Abstract
We retrospectively investigated the prognostic factors of acute myeloid leukemia (AML) in 152 Chinese patients with de novo AML who were older than 60 years of age and who received treatment at our hospital. Log-rank test showed that 6 parameters including older age, higher white blood cell (WBC) counts, lactate dehydrogenase (LDH) and bone marrow (BM) blasts at diagnosis, unfavorable risk cytogenetics, and non-mutated CEBPα were significant adverse prognostic factors of overall survival (OS) for elderly AML patients (P = 0.0013, 0.0358, 0.0132, 0.0242, 0.0236 and 0.0130, respectively). Moreover, older age and higher LDH were significant adverse predictors for relapse-free survival (RFS) (P = 0.0447 and 0.0470, respectively). Univariate analysis revealed similar results for OS to those of the log-rank test and only higher LDH at diagnosis was a significant adverse predictor for RFS (P = 0.028, HR: 1.979, 95%CI: 1.075–3.644). In multivariate analysis, we identified 2 trends towards independent prognostic factors for OS, including BM blasts at diagnosis (P = 0.057, HR: 1.676, 95%CI: 0.984–2.854) and mutation status of CEBPα (P = 0.064, HR: 4.173, 95%CI: 0.918–18.966). Our data indicated that older age, gender and a previous history of hematologic diseases resulted in lower complete remission rate (P = 0.012, 0.051 and 0.086, respectively). We further developed an easy scoring system for predicting prognosis and response to induction therapy in older AML patients. Patients who had lower scores showed significantly longer OS and RFS (P = 0.0006 and 0.1001, respectively) and higher CR rate (P = 0.014). Our research is limited by its retrospective nature and the results from our study need to be further validated by prospective randomized clinical trials.
Keywords: acute myeloid leukemia, elderly patients, prognosis factors
INTRODUCTION
Acute myeloid leukemia (AML) results from abnormal self-renewal and suppressed differentiation of hematopoietic progenitor cells, which leads to replacement of normal marrow elements[1]. AML usually afflicts elderly people with a median age of 67 years. Actually, patients older than 60 years represent the majority of patients with AML[2]. According to Brincker et al., the annual incidence of AML patients at 50 years is 4.1 cases per 10,000 and increases progressively into 14.9 cases per 10,000 at 80 years[3]. Currently, the world population is aging at an accelerated pace; therefore, the number of elderly patients presenting with AML are expected to continue to rise. However, elderly AML patients usually show a much worse prognosis than younger patients, and more than 50% of them die in the first year of diagnosis[4]. Clinical outcomes in this population remain dismal and have not made much progress over the previous three decades[5].
According to the World Health Organization (WHO) categorization of AML, cytogenetic and molecular analyses play an important role in predicting the remission and survival rates of AML patients. Unfortunately, the unfavorable characteristics are often amplified in elderly AML patients, such as a higher incidence of complex cytogenetics or a multidrug resistance phenotype[6],[7]. Meanwhile, a higher proportion of secondary AML arising from myelodysplastic syndrome (MDS) or other previous hematological diseases also leads to poor survival of elderly AML patients[8]. Moreover, the decreased physiologic reserve and functional impairment of elderly AML patients always contribute to a diminished response to chemotherapy and less tolerance of complications related to chemotherapy. Many previous studies reported numerous prognostic factors for these patients, such as age, relevant comorbidity, Eastern Cooperative Oncology Group (ECOG) performance status, serum lactate dehydrogenase (sLDH) at diagnosis, cytogenetics, gene mutations, immunophenotypes, and the French-American-British (FAB) subtypes[7],[9]-[12].
In the current study, we retrospectively analyzed 152 elderly de novo AML patients treated at a single tertiary care center. We also developed an easily manageable scoring system combining five host- or disease-related factors (age, sex, white blood cell (WBC) at diagnosis, LDH at diagnosis and bone marrow (BM) blasts at diagnosis) to classify elderly AML patients into groups with variable prognosis.
PATIENTS AND METHODS
Patients
From January 2006 to May 2013, 152 patients older than 60 years of age (median age: 68 years, range: 60–94) years with newly diagnosed AML (other than M3 subtypes) who were treated at the authors' affiliated institution were included in this retrospective cohort study. The study was conducted according to institutional guidelines and the Declaration of Helsinki. All data were collected with approval by the local institutional review board. All patients were unrelated ethnic Han Chinese and all of them dwell in mainland China. The diagnosis of AML was made according to the morphologic and cytochemical criteria of the FAB classification[13]. Follow-up information was obtained from the patient records at the hospital. The number of patients given hematopoietic stem cell transplantation was not clear as some patients were not treated in our hospital from beginning to end and they may have received transplantation in other hospitals.
Cytogenetic analysis
Conventional cytogenetic analysis was performed using non-stimulated short-term cultures according to the recommendations of the International System for Human Cytogenetic Nomenclature (ISCN) and at least 20 bone marrow metaphase cells were analyzed. According to the National Comprehensive Cancer Network (NCCN) guidelines of AML (version 1, 2012), the favorable risk cytogenetic group was defined as patients with inv16 or t (16; 16), t (8; 21), or t (15; 17). Patients with -5/5q-, -7/7q-, t (6, 9), t (9, 22), inv (3), t (3; 3), 11q23-non t (9; 11) or complex aberrations (≥3 independent clonal chromosomal abnormalities) were categorized as poor risk. Patients with +8, t (9; 11), normal or other non-defined cytogenetics were defined as the intermediate risk group.
