Abstract
Astrocyte elevated gene-1 (AEG-1) is associated with tumor genesis and progression in a variety of human cancers. This study aimed to explore the significance of AEG-1 in glioma and investigate whether it correlated with radioresistance of glioma cells. Immunohistochemical staining showed that the intensity of AEG-1, CD133 and PPP6c protein expression in glioma tissues increased significantly, mainly in the cytoplasm. The expression rate of AEG-1, CD133 and PPP6c were 85.9% (67/78), 60.3% (47/78) and 65.8% (51/78), respectively. AEG-1 expression was correlated with age (r = 0.227, P = 0.045), clinical stage (r = 0.491, P<0.001) and clinical grade (r = 0.450, P<0.001). No correlation was found between AEG-1 expression and other clinicopathologic parameters (P>0.05). The expression of AEG-1 was positively correlated with the expression of CD133 (r = 0.240, P = 0.035) and PPP6c (r = 0.250, P = 0.027). In addition, retrieved data on TCGA implied co-occurrence of genomic alterations of AEG-1 and PPP6c in glioblastoma. Our findings indicate that AEG-1 is positively correlated with CD133 and AEG-1 expression. It may play an important role in the progression of glioma and may serve as potential novel marker of chemoresistance and radioresistance.
Keywords: glioma, AEG-1, immunohistochemistry, radioresistance
INTRODUCTION
Malignant glioma is the most common primary brain tumor in adults and also one of the most deadly and least successfully treated solid tumors. Despite rapid advancement of multimodal treatments for patients afflicted with glioma, the prognosis has not been significantly improved, and the median survival for patients with malignant glioma is less than 1.5 years[1]. The modest increase in survival after radiotherapy has been ascribed to the high intrinsic resistance of malignant glioma to ionizing radiation (IR). Due to the limited number of cases available in most series of glioma, the molecular mechanism underlying the development and progression of glioma is still poorly understood. Therefore, it is of great value to find the etiology and to identify valuable diagnostic and prognostic markers as well as novel therapeutic strategies of the disease.
Previous studies have shown that many biological characteristics of tumor cells were associated with AEG-1 expression[2]. Lee et al. reported[3] that AEG-1 could increase Akt phosphorylation, promote FOXO3a translocation out of the nucleus and inhibit apoptosis. Interestingly, AEG-1 is also a downstream mediator of the PI3K/Akt signaling pathway; activation of the PI3K/Akt signaling pathway could increase AEG-1 transcription, leading to binding of c-Myc to the AEG-1 promoter[4]. AEG-1 is also closely associated with tumor metastasis. It has been reported that the up-regulation of AEG-1 increases MMP-9 and the down-regulation of AEG-1 decreases breast cancer cell metastasis in the lung by a lung homing domain (LHD)[5],[6]. In addition, the overexpression of AEG-1 promotes the migratory and invasive properties of glioma cells, while the depletion of endogenous AEG-1 in glioma cells significantly inhibits migration and invasion of glioma cells[4],[7]. AEG-1 was also found to activate Wnt/β-catenin signaling, and upregulated LEF1/TCF1, the ultimate executor of the Wnt signaling pathway, was important for progression of hepatocellular carcinoma (HCC). Additional studies further demonstrated that activation of Wnt signaling played a key role in mediating AEG-1 function[8].
Some studies reported that AEG-1 regulates chemosensitivity of tumor cells[9]. Chemoresistance analyses confirmed that knockdown of AEG-1 sensitized various types of tumors to multiple chemotherapeutic agents, such as 5-fluorouracil, cisplatin, paclitaxel and doxorubicin in vitro and vivo[10]-[12]. The hypothesis about the connection between AEG-1 and radioresistance was presumed from signaling network in theory[13]-[15]. Therefore, more evidence should be collected to prove their relationship in radiotherapy. However, the hypothesis and prevalence data appear weak at the molecular level; the study about cancer stem cells (CSCs) and DNA double-strand breaks (DSBs) open a window for the role of AEG-1 on radioresistance. Recent studies have demonstrated that CSCs could impact on tumor radioresistance. Jamal et al.[16] reported that CD133+ cells are relatively radioresistant under intracerebral growth conditions by regulating γH2AX and 53BP1. Piao et al.[17] also reported that CD133 contributed to radioresistance in HCC by the MAPK/PI3K pathway. On the other hand, it is well known that irradiation induces DSBs, which are predominantly repaired by non-homologous end joining, with DNA-PK a central player in the process[18],[19]. PPP6c could improve the sensitivity of glioblastoma multiforme (GBM) cells to radiation by regulating DNA-PK activity, and be a better biomarker for radiotherapy prognosis than DNA-PKcs (a catalytic subunit of DNA-PK)[20]. Therefore, delineation of AEG-1 and CD133/PPP6c will provide insight into the impact of AEG-1 on radioresistance. In this study, we investigated the expression of AEG-1 in glioma, correlated AEG-1 expression levels with glioma stem cell marker CD133 and glioma radioresistance marker PPP6c, and estimated its effect on glioma radioresistance.
