Abstract
Actinorhizal plants contain numerous antioxidants that may play a crucial role in preventing the formation of tumors. H-Ras p21, a member of the Ras-GTPase family, is a promising target to treat various kinds of cancers. An in silico docking study was carried out to identify the inhibitory potential of compounds of these plants against H-Ras by using Discovery Studio 3.5 and by using Autodock 4.2. Docking studies revealed that four compounds, isorhamnetin-7-rhamnoside, quercetin-3-glucoside-7-rhamnoside (present in H. rhamnoides), zeaxanthin, and translutein (present in H. salicifolia) significantly bind with binding energies −17.1534, −14.7936, −10.2105 and −17.2217 Kcal/mol, respectively, even though they slightly deviate from Lipinski's rule. Absorption, distribution, metabolism, excretion and toxicity (ADME/tox) analyses of these compounds and their stereoisomers showed that they were less toxic and non-mutagenic. Amongst them, isorhamntein-7-rhamnoside showed hepatotoxicity. Hence, these compounds can be further investigated in vivo to optimize their formulation and concentration and to develop potential chemical entities for the prevention and treatment of cancers.
Keywords: Hippophae, H-Ras, cancer, docking, Discovery Studio 3.5, H. rhamnoides, H. salicifolia
INTRODUCTION
Cancer is a complex disease that is characterized by aberrant cell division. It is caused by genetic variations and many environmental factors. It can invade vital organs and is a major harbinger of imminent patient death throughout the world[1]. Approximately 30% of the tumors are due to oncogenic mutations in any of the canonical Ras genes[2]. Ras belongs to the family of small GTPases, which are a group of enzymes that can bind to and hydrolyze guanosine triphosphate (GTP). Essentially, they work as molecular “on/off” switches. The guanosine diphosphate (GDP) bound conformation is the “off” state, and the GTP bound conformation is the “on” state that plays an important role in intracellular signaling which regulates processes such as cell proliferation, differentiation, cell apoptosis, and migration[3]. Hyperactivation of the Ras signaling pathway drives many cancers. The oncogenic mutations in many components of the Ras/MAPK signaling pathways, such as H-Ras, B-Raf, EGFR and NF-1, indicate that deregulation of Ras-dependent signaling is an essential requirement for tumorigenesis[2].
Hippophae (sea-buckthorn) is a deciduous, actinorhizal plant that belongs to the Elaeagnaceae family. The most commonly found species is Hippophae rhamnoides. It is followed by Hippophae salicifolia. H. salicifolia has a shrub-to-tree habit and is restricted to the Himalayan region[4], whereas H. rhamnoides is shrubby-to-bushy, grows at a higher altitude and is widely distributed in Eurasia[5],[6]. In India, this plant grows predominantly in the high-altitude areas of Sikkim, Himachal Pradesh, Jammu and Kashmir, and Uttar Pradesh[7]. Hippophae is a plant that is cultivated worldwide for its medicinal properties[8]. It has been established that compounds in Hippophae help wound healing, prevent ulcer formation, and have anti-atherogenic and anti-platelet aggregation activity[9],[10].
In general, Hippophae contains different antioxidants that can prevent oxidative stress. The leaves and fruits of the plant are rich sources of vitamins (A, B, C, E and K), carotenoids, flavonoids, glycerophospholipids, phytosterols, zeaxanthin esters, alpha-tocopherol and phenolic compounds[9]. A few of the flavonoids that are found in H. rhamnoides include isorhamnetin-3-glucoside, isorhamnetin-7-glucoside, isorhamnetin-5-glucoside, isorhamnetin-7-rhamnoside, quercetin-3-glucoside, quercetin-3-glucoside-7-rhamnoside and isorhamnetin-3-rhamnoside[10]. There are also reports of various anti-cancerous compounds, such as vitamin C, carotenoids, vitamin E, different flavonoids, kaempferol, fatty acids, triacylglycerol, phytosterols and phenolic compounds that are known to be present in Hippophae rhamnoides[11]. Similarly, Hippophae salicifolia was found to have a few compounds such as alpha carotene, beta carotene and zeaxanthin analogues that have antioxidant activity[8],[12].