Molecular analysis
Internal tandem duplications of FMS-like tyrosine kinase-3 (FLT3-ITD), mutation status of the nucleophosmin 1 gene (NPM1) and CCAAT/enhancer-binding protein alpha gene (CEBPα) were evaluated as previously described[14],[15].
Treatment
In each case, treatment choice was based on physician recommendation and patient preference. Based on investigational protocol availability, although the induction therapy was not uniform, they always included cytosine arabinoside (Ara-C). Among the 152 elderly patients with AML, 23 patients only received palliative care and the remaining 129 patients received various standard-intensity or low-intensity induction regimens according to their performance status.
Endpoints and definitions
Complete remission (CR) was defined by the presence of normal cellular BM with less than 5% blasts along with a neutrophil count ≥ 1x109/L, a platelet count ≥ 100 × 109/L in peripheral blood, and the patient was independent with transfusion[16]. Relapse was defined as the reappearance of more than 5% leukemic blasts in the BM or presence of blast infiltration in extramedullary organs such as the central nervous system. The period from the time of documented CR until relapse or death in CR (failure), or alive in CR until last follow-up (censored) was defined as relapse-free survival (RFS). Overall survival (OS) was defined as the period from the time of first diagnosis to death (failure) or censored on the last known alive date if the patients were still alive.
Statistical analysis
Clinical characteristics were described in numbers and frequency for qualitative variables, median and range for quantitative factors. Qualitative parameters were evaluated by χ2 test or Fisher's exact test. The cumulative survival rate was calculated by Kaplan-Meier method, and statistical significance was analyzed by log-rank test. Univariate and multivariate cox proportional hazard models were used for exploring various significant prognostic clinical variables. Two-sided P-value less than 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS) 13.0. Graphpad Prism 5.0 was used for plotting graphs.
RESULTS
Survival
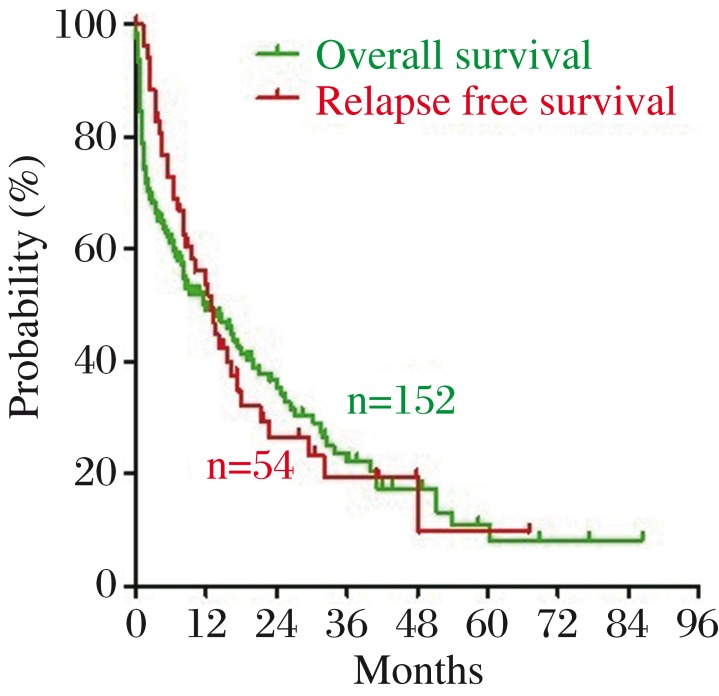
The baseline characteristics of the 152 patients are listed in Table 1. The 152 patients included 97 death cases and 55 censored cases. The median OS was 6.2 months (range: 0.07 to 86.33 months). The median OS for patients receiving induction therapy was 7.9 months. The estimated 1-, 3-, and 5-year survival rates of the patients older than 60 years were 49.1%, 22.2% and 8.2%, respectively (Fig. 1). In patients receiving chemotherapy, CR was achieved in 69 of 115 cases (60.0%). Among 69 patients who achieved CR, 15 were lost to follow up. In the remaining 54 patients, 37 (68.5%) relapsed. The RFS of the 54 patients ranged from 3 days to 5.6 years. The median RFS was 6.2 months (Fig. 1).
Table 1. Demographic and baseline characteristics of 152 elderly patients with AML.