PATIENTS AND METHODS
Patients and acquisition of tissue specimens
Paraffin embedded sections of glioma samples were obtained from 78 patients diagnosed with glioma between January 2008 and March 2012 at the authors' affiliated hospital. Archived glioma tissue samples, include 26 GBMs, 41 astrocytomas, 8 oligodendrogliomas and 3 gliosarcoma. The carcinoid group included 5 meningeomas and 2 shwannomas. The study protocol was approved by the institutional review board of the authors' affiliated institution.
Immunohistochemical (IHC) analysis
IHC analysis was carried out as previously described[21]. Briefly, tissue sections were incubated with a rabbit anti-AEG-1 antibody (Bioss, Beijing, China), a rabbit anti-CD133 antibody (Bioss) and a rabbit anti-PPP6c antibody (ABGENT, San Diego, CA, USA) overnight at 4°C. For negative controls, rabbit anti-AEG-1 antibody was replaced with normal none immune serum.
The staining index (SI) of paraffin-embedded sections was reviewed and scored independently by two observers, based on the proportion of positively stained tumor cells and the intensity of staining[22]. The proportion of positive tumor cells was scored as follows[2]: 0 (no positive tumor cells), 1 (< 10% positive tumor cells), 2 (10–50% positive tumor cells) and 3 (> 50% positive tumor cells). The intensity of staining was graded according to the following criteria: 0 (no staining); 1 (weak staining = light yellow), 2 (moderate staining = yellow brown) and 3 (strong staining = brown). The SI was calculated as staining intensity score × proportion of positive tumor cells. Using this method of assessment, we evaluated the expression of AEG-1 in benign brain tumors tissues and glioma tumors by determining the SI, which scores as 0, 1, 2, 3, 4, 6 and 9. An optimal cutoff value was identified: the SI score of ≥ 4 was used to define tumors as having high AEG-1 expression and ≤ 3 as having low expression of AEG-1.
TCGA data retrieval
To further validate the correlations between AEG-1 and PPP6c, we inspected glioma data on The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov), the methods to detect mRNA is Agilent microarray. We analyzed the relationship between AEG-1 and PPP6c and prognosis by cBio Portal (http://cbioportal.org). Increase/decrease of gene expression was defined as a sample exceeding the standard level of gene expression in all samples: EXP> 1.0 was upregulated, and EXP < −1.0 was downregulated. The scoring criteria and co-occurrence of genes was obtained by analysis of cBio Portal to TCGA data[23].
Statistical analyses
All statistical analyses were carried out using the SPSS 13.0 statistical software (SPSS Inc, Chicago, IL, USA). The chi-square test was performed to analyze the relationship between AEG-1 expression and clinicopathologic features. Bivariate correlations between variables were calculated by Spearman's correlation coefficients. P values < 0.05 were considered statistically significant.
RESULTS
Overexpression of AEG-1 protein in archived glioma samples
Immunohistochemical staining was performed to evaluate AEG-1 expression in formalin-fixed paraffin embedded sections of glioma and various brain tumors. The demographic and baseline characteristics of the patients are shown in Table 1. Among the 78 glioma samples, 67 scored positive for AEG-1 (85.9%) and high expression was 53.8% (42/78). However, benign tumor tissues showed no immunoreactivity for AEG-1. AEG-1 was mainly localized in the cytoplasm of primary cancer cells, which is consistent with previous reports on AEG-1 expression (Fig. 1)[2],[22],[24],[25].