In our early studies, we applied computer based prediction methods for the rational design of compounds presented in natural sources[12]. Complementary treatment approaches are increasingly requested by cancer patients and survivors to reduce side effects of conventional cancer treatment and enhance health related quality of life[13],[14]. As more herbs, such as Hippophae, are used in daily life, the safety of the co-administration of herbs and allopathic medicines should be investigated. Even though the pharmacokinetics and pharmacodynamics of Western medicines are well known, the corresponding knowledge of any co-administered herbal products has not been well studied, as well as their complex components and variable amounts of active components. Hence, the objective of this study was to discover new anti-cancerous candidates of the herb Hippophae by analyzing physicochemical properties, potential binding interactions and side effects.
MATERIALS AND METHODS
Generation of ligand dataset
Structures of compounds present in H. salicifolia and H. rhamnoides were attained from PubChem compound database (http://www.ncbi.nlm.nih.gov/pccompound) and redrawn using MarvinSketch[15]. The same was used to predict properties of ligands such as hydrogen donors, acceptors, LogP value, refractivity, pH and molecular weight. These compounds were screened for drug-likeliness according to Lipinski's rule of five[16]. A range of conformers for each ligand were created (energy minimization) by using MarvinSketch and the lowest energy conformer was chosen for further evaluation.
ADMET and Lipinski's rule of five
The 2D structures were subject to absorption, distribution, metabolism, excretion and toxicity (ADMET) analyses for solubility, intestinal absorption, hepatotoxicity, plasma protein binding ability, blood-brain barrier (BBB) penetration, cytochrome P450 inhibition, and AMES mutagenicity using Discovery Studio (DS) 3.5. ADMET describes the deposition of a pharmaceutical compound within an organism. Lead compounds can be identified through high throughput screening approaches or by virtual screening. To decrease cost and clinical failures of new drugs, the compound dataset is effectively screened in the initial stages for ADMET, since these ADMET technologies are becoming more sophisticated and reliable[17],[18]. All these criteria have a predictable influence on pharmacokinetic and pharmacological effects of drugs.
Preparing the receptor
The native crystal structure of a chain of H-Ras oncogene p21 complexed to the slowly hydrolyzing GTP analogue, phosphoaminophosphonic acid-guanylate ester (GNP) was retrieved from Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank (PDB, ID: 5P21) (http://www.rcsb.org/pdb/home/home.do). RCSB is the single, global archive for information about the 3D structure of macromolecules (proteins and DNA) and their complexes, as determined by X-ray crystallography, NMR spectroscopy and cryoelectron microscopy[19],[20]. The PDB file was cleaned and the hetero atoms (HETATM) of the receptor were removed manually, since these are non-standard residues of protein[21].
Docking simulations
Docking is virtual screening of a database of compounds and predicting the strongest binders based on various scoring functions. Accelrys Discovery Studio 3.5[22] and Autodock[17] were used for docking. Before docking, the ligands were prepared using the “prepare ligand” module. The ligands were primarily positioned in the binding site by using LibDock[22], which is a high throughput docking algorithm that positions catalyst generated ligand conformations in the protein active site based on polar interaction sites (hotspots). Other algorithms such as GOLD and CDOCKER generate random conformations by using CHARMm-based molecular dynamics. Hence, LibDock is a suitable algorithm to find various conformations of the ligands within the receptor[22]. Receptor-ligand interactions were further optimized by molecular dynamics using CHARMM[23] and Clean Geometry of Discovery Studio. Force fields applied in CHARMM are energies and forces on each particle of the system and also defines the positional relationships between atoms that determine their energy. AutoDock identifies the conformational space of the ligand by using Lamarkian genetic algorithm (LGA), which is a hybrid of a genetic algorithm (GA) with an adaptive local search (LS)[24]. In this study, the receptor and ligand coordinates were used in MOL2 format. Nonpolar hydrogen atoms were removed from the receptor file and their partial charges were added to the receptors. The program Mol2topdbqs was used to transform the receptor MOL2 file into the PDBQS format file containing the receptor atom coordinates, partial charges and solvation parameters. The grid calculations were set up with the utility Mkgpf3 and maps were calculated with the program AutoGrid. The grid maps were centered on the ligand's binding site obtained from PDBSum. For each complex, 10 docking simulations were performed with default parameters[17]. PDBSum was utilized to determine the binding sites with which the ligand can bind itself to the receptor. Following this, the natural compounds and receptors were docked. The LibDock scores and binding energies of the docked ligands were estimated.