| Characteristic | Value |
| Age, year (median, range) | 68, 60–94 |
| 60–69 years, n (%) | 93(61.2) |
| 70–79 years, n (%) | 49(32.2) |
| >80 years, n (%) | 10(6.6) |
| Male gender, n (%) | 91(59.9) |
| Previous hematologic diseases, n (%) | 20(13.2) |
| MDS | 14(9.2) |
| CMML | 2(1.3) |
| ITP | 1(0.7) |
| PV | 1(0.7) |
| IMF | 1(0.7) |
| NHL | 1(0.7) |
| Previous tumors of other systems, n (%) | 8(5.3) |
| Extramedullary presentation, n (%) | 39(25.7) |
| WBC at diagnosis, ×109/L (median, range) # | 7.60, 0.41–272.3 |
| ≤ 30, n (%) | 105(69.5) |
| Hemoglobin at diagnosis, g/L (median, range) # | 76.0, 34.0–131.0 |
| Normal (male 120–160, female 110–150), n (%) | 5(3.3) |
| Anemia (male < 120, female < 110), n (%) | 146(96.7) |
| Platelet at diagnosis, ×109/L (median, range) # | 46.0, 2.0–463.0 |
| Normal (100–300), n (%) | 25(16.6) |
| Thrombocytopenia (< 100), n (%) | 124(82.1) |
| Thrombocythemia (> 300), n (%) | 2(1.3) |
| Missing data, n | 1 |
| LDH at diagnosis, U/L (median, range) $ | 329, 92–2899 |
| ≤ 250 U/L, n (%) | 46(32.2) |
| > 250 U/L, ≤ 1000 U/L, n (%) | 79(55.2) |
| > 1,000 U/L, n (%) | 18(12.6) |
| BM blasts at diagnosis, % (median, range) & | 59.0, 20.0–96.2 |
| ≥ 20%, ≤ 50%, n (%) | 62(41.6) |
| > 50%, ≤ 80%, n (%) | 49(32.9) |
| > 80%, n (%) | 38(25.5) |
| FAB subtype, n (%) | |
| M0 | 6(3.9) |
| M1 | 24(15.8) |
| M2 | 73(48.0) |
| M4 | 15(9.9) |
| M5 | 18(11.8) |
| M6 | 10(6.6) |
| M7 | 2(1.3) |
| Unclassified | 4(2.6) |
| FLT3-ITD mutation status, mutated +/total (%) | 7/72(9.7) |
| NPM1 mutation status, mutated +/total (%) | 16/67(23.9) |
| CEBPα mutation status, mutated +/total (%)* | 10/62(16.1) |
| Cytogenetics, n (%) | |
| Favorable | 5(4.6) |
| Intermediate | 92(85.2) |
| Unfavorable | 11(10.2) |
AML, acute myeloid leukemia; MDS: myelodysplastic syndromes; CMML: Chronic myelomonocytic leukemia; ITP: Idiopathic thrombocytopenic purpura; PV: polycythemia vera; IMF: idiopathic myelofibrosis; NHL: non-Hodgkin's lymphoma; WBC: white blood cell, normal range: 4–10×109/L; LDH: lactate dehydrogenase, normal range: 110–250 U/L; BM: bone marrow; FAB: the French American British; FLT3-ITD: internal tandem duplications of FMS-like tyrosine kinase-3; NPM1: nucleophosmin 1; CEBPα: CCAAT/enhancer-binding protein alpha; Missing data: # n = 1; $ n = 9; & n = 3; * n = 44.
Fig. 1. The overall survival (OS) (green line) and relapse free survival (RFS) (red line) of 152 elderly patients with AML.
Kaplan-Meier method and log-rank test
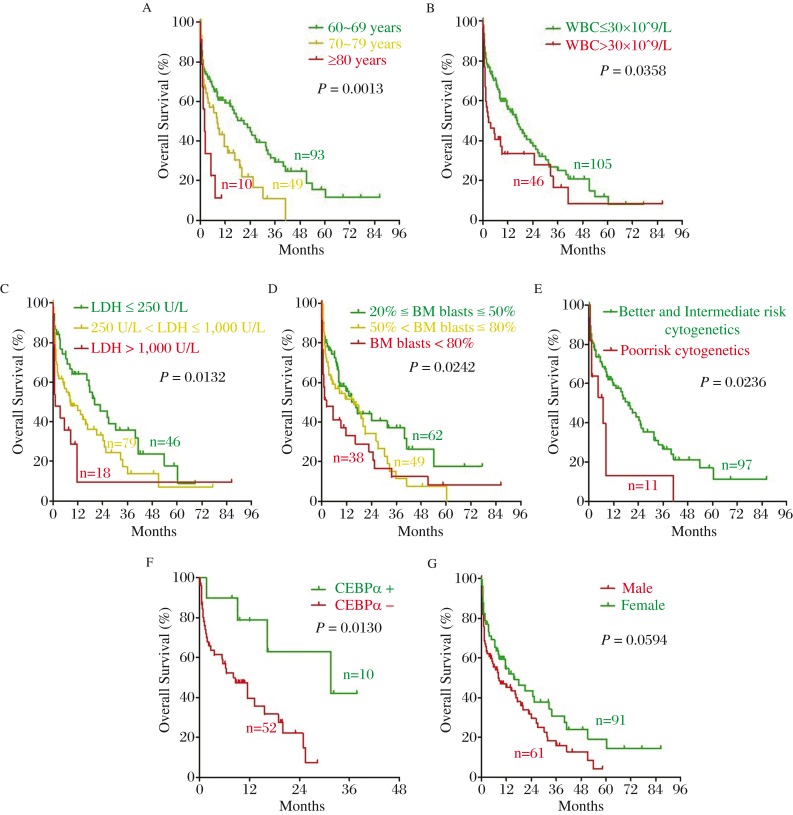
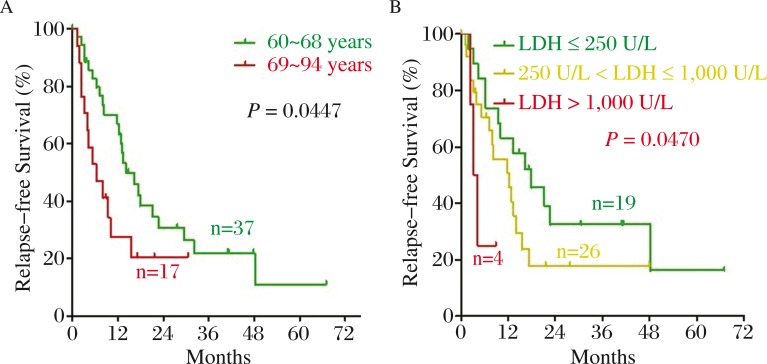
To identify the clinical prognostic factors for elderly AML patients, we performed survival analysis of 152 AML patients. We examined prognostic factors involved in OS and RFS by Kaplan-Meier method and log-rank test. As shown in Fig. 2A–2F, older age, higher level of WBC, LDH and BM blasts at diagnosis, the poor risk group of cytogenetics, and non-mutated CEBPα were significant adverse prognostic factors of OS for elderly AML patients (P = 0.0013, 0.0358, 0.0132, 0.0242, 0.0236 and 0.0130, respectively). Meanwhile, there was a trend toward unfavorable OS in male patients (P = 0.0594) (Fig. 2G). Older age and higher LDH at diagnosis were significant adverse predictors for RFS (P = 0.0447 and 0.0470, respectively) (Fig. 3A and 3B). Previous hematologic diseases, hemoglobin and platelet count and mutation of NPM1 and FLT3-ITD had no significant impact on OS and RFS in elderly AML patients (all P > 0.05).