Table 1. Clinicopathological characteristics of patient samples and expression of AEG-1 in glioma.
| Clinicopathological characteristics | All cases, n (%) |
| Gender | |
| Male | 33(42.3) |
| Female | 45(57.7) |
| Age (years) | |
| <42 | 42(53.9) |
| ≥42 | 36(46.1) |
| Clinical stage | |
| I | 12(15.4) |
| II | 16(20.5) |
| III | 22(28.2) |
| IV | 28(35.9) |
| Clinical grade | |
| Low-grade | 28(35.9) |
| High-grade | 50(64.1) |
| Histological types | |
| Glioblastoma | 26(33.3) |
| Astrocytomas | 41(52.6) |
| Oligodendrogliomas | 8(10.3) |
| Gliosaromas | 3(3.8) |
| Expression of AEG-1 | |
| Negative | 11(14.1) |
| Positive | 67(85.9) |
| Low expression | 36(46.2) |
| High expression | 42(53.8) |
| Tumor diameter (cm) | |
| <4.5 | 29(37.2) |
| ≥4.5 | 49(62.8) |
| Hyperspasmia | |
| No | 62(79.5) |
| Yes | 16(20.5) |
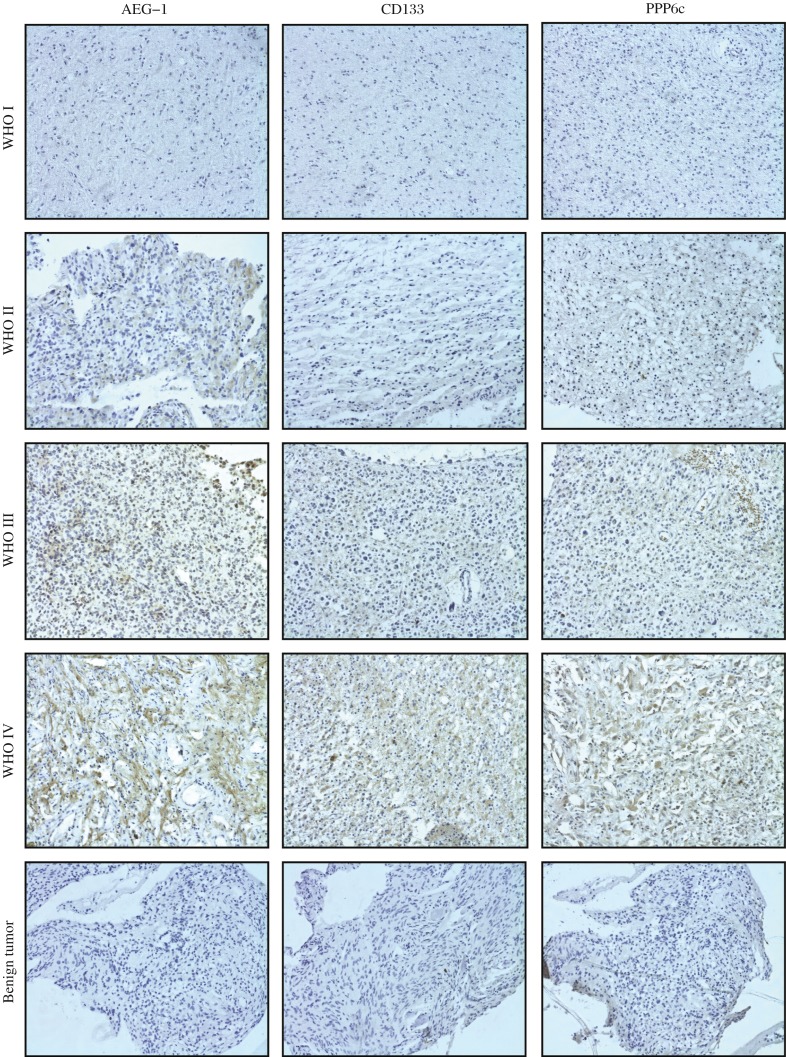
Fig. 1. Immunohistochemical staining of AEG-1, CD133 and PPP6c protein (SD, magnification×200).
