut, Michel Galinier, Matthieu Berry, Celine Caubere, Maria Evaristi, Pauline Fournier, Annie Turkieh, Fatima Smih |
| Inserm Lille, CHRU, Lille, France | Nicolas Lamblin, Marie Fertin, Gilles Lemesle |
| Graz, Austria | Andreas Meinitzer, Caterina Colantonio, Albrecht Schmidt, Elisabeth Kraigher-Krainer, Rolf Wachter, Frank Edelmann |
| Manchester, UK | Ludwig Neyses, Christi Deaton, Martin Yuille, Muhammad Khan, Robert Oliver |
| Pamplona, Spain | Arantxa González, Begoña López, Ramon Querejeta, Elena Zubillaga |
| Dublin, Ireland | Mark Ledwidge, Eoin O'Connell, Chris Watson, Kenneth McDonald |
| Hull, UK | Andrew Clark, Rachel Nicholls, Syed Kazmi |
| Maastricht, Netherlands | Mark Hazebroek, Sema Bektas, Sandra Sanders-van Wijk, Kimberly Everaerts, Desiree Rutten, Matthias E. Pfisterer (Basle Switzerland), TIME-CHF investigators |
| Milan, Italy | Nera Agabiti, Alessandro Boccanelli, Marco Comaschi, Marina Davoli, Carlo B. Giorda, Roberto Latini, Donata Lucci, Aldo P. Maggioni, Serge Masson, Flavia Mayer, Gian Francesco Mureddu |
| London, UK | Neil Poulter, Bjorn Dahlof, Eoin O'Brien |
| Glasgow, UK | Naveed Sattar, Paul Welsh, Ian Ford (PROSPER), Wouter Jukema (Eindhoven, The Netherlands; PROSPER), Brendan Buckley (Cork, Ireland; PROSPER) |
Supplementary Table 2. Work description and work package leaders within the HOMAGE consortium.
| Work package title | Principal investigator | Institute |
| General coordination | Faiez Zannad | Inserm, France |
| Coordination of clinical studies and centres | Stéphanie Grojean | Fondation Transplantation EDDH, France |
| Cohorts studies for diagnosis and risk prediction | Faiez Zannad | Inserm, France |
| Prediction of therapeutic response | John Cleland | University of Hull, UK |
| Biobanking and bio-informatics | Stephane Heymans | Maastricht University, Netherlands |
| Bio-assay | Jim Curry | Randox Testing Service, UK |
| Data management and statistical analysis | Jan A. Staessen / Stuart Pocock | University of Leuven, Belgium / London School of Hygiene, UK |
| Industrial application, regulatory consultation and market research | Yigal Pinto / Joost Leenders | ACS Biomarker, Netherlands |
| Dissemination and changing clinical practices | Faiez Zannad / Patricia Joseph-Mathieu | Inserm, France / Inserm Transfert, France |
| Management and coordination | Patricia Joseph-Mathieu | Inserm-Transfert SA, France |
Supplementary Table 3. List of candidate proteomics-based biomarkers.
| Domain | Candidate marker | Processes | Biofluid (volume needed) |
| miRNA-omics | miR-208, -499, -423-5p, -221, -132, -21, -126, -18 &- 19 and -22 & -24 | F, I, M, A | Serum/plasma (0.5 mL) |
| Transcriptomics | Neutrophil gelatinase-associated lipocalin (NGAL) | I, M | Serum/plasma (0.1 mL) |
| Anaplastic lymphoma receptor tyrosine kinase (ALK) | M | PBMC (5 mL for all) | |
| Nerve growth factor beta (NGFb) | M | ||
| F-box and WD repeat domain containing 7 (FBXW7) | M | ||
| Ferrochelatase (FECH) | M | ||
| CX3C chemokine receptor 1 (CX3CR1) | I, M | PBMC (1 mL for all) | |
| Chemokine (C-C motif) receptor 2 (CCR2) | I, M | ||
| CD28 | F, I, M, A | PBMC (2 mL for all) | |
| CD69 | F, I, M, A | ||
| Lymphocyte-specific protein tyrosine kinase (LCK) | F, I, M, A | ||
| Heme oxygenase 1 (HMOX1) | F, I, M, A | ||
| TNF receptor superfamily member 1A (TNFRSF1A) | F, I, M, A | ||
| B-cell CLL lymphoma 2 (BCL2) | F, I, M, A | ||
| Caspase 8 | F, I, M, A | ||
| Chemokine (C-C motif) ligand 5 (CCL5) | F, I, M, A | ||
| DNA damage-inducible transcript 3 (DDIT3) | F, I, M, A | ||
| Early growth response 3 (EGR3) | F, I, M, A | ||
| IL10RB | F, I, M, A | ||
| IL1R2 | F, I, M, A | ||
| Serpin peptidase inhibitor B2 (SERPINB2) | F, I, M, A | ||
| TIMP1 | F, I, M, A | ||
| Thrombospondin 2 | F | Serum/plasma (0.2 mL) | |
| Glycoprotein-Nmb | F | ||
| COL4A1 | F, M | Serum/plasma (0.2 mL) | |
| Pentraxin | F, M | ||