Fig. 2. Significant prognostic factors of OS for elderly AML patients.
Older age (green line: 60-69 years, yellow line: 70-79 years, red line: ≥80 years), higher level of WBC (green line: ≤30×109/L, red line: >30×109/L), LDH (green line: ≤250U/L, yellow line: >250U/L, ≤1000U/L, red line: >1000U/L) and BM blasts (green line: ≥20%, ≤50%, yellow line: >50%, ≤80%, red line: <80%) at diagnosis, poor-risk group of cytogenetics (green line: better- and intermediated-risk, red line: poor-risk), non-mutated CEBPα (green line: mutated; red line: non-mutated) were significant adverse prognostic factors of OS for elderly AML patients. Meanwhile, there was a trend toward unfavorable OS in male patients (green line: male; red line: female). A: Age; B: WBC at diagnosis; C: LDH at diagnosis; D: BM blast at diagnosis; E: Cytogenetic; F: CEBPα; G: Sex.
Fig. 3. Significant prognostic factors of RFS for elderly AML patients.
Older age (green line: 60-68 years, red line: 69-94 years) and higher level of LDH at diagnosis (green line: ≤250 U/L, yellow line: >250 U/L, ≤1000U/L, red line: >1000 U/L) were shown to be significant poor prognostic factors of RFS. A: Age; B: LDH at diagnosis.
Univariate and multivariate analyses
In univariate analysis, the results were similar to those of the log-rank test, as shown in Table 2. Older age (P = 0.000, HR: 1.842, 95%CI: 1.321–2.568), higher WBC (P = 0.038, HR: 1.588, 95%CI: 1.027–2.455), higher LDH (P = 0.004, HR: 1.598, 95%CI: 1.164–2.193) and higher BM blasts (P = 0.007, HR: 1.419, 95%CI: 1.101–1.830), the poor risk group of cytogenetics (P = 0.028, HR: 1.496, 95%CI: 1.045–2.141) and non-mutated CEBPα (P = 0.021, HR: 4.084, 95%CI: 1.233–13.524) at diagnosis were shown to be significant poor prognostic factors of OS for elderly AML patients. Moreover, there was a trend toward unfavorable OS in male patients (P = 0.061). Only higher LDH at diagnosis was a significant poor predictor for RFS (P = 0.028, HR: 1.979, 95%CI: 1.075–3.644).
Table 2. Univariate analysis in the primary cohort of 152 AML patients.
| Variable | RFS (n = 38) | OS (n = 151) | ||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (60–69 years vs. 70–79 years vs. ≧80 years) | 1.488 (0.711–3.114) | 0.292 | 1.842 (1.321–2.568) | 0.000 |
| Sex (male vs. female) | 0.773 (0.403–1.486) | 0.441 | 0.670 (0.441–1.019) | 0.061 |
| WBC at diagnosis (≦30×10 ˆ9/L vs. > 30×10ˆ9/L) | 1.507 (0.680–3.343) | 0.313 | 1.588 (1.027–2.455) | 0.038 |
| LDH at diagnosis (≦250 U/L vs. > 250 U/L, ≦1,000 U/L vs. >1,000 U/L) | 1.979 (1.075–3.644) | 0.028 | 1.598 (1.164–2.193) | 0.004 |
| BM blasts at diagnosis (≧20%, ≦50% vs. > 50%, ≦80% vs. > 80%) | 1.536 (0.994–2.373) | 0.054 | 1.419 (1.101–1.830) | 0.007 |
| Cytogenetics (good and intermediate vs. poor) | 1.265 (0.612–2.613) | 0.526 | 1.496 (1.045–2.141) | 0.028 |
| CEBPα (mutated vs. unmutated) | 1.803 (0.402–8.086) | 0.441 | 4.084 (1.233–13.524) | 0.021 |
| Type of AML (primary vs. secondary) | 0.472 (0.165–1.354) | 0.163 | 1.044 (0.617–1.766) | 0.873 |
BM: bone marrow; HR: hazards ratio; LDH: lactate dehydrogenase; OS: overall survival; RFS: relapse free survival; WBC: white blood cell.