Immunoreactivity of AEG-1, CD133 and PPP6c proteins was observed in the cytoplasm of glioma tissues. The figure rows show representative pictures of glioma sections according to the World Health Organization (WHO) criteria.
As shown in Fig. 1, the benign brain tumor tissues were negative for AEG-1 except for sparse cytoplasmic staining in a few glial cells and neurons. In contrast, moderate to strong cytoplasmic staining of AEG-1 protein was observed in tumor cells in the primary glioma tissues. We observed that the intensity of AEG-1 staining in all primary glioma were increased along with the progression of tumor stages I to IV. These findings indicated that AEG1 expression was gradually increased with tumor progression as well as with loss of differentiation.
Increased AEG-1 expression correlates with clinicopathologic features of glioma
We further examined possible correlations between expression levels of AEG-1 and clinical features of glioma patients. As summarized in Table 2, in 78 primary glioma samples, AEG-1 expression was strongly correlated with age (P = 0.035), clinical stage (P = 0.001), clinical grade (P < 0.001) and histological type (P = 0.020). For histological type, there was a statistical difference between astrocytomas and other groups (P = 0.021), but not in the other three histological types (P = 0.078). Spearman correlation analysis (Table 3) proved that high AEG-1 expression was strongly correlated with age (R = 0.227, P = 0.045), advanced clinical stage (R = 0.491, P < 0.001) and clinical grade (R = 0.450, P < 0.001). However, the results showed no significant association between AEG-1 expression and other clinical features, including gender, diameter of the tumor and history of epilepsy.
Table 2. Correlation between AEG-1 expression and clinicopathologic characteristics of the patients.
| Characteristics | AEG-1 expression | χ2 | P | |
| Low, n(%) | High, n(%) | |||
| Age (years) | ||||
| <42 | 24(30.8) | 18(23.1) | 4.422 | 0.035 |
| ≥42 | 12(15.4) | 24(30.8) | ||
| Gender | ||||
| Male | 18(23.1) | 15(19.2) | 1.621 | 0.203 |
| Female | 18(23.1) | 27(34.6) | ||
| Clinical stage | ||||
| I | 10(12.8) | 2(2.6) | 15.852 | 0.001 |
| II | 11(14.1) | 5(6.4) | ||
| III | 8(10.3) | 14(17.9) | ||
| IV | 7(9.0) | 21(26.9) | ||
| Clinical grade | ||||
| Low | 21(26.9) | 8(10.3) | 12.809 | 0.000 |
| High | 15(19.2) | 34(43.6) | ||
| Histological types | ||||
| Glioblastoma | 7(9.0) | 19(24.4) | 9.830 | 0.020 |
| Astrocytomas | 24(30.8) | 17(21.8) | ||
| Oligodendrogliomas | 5(6.4) | 3(3.8) | ||
| Gliosaromas | 0(0.0) | 3(3.8) | ||
| Diameter (cm) | ||||
| <4.5 | 12(15.4) | 17(21.8) | 0.423 | 0.515 |
| ≥4.5 | 24(30.8) | 25(32.0) | ||
| Hyperspasmia | ||||
| No | 27(34.6) | 35(44.9) | 0.826 | 0.364 |
| Yes | 9(11.5) | 7(9.0) | ||
Table 3. Spearman correlation analysis between AEG-1, clinicopathologic factors, CD133 and PPP6c expression.
| Variables | AEG-1 expression level | |
| Correlation coefficient | P | |
| Age (years) | 0.227 | 0.045 |
| Clinical stage | 0.491 | 0.000 |
| Clinical grade | 0.450 | 0.000 |
| CD133 expression level | 0.240 | 0.035 |
| PPP6c expression level | 0.250 | 0.027 |
AEG-1 expression correlates with CD133 and PPP6c
To determine whether AEG-1 is related to cancer stem cell or cell radioresistance in glioma, we examined the correlation of AEG-1 and a glioma stem cell (GSC) marker CD133 and glioma cell radioresistance maker PPP6c (Fig. 1). The positive expression rates of CD133 (60.3%, 47/78) and PPP6c (65.8%, 51/78) in patients with glioma were higher than those in benign brain tumors tissues (both 28.6%, 2/7), and high expression were 21.8% (17/78) and 32.0% (25/78). To evaluate whether there was a correlation between expression of CD133, PPP6c and AEG-1, a bivariate correlation analysis (Spearman correlation coefficients) was carried out. Statistically significant correlations between the percentages of immunostained cells and expression of CD133 and PPP6c in glioma were found for AEG-1 (R = 0.240 and 0.250, P = 0.035 and 0.027, respectively) (Table 3), suggesting that the greater the number of stem cells or radioresistant cells in glioma tissues was, the higher the level of AEG-1 was.