| Proteomics | Senescence associated secreted proteins including IL-8, GRO-a, GM-CSF, hsIL6 and MCP1 | I, A | Serum/plasma (0.5 mL) |
| Phosphorylated troponin T | M | Serum/plasma (0.1 mL) | |
| Quiescin-sulfhydryl oxidase 1 (QSOX-1 | M | Serum/plasma (0.2 mL) | |
| CD146 | M | ||
| Soluble TWEAK (sTWEAK) | I, M | Serum/plasma (0.1 mL) | |
| Cathelicidin-related antimicrobial protein (CRAMP), | F, A | Serum/plasma (0.5 mL) | |
| Stathmin | F, A | ||
| Elongation factor 1 alpha (EF-1) | F, A | ||
| Chitinase 3-like protein 3 | F, A | ||
| 243 urinary peptides | F, I, M | Urine (1.5 mL) | |
| Metabolomics | α-Ketoglutarate | M | Serum (0.25 mL) |
| Pseudouridine | M | ||
| γ-Glutamyl leucine and valine | F, I, M | ||
| Asymmetric and symmetric dimethylarginin | F, I, M | ||
| 5-Hydroxy-tryptamine (Serotonin) | M | ||
| Amino acids and derivates | F, I, M, A | Serum/plasma (20 μL) | |
| Biogenic amines | F, I, M, A | ||
| Acylcarnitines | M, A | ||
| Sphingolipids | F, I, M | ||
| Glycerolphospholipids | F, I, M |
The processes, in which proposed biomarkers are involved in, include ageing (A), inflammation (I), fibrosis (F) myocyte damage (M) or combinations. PBMC: peripheral blood mononuclear cell.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e319. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 3.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3:315–22. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 5.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 6.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, et al. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J. 2001;22:1318–27. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 7.Mosterd A, Hoes AW. Clinical Epidemiology of Heart Failure. Heart. 2007;93:1137–46. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, et al. Hospitalization of patients with heart failure: a population-based study. Eur Heart J. 2002;23:877–85. doi: 10.1053/euhj.2001.2973. [DOI] [PubMed] [Google Scholar]
- 9.Oudejans I, Mosterd A, Bloemen JA, Valk MJ, van VE, Wielders JP, et al. Clinical evaluation of geriatric outpatients with suspected heart failure: value of symptoms, signs, and additional tests. Eur J Heart Fail. 2011;13:518–27. doi: 10.1093/eurjhf/hfr021. [DOI] [PubMed] [Google Scholar]
- 10.Rich MW. Office management of heart failure in the elderly. Am J Med. 2005;118:342–8. doi: 10.1016/j.amjmed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca C. Diagnosis of heart failure in primary care. Heart Fail Rev. 2006;11:95–107. doi: 10.1007/s10741-006-9481-0. [DOI] [PubMed] [Google Scholar]
- 12.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 13.deFilippi CR, Christenson RH, Kop WJ, Gottdiener JS, Zhan M, Seliger SL. Left ventricular ejection fraction assessment in older adults: an adjunct to natriuretic peptide testing to identify risk of new-onset heart failure and cardiovascular death? J Am Coll Cardiol. 2011;58:1497–506. doi: 10.1016/j.jacc.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siest G, Visvikis S, Herbeth B, Gueguen R, Vincent-Viry M, Sass C, et al. Objectives, design and recruitment of a familial and longitudinal cohort for studying gene-environment interactions in the field of cardiovascular risk: the Stanislas cohort. Clin Chem Lab Med. 1998;36:35–42. doi: 10.1515/CCLM.1998.007. [DOI] [PubMed] [Google Scholar]
- 15.Visvikis-Siest S, Siest G. The STANISLAS Cohort: a 10-year follow-up of supposed healthy families. Gene-environment interactions, reference values and evaluation of biomarkers in prevention of cardiovascular diseases. Clin Chem Lab Med. 2008;46:733–47. doi: 10.1515/CCLM.2008.178. [DOI] [PubMed] [Google Scholar]