In multivariate analysis, we constructed a model to evaluate the prognostic significance of age, WBC, LDH, BM blasts, group of cytogenetics and mutation status of CEBPα at diagnosis. However, our multivariable analysis failed to define any independent significant prognostic parameters for OS. Nevertheless, we identified two trends towards independent prognostic factors for OS, including BM blasts at diagnosis (P = 0.057, HR: 1.676, 95%CI: 0.984–2.854) and mutation status of CEBPα (P = 0.064, HR: 4.173, 95%CI: 0.918–18.966) (Table 3). Additionally, higher BM blasts at diagnosis was a significant independent adverse predictor for RFS (P = 0.045, HR: 3.747, 95%CI: 1.028–13.662).
Table 3. Multivariate analysis of clinicopathologic factors in the primary cohort of 152 AML patients.
| Variable | RFS (n = 38) | OS (n = 151) | ||
| HR (95%CI) | P | HR (95%CI) | P | |
| Age (60–69 years vs. 70–79 years vs. ≧80 years) | 0.631 (0.176–2.266) | 0.480 | 0.779 (0.342–1.773) | 0.552 |
| WBC at diagnosis (≦30×10 ˆ9/L vs. >30×10 ˆ9/L) | 0.226 (0.025–2.015) | 0.183 | 0.971 (0.353–2.677) | 0.955 |
| LDH at diagnosis (≦250 U/L vs. >250 U/L, ≦1,000 U/L vs. > 1,000 U/L) | 1.338 (0.421–4.248) | 0.621 | 1.078 (0.535–2.172) | 0.833 |
| BM blasts at diagnosis (≧20%, ≦50% vs. >50%, ≦80% vs. > 80%) | 3.747,5 (1.028–13.662) | 0.045 | 1.676 (0.984–2.854) | 0.057 |
| Cytogenetics (good and intermediate vs. poor) | n.d.* | 1.879 (0.842–4.195) | 0.124 | |
| CEBPα (mutated vs. unmutated) | 5.084 (0.385–67.147) | 0.217 | 4.173 (0.918–18.966) | 0.064 |
Due to the high number of missing values, this variable could not be included in the model for RFS.
A new prognostic scoring system for stratifying elderly AML patients into three risk groups
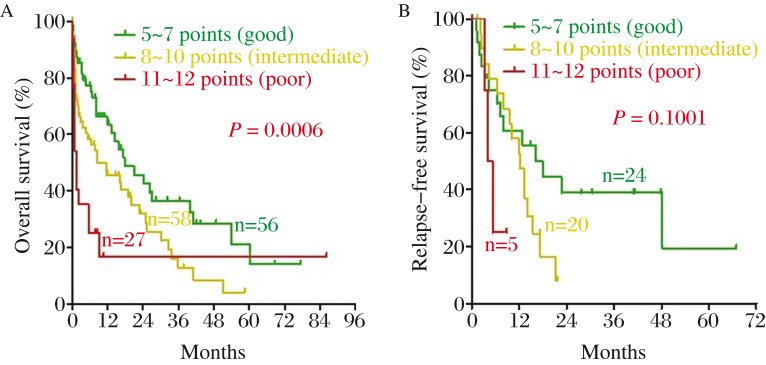
According to the results of log-rank test, univariate and multivariate analysis, we further developed a convenient five-factor scoring system. A score of 1 was assigned to female sex, age from 60 to 69 years, WBC at diagnosis ≤ 30×109/L, LDH at diagnosis ≤ 250 U/L, or BM blasts at diagnosis at 20%–50%. A score of 2 was assigned to male sex, age from 70 to 79 years, LDH at diagnosis at 250–1,000 U/L, or BM blasts at diagnosis at 50%–80%. A score of 3 was assigned to age older than 80 years, WBC at diagnosis more than 30 × 109/L, LDH at diagnosis more than 1,000 U/L, or BM blasts at diagnosis more than 80%. Table 4 shows this scoring system in a more intuitive way. As shown in Fig. 4, the novel scoring system stratified the patients into three risk groups: a score of 5 to 7, goodrisk (n = 56); a score of 8 to 10, intermediaterisk (n = 58); a score of 11 to 12, poorrisk (n = 27). The median OS was 9.48 months (range, 0.13–77.30 months) for the goodrisk group, 5.30 months (0.07–58.40 months) for the intermediate risk group and 0.8 month (0.10–86.33 months) for the poor risk group, respectively. Our scoring system was shown to be a significant prognostic factor of OS for elderly AML patients (P = 0.0006), but not for RFS (P = 0.1001). To validate our risk score model, we tested three independent samples from other hospitals to avoid overfit and the results turned out to be good (Table 5).
Table 4. The prognostic scoring system.
| Factors | 1 | 2 | 3 |
| Sex | Female | Male | |
| Age | 60–69 years | 70–79 years | ≧80 years |
| WBC at diagnosis | ≦30×10 ˆ9/L | > 30×10 ˆ9/L | |
| LDH at diagnosis | ≦250 U/L | >250 U/L, ≦1,000 U/L | >1,000 U/L |
| BM blasts at diagnosis | ≧20%, ≦50% | >50%, ≦80% | > 80% |
5–7 points = good& risk, 8– points = intermediate risk, 11–12 points = poor risk.
Fig. 4. The novel scoring system performed well in stratifying elderly AML patients into three risk groups.
(green line: good, yellow line: intermediated, red line: poor). A: OS; B: RFS.