Co-occurrence of genomic alterations of AEG-1 and PPP6c in GBM
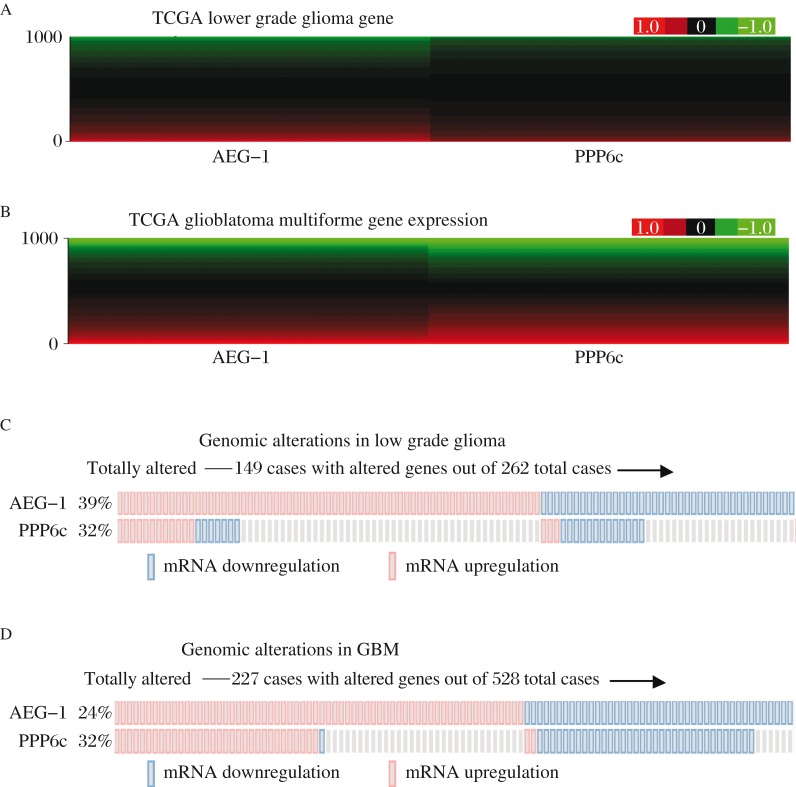
To further validate the correlation between AEG-1 and PPP6c, we retrieved more information from TCGA. According to the results of one of the earliest reports, the data of glioma in the TCGA and the tools net the cBio portal, has been widely recognized. There was a similar trend of AEG-1 and PPP6c mRNA expression in GBM (Fig. 2A and 2B). Fig. 2C and 2D showed that alterations of AEG-1 and PPP6c in GBM tend to be compatible. Statistical tests also pointed out that the tendency toward co-occurrence of AEG-1 and PPP6c in GBM (P < 0.001). However, similar trend was not found in LGG (Table 4).
Fig. 2. The data of AEG-1 and PPP6c expression in TCGA.
Microarray shows that AEG-1 and PPP6c gene expression in brain lower grade glioma (LGG) (Fig. 2A) and Glioblastoma Multiforme (GBM) (Fig. 2B) have the similar trends. Fig. 2C and 2D show an overview of genomic alterations (legend) in AEG-1 and PPP6c (rows) affecting particular individual samples (columns). Genomic alterations of AEG-1 and PPP6c in GBM are compatible.