RESULTS
Lipinski's rule of five
The drug likeness scores of the compounds of H. salicifolia and H. rhamnoides were evaluated with the help of Lipinski's rule of five. The physicochemical properties of the compounds that accept the Lipinski's rule, TPSA (topological polar surface area) and number of rotatable bonds are tabulated in Table 1. Zeaxanthin and vitamin C were the only compounds that showed drug-like properties. All the compounds of the ligand dataset were also included in addition to vitamin C and zeaxanthin for docking with the H-Ras receptor. The binding energies and the highest LibDock score of the docked ligands are shown in Table 2.
Table 1. Physicochemical properties of compounds accepting the Lipinski's rule.
| Compound | Plant | logP | Hydrogen bond donors | Hydrogen bond acceptors | Molecular weight [g/mol] |
| Zeaxanthin | H. salicifolia | 4.92 | 1 | 2 | 366.5363 |
| Vitamin C | H. rhamnoides | −1.91 | 4 | 5 | 176.1241 |
Table 2. Binding energies of docked ligands using LibDock.
| Compounds | Binding energy (kcal/mol) | LibDock score |
| Alpha carotene | −1.54354 | 176.40 |
| Beta cryptoxanthin | 1.31813 | 180.79 |
| Beta carotene | −5.42891 | 180.96 |
| Gamma carotene | −1.35E+09 | 163.80 |
| Lutein D | −2.71363 | 183.49 |
| Lycopene | 8.91E+06 | 183.11 |
| Translutein | −14.7936 | 174.84 |
| Zeaxanthin | −17.1534 | 150.00 |
| Isorhamnetin 3-glucoside | 5.88714 | 142.29 |
| Isorhamnetin 3-rhamnoside | 7.48074 | 141.69 |
| Isorhamnetin 5-glucoside | 16.8981 | 148.43 |
| Isorhamnetin 7-glucoside | −4.58523 | 158.33 |
| Isorhamnetin 7-rhamnoside | −10.2105 | 161.66 |
| Quercetin 3-glucoside-7-rhamnoside | −17.2217 | 175.05 |
| Quercetin 3-glucoside | 4.32627 | 149.05 |
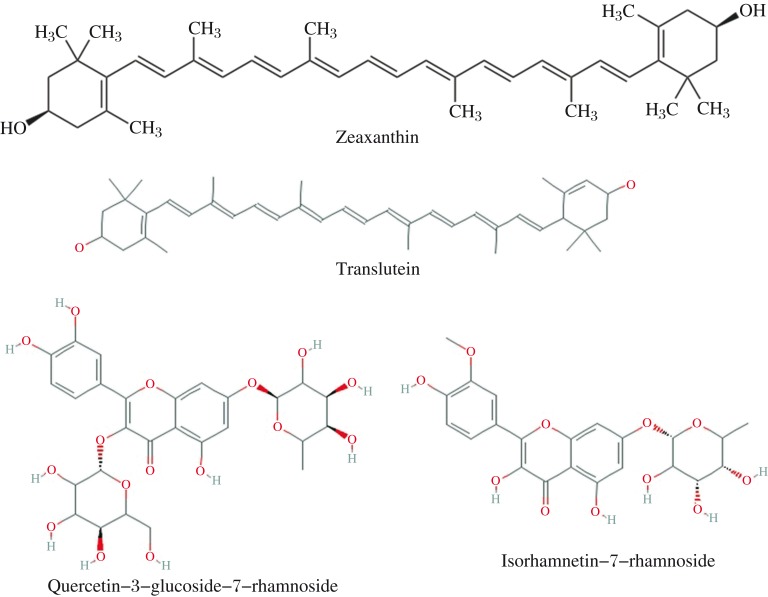
Vitamin C and E did not show interactions with the receptor. As a result, no LibDock scores or binding energies were observed. Two compounds from H. salicifolia, namely translutein and zeaxanthin (Fig. 1) and two compounds from H. rhamnoides, namely quercetin-3-glucoside-7-rhamnoside and isorhamnetin-7-rhamnoside (Fig. 1), bind significantly to the active site of the receptor compared to the other compounds as shown by their negative binding energies (Table 2).