Table 5. Characteristics of three representative patients.
| Patients | 1 | 2 | 3 |
| Sex | Female | Female | Male |
| Age | 61 years | 65 years | 69 years |
| WBC at diagnosis | 2.10×10ˆ9/L | 65.70×10ˆ9/L | 239.24×10ˆ9/L |
| LDH at diagnosis | 154 U/L | 630 U/L | 736 U/L |
| BM blasts at diagnosis | 44.00% | 52.80% | 90.80% |
| Score | 5 points | 9 points | 11 points |
| OS | 68.73 months | 32.67 months | 5.6 months |
| RFS | 66.97 months | 12.33 months | 4.01 months |
Factors associated with response to induction treatment
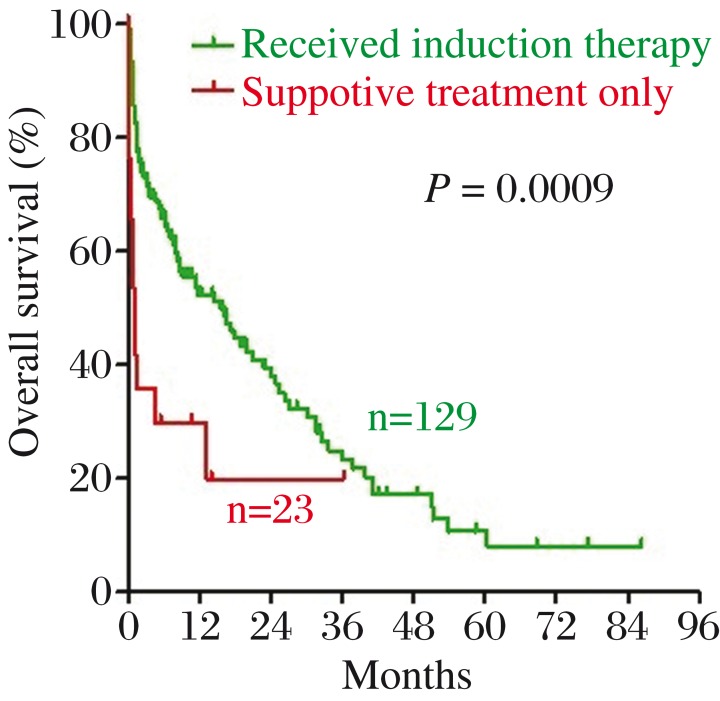
Among the 152 patients, 129 (84.9%) were treated with induction chemotherapy while the remaining 23 (15.1%) received only supportive management. There was a statistically significant difference in OS between the two groups (P = 0.0009, Fig. 5). Among the 129 patients who received chemotherapy, the CR rate was 60.0%. As shown in Table 6, our prognostic scoring system predicted response to induction therapy successfully in the current cohort of patients.
Fig. 5. There was a statistically significant difference in OS between patients treated with induction chemotherapy (green line) and patients received only supportive management (red line).
Table 6. CR rate in the 129 elderly patients who received induction treatment.
| Characteristic | Achieved CRn/all (%) | P value |
| All patients | 69/115 (60.0) | |
| Age | ||
| 60–69 years | 50/76 (65.8) | 0.012 |
| 70–79 years | 19/34 (55.9) | |
| >80 years | 0/5 (0.0) | |
| Sex | ||
| Male | 37/70 (52.9) | 0.051 |
| Previous hematologic diseases | ||
| Yes | 7/17 (41.2) | 0.086 |
| WBC at diagnosis, ×109/L | ||
| ≤ 30 | 54/83 (65.1) | 0.105 |
| LDH at diagnosis, U/L | ||
| ≤ 250 | 27/39 (69.2) | 0.266 |
| > 250, ≤ 1,000 | 31/54 (57.4) | |
| > 1,000 | 7/15 (46.7) | |
| BM blasts at diagnosis, % | ||
| ≥ 20%, ≤ 50% | 31/49 (63.3) | 0.235 |
| > 50%, ≤ 80% | 25/38 (65.8) | |
| > 80% | 13/28 (46.4) | |
| Cytogenetics | ||
| Favorable | 3/3 (100.0) | 0.528 |
| Intermediate | 47/68 (69.1) | |
| Unfavorable | 4/7 (57.1) | |
| CEBPα mutation status | ||
| mutated | 4/6 (66.7) | 1.000 |
| unmutated | 18/27 (66.7) | |
| The scoring system | ||
| 5–7, good risk | 33/47 (70.2) | 0.014 |
| 8–10, intermediate risk | 26/42 (61.9) | |
| 11–12, poor risk | 6/19 (31.6) |
DISCUSSION
AML is predominantly a disease of elderly people. Collaborative group study and large center experience have rarely proved an increase in the cure rate for elderly AML patients over the recent three decades[5]. During the last 15 years, only slight improvement on CR rates has been made. According to the previous reports, the 2-year survival rate of elderly AML patients was approximately 20%[17],[18]. In the study by Iwakiri et al.[19], the 3-year survival rate was 28%, which is close to our data (22.2%). The poor outcomes of elderly AML were attributed to patient and disease factors including poor physical status, decreased organ functional reserve, poor tolerance for chemotherapy drug toxicity, more occurrence of drug resistance, more comorbidities, and more chance to get poor risk cytogenetics[20],[21]. For elderly AML patients, treatment choices include standard dose regimen, reduced dose chemotherapy, and palliative care. The selection mechanism of treatment remains controversial. Our results showed that people treated with chemotherapy for remission induction have significantly longer OS than those who received only supportive treatment. The EORTC leukemia cooperative group also indicated that in AML patients older than 65 years, the wait-and-see treatment leads to a shorter median OS comparing with the group of immediate induction (11 weeks vs. 21 weeks)[22].