Table 4. Co-occurrence analyses between AEG-1 and PPP6c.
| Co-occurrence of AEG-1 and PPP6c | |||
| OR | 95% Confidence Interval | P | |
| Low grade glioma | 1.25 | 0.73–2.12 | 0.247 |
| Glioblastoma multiforme | 3.73 | 2.45–5.68 | 0.000 |
DISCUSSION
Malignant gliomas are highly recurrent tumors even after standard treatment. Ionizing radiation is considered to be the most effective therapy for glioma, but radiotherapy remains only palliative because of radioresistance. Our study demonstrated that AEG-1 was widely expressed in many histological types of glioma, and correlated with clinical course and the histological grade, as previously reported[28],[29]. Moreover, the data showed that AEG-1 protein expression was lower in astrocytomas than other histological types, which may be due to that many low grade (I and II) gliomas were well differentiated with clear histological similarity to the astrocytic lineage. High grade (III and IV) gliomas were more anaplastic, with features resembling immature astrocytes, oligodendrocytes or a mixture of both types[26]. By the same token, low-grade gliomas were frequently diagnosed in younger patients and our data also indicated that AEG-1 was correlated with age. These results demonstrated that AEG-1 protein expression was significantly correlated with the malignant degree of glioma: the higher degree the malignancy was, the higher the expression of AEG-1 protein was.
Many reports demonstrated that high grade gliomas are associated with radioresistance and poor prognosis[30]-[32]. Thus, finding a way to enhance the efficacy of radiotherapy is critical to improving the survival of high-grade glioma patients. Several recent reports suggested that AEG-1 was a multifunctional regulator of tumor physiology by promoting proliferation, invasion, angiogenesis and metastasis[33]. We were interested in whether AEG-1 was correlated with radioresistance. To address this problem, we investigated the expression of two radioresistance markers CD133 and PPP6c in this study. We found that there was positive correlation between the expression of AEG-1 and CD133/PPP6c, which suggested that AEG-1 might play a role in glioma radioresistance. Moreover, we retrieved more data from the TCGA and found co-occurrence of genomic alterations of AEG-1 and PPP6c in GBM. All these results suggested that AEG-1 may play a role in glioma radiosensitivity. According to previous studies[4],[7], AEG-1 is a key player in the complex oncogenic signaling networks. What's more, the relationship among some of these signaling pathways and cancer radioresistance has been confirmed. In a recent study, we found that the PI3K/AKT pathway was activated by enhancing radioresistance of esophageal carcinoma cell line Eca109. PT-PCR and Western blotting assays showed that radiation induced the expression of stem cell markers p75NTR[13]. These results suggested that there would be more cancer stem cells in esophageal radioresistance cells, and radioresistance was positively correlated with the PI3K/AKT pathway. We further found that Cox-2 expression was increased in ECA109 cells repeatedly exposed to X rays[14]. Other report indicated[15] that Cox-2 is a downstream target of Wnt/β-catenin. Interestingly, the frequency of alterations of AEG-1 in this study was lower than that in immunohistochemistry. It may be due to the fact that the samples were from different populations (Asians vs. Americans and Europeans), and different kind of test methods (immunohistochemistry vs. microarray).
In summary, AEG-1 expression is correlated with CD133 and PPP6c in glioma. Our data provide compelling evidence that AEG-1 may play a role during the development and progression of glioma and specifically targeting AEG-1 may have important therapeutic implications when used in combination with radiation in the treatment of glioma patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81272775). We gratefully acknowledge the Cancer Genome Atlas (TCGA), Memorial Sloan-Kettering Cancer Center (MSKCC) and University of California Santa Cruz (UCSC) for their generous feedback and promotion of the portal within the cancer genomics community.