Fig. 1. Chemical structures of zeaxanthin, translutein, quercetin-3-glucoside-7-rhamnoside and isorhamnetin-7-rhamnoside.
PDBSum interactions
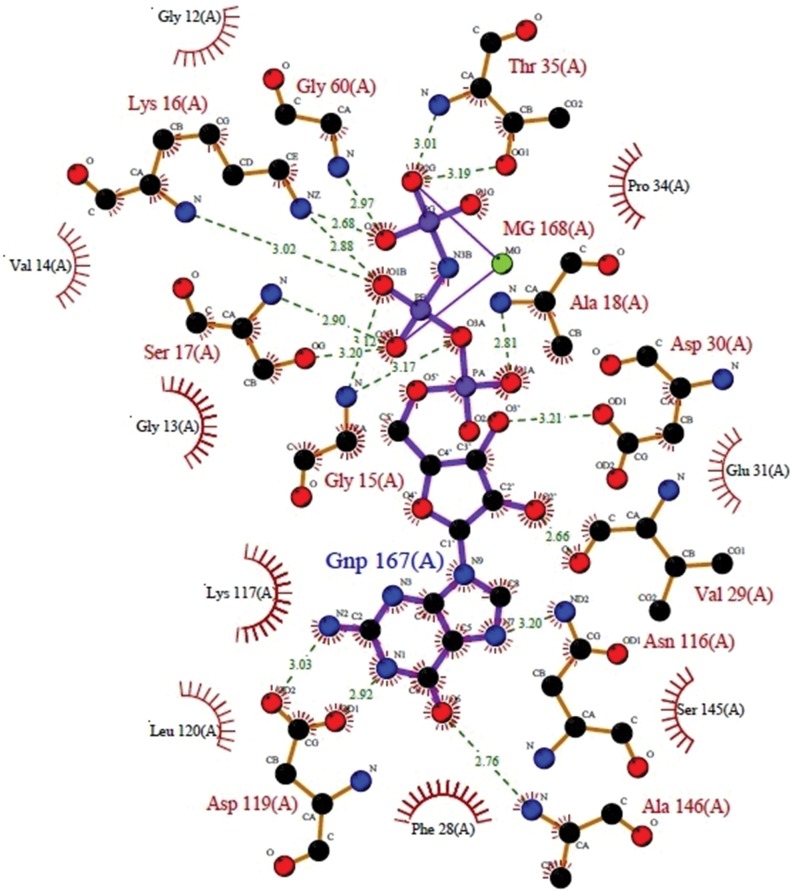
A 3D image generated of H-Ras (5p21) bound to a ligand molecule phosphoaminophosphonic acid-guanylate ester (GNP) by Raster3D in PDBsum was used to study the ligand and receptor interactions. The ligplot (Fig. 2) clearly indicates the hydrophilic interactions of the ligand with the residues like ASP119 (A), GLY15(A), SER17(A), LYS16(A), GLY60(A), THR35(A), ALA18(A), ASP30(A), VAL 29(A), ASN116(A), and ALA146(A) (Fig. 2). Some of these amino acid residues were also found to be bound to the ligand molecules of H. salicifolia and H. rhamnoides after docking studies.
Fig. 2. A LIGPLOT of the interactions of GNP ligand with the residues of the H-Ras 5p21.
The atoms are color-coded as follows: carbon, black; nitrogen, blue; oxygen, red; and phosphorus, purple. The ligand's bonds are shown in purple, and those of the protein residues are in orange. Hydrogen bonds between protein and ligand are represented by green dotted lines, lengths in angstroms, residues involved in non-bonded contacts with the ligand are represented by the arcs, with corresponding arcs on the relevant atoms of the ligand.