In a long-term follow-up from five hematological intensive care centers, age was one of the most important prognostic factors for overall AML patients[23],[24]. Our data from 152 elderly patients also confirmed that older age predicts shorter OS and RFS and lower CR rate in elderly AML patients. In nine patients older than 80 years who received induction therapy, OS ranged from 0.17 to 10.17 months. Besides age, higher WBC at diagnosis (>30×109/L) also infers shorter OS, which is in accordance with the previous studies[6],[25],[26]. Moreover, the results showed that higher LDH at initial diagnosis was associated with shorter OS and RFS in our cohort. As a marker of tumor burden and cell turnover, LDH is an acknowledged prognostic element in AML[6],[7]. Behringer et al. also demonstrated this finding in their single-center retrospective study[11]. In our retrospective study, both univariate and multivariate analysis identified lower BM blasts at diagnosis as a significantly favorable prognostic factor for elderly patients with AML.
According to the current WHO categorization of AML, cytogenetic and molecular analyses play a more important role in predicting remission rate and survival outcome for AML patients. Since elderly patients are more likely to carry poor risk cytogenetics at diagnosis[27], one may question the availability of these prognostic factors in this population of patients. We proved that patients with poor risk cytogenetics presented statistically significant shorter survival time in comparison to those with better or intermediate risk cytogenetics. However, we failed to find differences in survival and CR rates between patients with favorable and intermediate risk cytogenetics. In molecular analysis, only CEBPα mutation turned out to have good influence on survival of elderly AML patients. There is a general consensus now that only patients with biallelic CEBPα mutations have a favorable outcome, with limited or no impact for monoallelic CEBPα. However, our research failed to assess with each mutant patterns caused by the limitation of its retrospective nature and our clinical lab.
Although cytogenetic and molecular evaluations play key roles in predicting prognosis for AML patients, the entire information from those laboratory tests is usually not available until 1–2 weeks following diagnosis. In the current study, we developed a novel scoring system for elderly AML patients based on five clinicopathologic characteristics including age, sex, WBC, LDH and BM blasts at initial diagnosis, which could be collected easily within several hours after diagnosis. De novo elderly AML patients may be categorized into three groups according to this scoring system. As mentioned above, our system performed well in stratifying elderly AML patients into groups with variable treatment response and survival. To validate our risk score model, we tested three independent samples to avoid overfit and the results turned out to be good. However, three cases are too small to be sufficient and the scoring system needs to be validated by more cases in the future. Some authors have succeeded in establishing a prognostic scoring system for adult and elderly AML patients based on different clinical factors. Malagola et al.[28] developed a prognostic index score to stratify adult patients (≤65 years) with cytogenetically normal AML into three prognostic groups using three independent adverse prognostic parameters, including age ≥ 50 years, secondary AML and WBC ≥ 20×109/L. Wheatley et al.[29] created a risk score system for survival of elderly AML, which contained five prognostic factors: the cytogenetic group, WBC, performance status, age and AML type (primary or secondary). Similarly, our scoring system contains two well-known prognostic clinical variables: age and WBC at diagnosis, and our approach may be novel due to the combination of LDH and BM blasts at diagnosis for elderly AML. As to the type of AML, we only found that previous history of hematologic diseases resulted in lower CR rate in our cohort.
Our research is limited by its retrospective nature and lack of unified treatment principles. The results from our study including the scoring system need to be validated by prospective randomized clinical trials.
References
- 1.Estey EH. Acute myeloid leukemia: 2013 update on risk-stratification and management. Am J Hematol. 2013;88:318–27. doi: 10.1002/ajh.23404. [DOI] [PubMed] [Google Scholar]
- 2.Juliusson G, Lazarevic V, Horstedt AS, Hagberg O, Hoglund M. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119:3890–9. doi: 10.1182/blood-2011-12-379008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brincker H. Estimate of overall treatment results in acute nonlymphocytic leukemia based on age-specific rates of incidence and of complete remission. Cancer Treat Rep. 1985;69:5–11. [PubMed] [Google Scholar]
- 4.Ferrara F. Treatment of unfit patients with acute myeloid leukemia: a still open clinical challenge. Clin Lymphoma Myeloma Leuk. 2011;11:10–6. doi: 10.3816/CLML.2011.n.001. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program. 2012;2012:1–6. doi: 10.1182/asheducation-2012.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Yanada M, Naoe T. Acute myeloid leukemia in older adults. Int J Hematol. 2012;96:186–93. doi: 10.1007/s12185-012-1137-3. [DOI] [PubMed] [Google Scholar]