References
- 1.Ding D, Han S, Wang Z, Guo Z, Wu A. Does the existence of HCMV components predict poor prognosis in glioma? J Neurooncol. 2014 doi: 10.1007/s11060-013-1350-9. [DOI] [PubMed] [Google Scholar]
- 2.Liao WT, Guo L, Zhong Y, Wu YH, Li J, Song LB. Astrocyte elevated gene-1 (AEG-1) is a marker for aggressive salivary gland carcinoma. J Transl Med. 2011;9:205. doi: 10.1186/1479-5876-9-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–21. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 4.Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–5. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–74. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 6.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 2008;68:1478–84. doi: 10.1158/0008-5472.CAN-07-6164. [DOI] [PubMed] [Google Scholar]
- 8.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–77. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen D, Forcier T, et al. Identification of genes conferring resistance to 5-fluorouracil. Proc Natl Acad Sci U S A. 2009;106:12938–43. doi: 10.1073/pnas.0901451106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Hu G, Kang Y. Metadherin as a link between metastasis and chemoresistance. Cell Cycle. 2009;8:2132–3. [PubMed] [Google Scholar]
- 11.Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, et al. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Che SM, Zhang XZ, Liu XL, Chen X, Hou L. The radiosensitization effect of NS398 on esophageal cancer stem cell-like radioresistant cells. Dis Esophagus. 2011;24:265–73. doi: 10.1111/j.1442-2050.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 14.Che SM, Zhang XZ, Hou L, Song TB. Cyclooxygenase-2 inhibitor NS398 enhances radiosensitivity of radioresistant esophageal cancer cells by inhibiting AKT activation and inducing apoptosis. Cancer Invest. 2010;28:679–88. doi: 10.3109/07357907.2010.483504. [DOI] [PubMed] [Google Scholar]
- 15.Shafie NH, Mohd Esa N, Ithnin H, Md Akim A, Saad N, Pandurangan AK. Preventive inositol hexaphosphate extracted from rice bran inhibits colorectal cancer through involvement of Wnt/beta-catenin and COX-2 pathways. Biomed Res Int. 2013;2013:681027. doi: 10.1155/2013/681027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamal M, Rath BH, Tsang PS, Camphausen K, Tofilon PJ. The brain microenvironment preferentially enhances the radioresistance of CD133(+) glioblastoma stem-like cells. Neoplasia. 2012;14:150–8. doi: 10.1593/neo.111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, et al. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–37. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Ciszewski WM, Tavecchio M, Dastych J, Curtin NJ. DNA-PK inhibition by NU7441 sensitizes breast cancer cells to ionizing radiation and doxorubicin. Breast Cancer Res Treat. 2014;143:47–55. doi: 10.1007/s10549-013-2785-6. [DOI] [PubMed] [Google Scholar]
- 19.Tomita M. Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death. J Radiat Res. 2010;51:493–501. doi: 10.1269/jrr.10039. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Wang Y, Sheng K, Fei X, Guo Q, Larner J, et al. Serine/threonine protein phosphatase 6 modulates the radiation sensitivity of glioblastoma. Cell Death Dis. 2011;2:e241. doi: 10.1038/cddis.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni X, Zhao Y, Ma J, Xia T, Liu X, Ding Q, et al. Hypoxia-induced factor-1 alpha upregulates vascular endothelial growth factor C to promote lymphangiogenesis and angiogenesis in breast cancer patients. J Biomed Res. 2013;27:478–85. doi: 10.7555/JBR.27.20130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–26. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, et al. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Res. 2010;70:3750–9. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- 25.Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, et al. Astrocyte elevated gene-1: a novel target for human glioma therapy. Mol Cancer Ther. 2010;9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E, et al. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 2008;3:e1936. doi: 10.1371/journal.pone.0001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr Med Chem. 2002;9:2055–75. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Wu J, Guan H, Cai J, Fang L, Li J, et al. MiR-136 promotes apoptosis of glioma cells by targeting AEG-1 and Bcl-2. FEBS Lett. 2012;586:3608–12. doi: 10.1016/j.febslet.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, et al. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Res. 2011;71:6514–23. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedrigo CA, Grivicich I, Schunemann DP, Chemale IM, dos Santos D, Jacovas T, et al. Radioresistance of human glioma spheroids and expression of HSP70, p53 and EGFr. Radiat Oncol. 2011;6:156. doi: 10.1186/1748-717X-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim YC, Roberts TL, Day BW, Harding A, Kozlov S, Kijas AW, et al. A role for homologous recombination and abnormal cell-cycle progression in radioresistance of glioma-initiating cells. Mol Cancer Ther. 2012;11:1863–72. doi: 10.1158/1535-7163.MCT-11-1044. [DOI] [PubMed] [Google Scholar]
- 32.Khalil AA, Jameson MJ, Broaddus WC, Lin PS, Chung TD. Nicotine enhances proliferation, migration, and radioresistance of human malignant glioma cells through EGFR activation. Brain Tumor Pathol. 2013;30:73–83. doi: 10.1007/s10014-012-0101-5. [DOI] [PubMed] [Google Scholar]
- 33.Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, et al. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol Ther. 2011;130:1–8. doi: 10.1016/j.pharmthera.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]