Docking simulations
Docking with DS showed the top four drug candidates: zeaxanthin, translutein, quercetin-3-glucoside-7-rhamnoside, and isorhamnetin-7-rhamnoside. This was also validated using AutoDock tool (vertion). The binding energies of the candidates are shown in Table 3.
Table 3. Binding energies of docked ligands using Autodock.
| Compounds | Binding Energy (kcal/mol) |
| Translutein | −3.56 |
| Zeaxanthin | −6.29 |
| Isorhamnetin 7-rhamnoside | −5.54 |
| Quercetin 3-glucoside-7-rhamnoside | −6.61 |
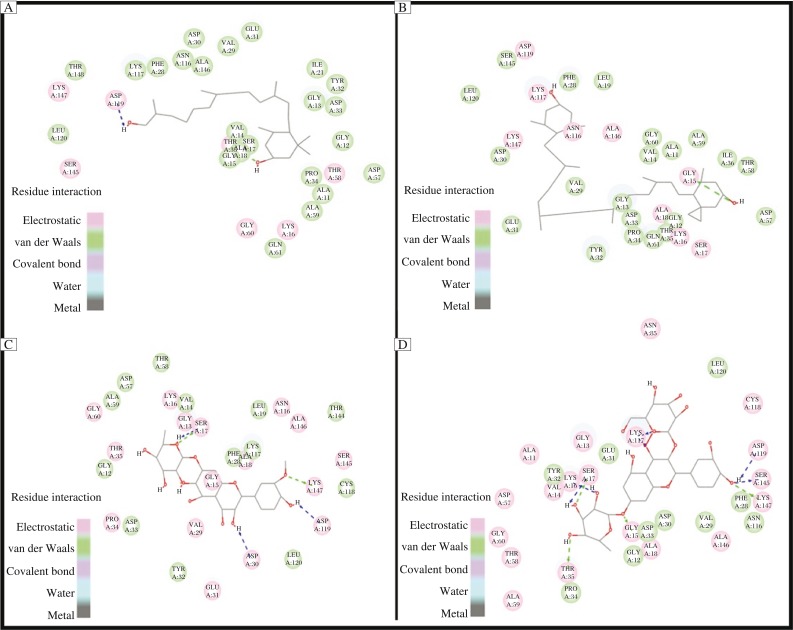
The docking results showed that all the ligands with the best binding energies viz, zeaxanthin, translutein, isorhamnetin-7-glucoside, isorhamnetin-7-glucoside and quercetin-3-glucoside-7-rhamnoside, occupied the same active site of H-Ras like that of GNP with LibDock score of 150,174,161.66, and 175.05 and binding energy is −17.1534, −14.7936, −10.2105, and −17.2217 Kcal/mol, respectively. Simulation studies carried out through AutoDock showed the binding energies of quercetin 3-glucoside 7-rhamnoside, zeaxanthin, isorhamnetin 7-rhamnoside, translutein were −6.61, −6.29, −5.54 and −3.56 Kcal/mol, respectively. The binding mode of the ligands within the active site of H-Ras was also analyzed using DS 3.5. It provides a 2D visualization of the drug-receptor interaction. The binding interactions of the zeaxanthin, (Fig. 3A) with the H-Ras, with ASP A:119 and ALA A:18 was noted. The potential binding sites of translutein (Fig. 3B) were found to bind with LYS A: 117 and GLY A:15. For isorhamnetin-7-glucoside (Fig. 3C), SER A:117, ASP A:30, ASP A:119 and LYS A:147. For quercetin-3-glucoside-7-rhamnoside (Fig. 3D), the amino acids associated were ASP A:119, SER A:145, LYS A:147, THR A:35, SER A:17, LYS A:16 and GLY A:15. These amino acid residues are actively involved in forming hydrophobic and hydrophilic contacts and were found to play an important role in the binding of inhibitors within the active site of H-Ras. The interactions found using DS were confirmed with Autodock, which showed similar association of the amino acids with the ligands.