- 7.Vey N. Targeting age-related changes in the biology of acute myeloid leukemia: is the patient seeing the progress? Interdiscip Top Gerontol. 2013;38:73–84. doi: 10.1159/000343623. [DOI] [PubMed] [Google Scholar]
- 8.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89:3323–9. [PubMed] [Google Scholar]
- 9.Djunic I, Suvajdzic-Vukovic N, Virijevic M, Novkovic A, Colovic N, Vidovic A, et al. Prognostic risk score for the survival of elderly patients with acute myeloid leukaemia comprising comorbidities. Med Oncol. 2013;30:394. doi: 10.1007/s12032-012-0394-6. [DOI] [PubMed] [Google Scholar]
- 10.Sherman AE, Motyckova G, Fega KR, Deangelo DJ, Abel GA, Steensma D, et al. Geriatric assessment in older patients with acute myeloid leukemia: A retrospective study of associated treatment and outcomes. Leuk Res. 2013;37:998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behringer B, Pitako JA, Kunzmann R, Schmoor C, Behringer D, Mertelsmann R, et al. Prognosis of older patients with acute myeloid leukemia receiving either induction or noncurative treatment: a single-center retrospective study. Ann Hematol. 2003;82:381–9. doi: 10.1007/s00277-003-0650-0. [DOI] [PubMed] [Google Scholar]
- 12.Walter RB, Othus M, Burnett AK, Lowenberg B, Kantarjian HM, Ossenkoppele GJ, et al. Significance of FAB subclassification of “acute myeloid leukemia, NOS” in the 2008 WHO classification: analysis of 5848 newly diagnosed patients. Blood. 2013;121:2424–31. doi: 10.1182/blood-2012-10-462440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SJ, Shi JY, Zhu YM, Shi ZZ, Yan S, Gu BW, et al. The investigation of mutation and single nucleotide polymorphism of receptor tyrosine kinases and downstream scaffold molecules in acute myeloid leukemia. Leuk Lymphoma. 2006;47:2610–6. doi: 10.1080/10428190600948048. [DOI] [PubMed] [Google Scholar]
- 15.Qiao C, Zhang R, Hong M, Wang L, Zhang JF, Wu YJ, et al. Heterogeneous leukemic clones identified by NPM1 mutation analysis in patient with acute monocytic leukemia. Leuk Lymphoma. 2012;53:886–90. doi: 10.3109/10428194.2011.635860. [DOI] [PubMed] [Google Scholar]
- 16.Creutzig U, Kaspers GJ. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2004;22:3432–3. doi: 10.1200/JCO.2004.99.116. [DOI] [PubMed] [Google Scholar]
- 17.Menzin J, Lang K, Earle CC, Kerney D, Mallick R. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med. 2002;162:1597–603. doi: 10.1001/archinte.162.14.1597. [DOI] [PubMed] [Google Scholar]
- 18.Burnett AK, Russell NH, Hunter AE, Milligan D, Knapper S, Wheatley K, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122:1384–94. doi: 10.1182/blood-2013-04-496596. [DOI] [PubMed] [Google Scholar]
- 19.Iwakiri R, Ohta M, Mikoshiba M, Tsutsumi H, Kumakawa T, Mori M. Prognosis of elderly patients with acute myelogenous leukemia: analysis of 126 AML cases. Int J Hematol. 2002;75:45–50. doi: 10.1007/BF02981978. [DOI] [PubMed] [Google Scholar]
- 20.Abe S, Kanaya K, Kikukawa M, Sakai M, Akai T, Takata Y, et al. Clinical results and issues of acute myeloid leukemia in elderly patients aged 75 years and older. Geriatr Gerontol Int. 2011;11:290–6. doi: 10.1111/j.1447-0594.2010.00682.x. [DOI] [PubMed] [Google Scholar]
- 21.Burnett AK. The Challenge of AML in Older Patients. Mediterr J Hematol Infect Dis. 2013;5:e2013038. doi: 10.4084/MJHID.2013.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7:1268–74. doi: 10.1200/JCO.1989.7.9.1268. [DOI] [PubMed] [Google Scholar]
- 23.Szotkowski T, Muzik J, Voglova J, Koza V, Maaloufova J, Kozak T, et al. Prognostic factors and treatment outcome in 1,516 adult patients with de novo and secondary acute myeloid leukemia in 1999–2009 in 5 hematology intensive care centers in the Czech Republic. Neoplasma. 2010;57:578–89. doi: 10.4149/neo_2010_06_578. [DOI] [PubMed] [Google Scholar]
- 24.Shuichi M, Hisashi S, Shigeki O, Nobuhiko E, Fumiharu Y, Kinuko M, et al. A Randomized, Postremission Comparison of Four Courses of Standard-Dose Consolidation Therapy without Maintenance Therapy versus Three Courses of Standard-Dose Consolidation with Maintenance Therapy in Adults with Acute Myeloid Leukemia. Cancer. 2005;104:2726–34. doi: 10.1002/cncr.21493. [DOI] [PubMed] [Google Scholar]
- 25.Thomas X, Chelghoum Y, Cannas G, Elhamri M, Labussiere H, Tigaud I, et al. Leukocytosis and circulating blasts in older adults with newly diagnosed acute myeloid leukemia: are they valuable factors for therapeutic decision-making? Clin Lymphoma Myeloma Leuk. 2011;11:342–9. doi: 10.1016/j.clml.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Bertoli S, Berard E, Huguet F, Huynh A, Tavitian S, Vergez F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121:2618–26. doi: 10.1182/blood-2012-09-454553. [DOI] [PubMed] [Google Scholar]
- 27.Ustun C, Lazarus H, Weisdorf D. To transplant or not: a dilemma for treatment of elderly AML patients in the twenty-first century. Bone Marrow Transplant. 2013;48:1497–505. doi: 10.1038/bmt.2013.67. [DOI] [PubMed] [Google Scholar]
- 28.Michele M, Cristina S, Marco V, Alfonso P, Giovanni M, Giuliana A, et al. A simple prognostic scoring system for newly diagnosed cytogenetically normal acute myeloid leukemia: retrospective analysis of 530 patients. Leuk Lymphoma. 2011;52:2329–35. doi: 10.3109/10428194.2011.596965. [DOI] [PubMed] [Google Scholar]
- 29.Keith W, Cassandra L, Andrew J, Anthony H, Donald W, Archibald G, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. doi: 10.1111/j.1365-2141.2009.07663.x. [DOI] [PubMed] [Google Scholar]