- 16.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhager WH, Herrmann SM, et al. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–58. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zagato L, Kuznetsova T, Tripodi G, Zerbini G, Richart T, et al. Angiotensin-converting enzyme I/D and alpha-adducin Gly460Trp polymorphisms: from angiotensin-converting enzyme activity to cardiovascular outcome. Hypertension. 2007;49:1291–7. doi: 10.1161/HYPERTENSIONAHA.106.085498. [DOI] [PubMed] [Google Scholar]
- 18.Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, et al. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur J Heart Fail. 2012;14:718–29. doi: 10.1093/eurjhf/hfs052. [DOI] [PubMed] [Google Scholar]
- 19.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–36. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 20.Joly L, Perret-Guillaume C, Kearney-Schwartz A, Salvi P, Mandry D, Marie PY, et al. Pulse wave velocity assessment by external noninvasive devices and phase-contrast magnetic resonance imaging in the obese. Hypertension. 2009;54:421–6. doi: 10.1161/HYPERTENSIONAHA.109.133645. [DOI] [PubMed] [Google Scholar]
- 21.Seronde MF, Gayat E, Logeart D, Lassus J, Laribi S, Boukef R, et al. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Int J Cardiol. 2013;168:3404–11. doi: 10.1016/j.ijcard.2013.04.164. [DOI] [PubMed] [Google Scholar]
- 22.Smih F, Desmoulin F, Berry M, Turkieh A, Harmancey R, Iacovoni J, et al. Blood signature of pre-heart failure: a microarrays study. PLoS One. 2011;6:e20414. doi: 10.1371/journal.pone.0020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savoye C, Equine O, Tricot O, Nugue O, Segrestin B, Sautiere K, et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am J Cardiol. 2006;98:1144–9. doi: 10.1016/j.amjcard.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Fertin M, Hennache B, Hamon M, Ennezat PV, Biausque F, Elkohen M, et al. Usefulness of serial assessment of B-type natriuretic peptide, troponin I, and C-reactive protein to predict left ventricular remodeling after acute myocardial infarction (from the REVE-2 study). Am J Cardiol. 2010;106:1410–6. doi: 10.1016/j.amjcard.2010.06.071. [DOI] [PubMed] [Google Scholar]
- 25.Pilz S, Tomaschitz A, Stepan V, Obermayer-Pietsch B, Fahrleitner-Pammer A, Schweighofer N, et al. Graz Endocrine Causes of Hypertension (GECOH) study: a diagnostic accuracy study of aldosterone to active renin ratio in screening for primary aldosteronism. BMC Endocr Disord. 2009;9:11. doi: 10.1186/1472-6823-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giorda CB, Cioffi G, de SG, Di LA, Faggiano P, Latini R, et al. Predictors of early-stage left ventricular dysfunction in type 2 diabetes: results of DYDA study. Eur J Cardiovasc Prev Rehabil. 2011;18:415–23. doi: 10.1177/1741826710389402. [DOI] [PubMed] [Google Scholar]
- 27.Tomaschitz A, Fahrleitner-Pammer A, Pieske B, Verheyen N, Amrein K, Ritz E, et al. Effect of eplerenone on parathyroid hormone levels in patients with primary hyperparathyroidism: a randomized, double-blind, placebo-controlled trial. BMC Endocr Disord. 2012;12:19. doi: 10.1186/1472-6823-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 29.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. ASCOT investigators. J Hypertens. 2001;19:1139–47. doi: 10.1097/00004872-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–7. doi: 10.1016/s0002-9149(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 32.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 33.Brunner-La Rocca HP, Buser PT, Schindler R, Bernheim A, Rickenbacher P, Pfisterer M. Management of elderly patients with congestive heart failure–design of the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Am Heart J. 2006;151:949–55. doi: 10.1016/j.ahj.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 35.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–64. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 36.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–67. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]