Fig. 3. Interaction of the ligands (A: zeaxanthin; B: translutein; C: isorhamnetin 7-glucoside; D: quercetin 3-glucoside-7-rhamnoside) with the receptor (H-Ras).
Toxicity prediction
The ligands with comparable scores to other molecules were subjected to predict ADME properties using the DS client, the toxicity prediction module of the software. The predicted ADME properties are tabulated in Table 4. The compounds showed partial plasma binding ability (Table 4).
Table 4. The toxicity prediction of the compounds present in the herbs and their stereoisomers.
| Parameters considered | Isorhamnetin-7-rhamoside | Isorhamentin(pure) | Quercetin-3-glucoside-7-rhamnoside | Quercetin (pure) | Translutein | Lutein | Zeaxanthin | Zeaxanthin (stereoisomer) |
| Solubility level | 4 | 3 | 3 | 3 | 0 | 2 | 1 | 1 |
| Blood brain barrier Level | 4 | 4 | 4 | 4 | 4 | 0 | 4 | 4 |
| Hepatotoxicity prediction | True | True | False | True | False | True | False | False |
| CYP2D6 | False | False | False | False | False | False | False | False |
| Plasma protein Binding prediction | False | False | False | False | True | True | True | True |
| Ames mutagenicity | Non-mutagen | Mutagen | Non-mutagen | Mutagen | Non-mutagen | Non-mutagen | Non-mutagen | Non-mutagen |
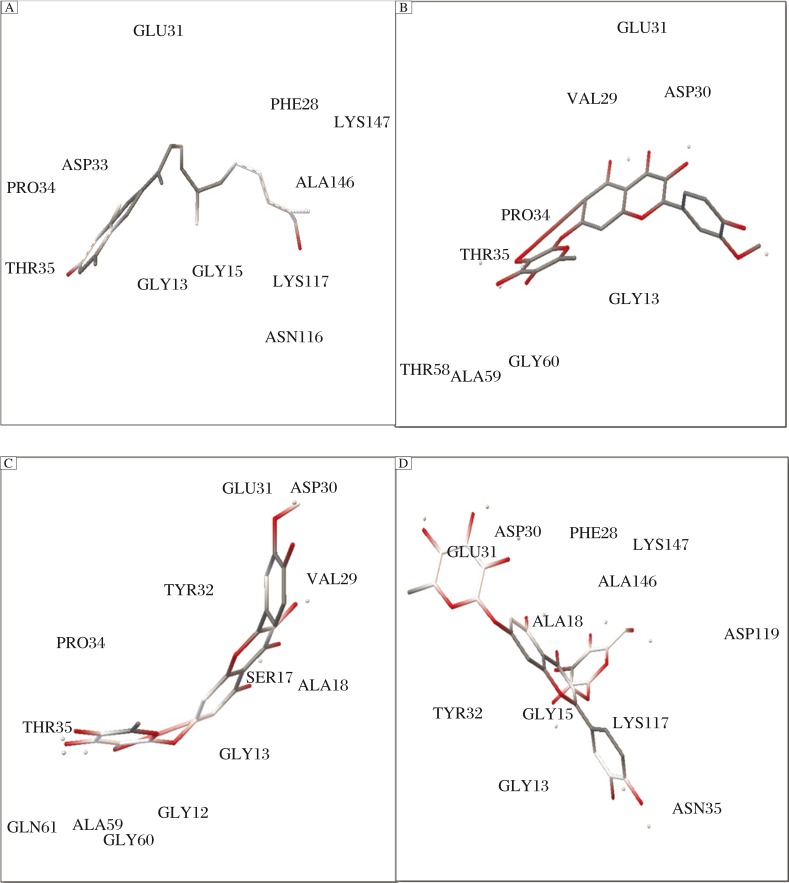
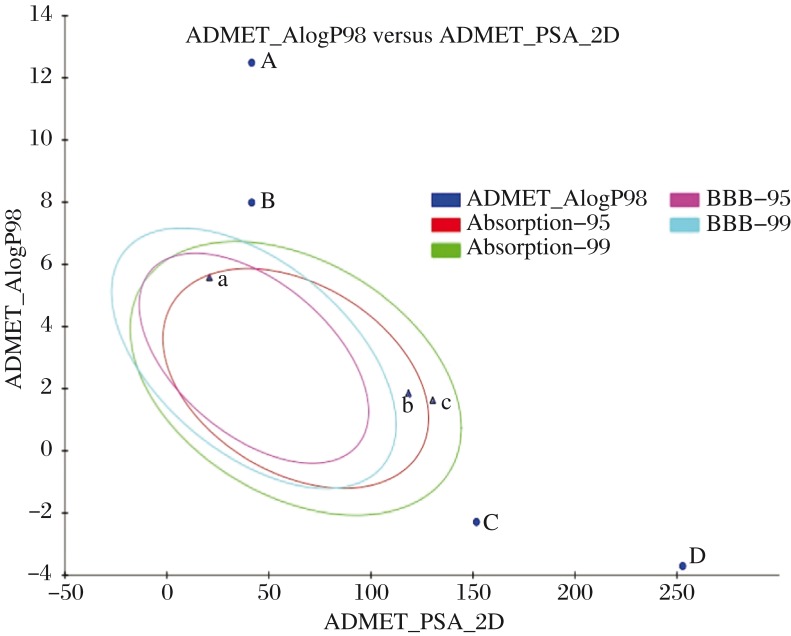
Fig. 4 depicts that all the compounds have poor intestinal absorption levels and may prove to be toxic. All the compounds except isorhamnetin and quercetin in their pure forms exhibited mutagenicity as predicted by TOPKAT Ames mutagenicity test of DS. Vitamin C being naturally present in the human body proves to be easily soluble in the intestine. As a result, to increase the safety and efficacy of the ligand molecules, the stereoisomers and pure forms of the drugs obtained from PubChem were also checked for their ADMET properties. As shown in Fig. 5, the stereoisomers have shown better intestinal absorption, since they lie in 99% confidence ellipses in comparison to the ligand forms available in herbs. As shown in Table 4, zeaxanthin in both forms exhibited the same pharmacological properties. Lutein, a stereoisomer of translutein is more toxic because of high penetrance through BBB. Table 4 also represents that the pure forms of isorhamnetin and quercetin show better absorption.
Fig. 4. Interaction of the ligands (A: zeaxanthin; B: translutein; C: isorhamnetin 7-glucoside; D: quercetin 3-glucoside-7-rhamnoside) with the receptor (H-Ras) using AutoDock 4.2.
Fig. 5. Prediction of drug absorption for various compounds.
A: translutein B: zeaxanthin C: isorhamnetin 7-rhamnoside, D: quercetin 3-glucoside-7-rhamnoside, a: lutein stereoisomer, b: isorhamnetin (pure) and c: quercetin (pure) [x axis indicates the solubility of the compounds; y axis indicates the log P values] considered using Discovery Studio 2.1 (Accelrys, San Diego, CA). ADMET Descriptors, 2D polar surface area (PSA_2D) in Å2 for each compound is plotted against their corresponding calculated atom-type partition coefficient (ALogP98). The area covered by the ellipse is a prophecy of excellent absorption without any violation of ADMET properties. Ellipses indicate the absorption model at 95% and 99% confidence limit to the blood brain barrier (BBB) and intestinal absorption models.
DISCUSSION
Molecular docking discovers the binding geometry of two interacting molecules with known structures. It predicts the preferred orientation of receptor and ligand to each other to form a stable complex[17],[23],[25]. Currently, the use of computers to determine the binding of datasets of small molecules to known receptors is a major component of drug discovery. Rule of five evaluates certain pharmacological, biological and ADME properties of the ligand [16],[17]. The compound that exceeds molecular weight (Mw) > 500 Da, calculated log P > 5, hydrogen-bond donors > 5, hydrogen-bond acceptors > 10 is unlikely to be further pursued as a potential drug, because it would likely lack properties essential in its absorption, distribution, metabolism and excretion. Zeaxanthin and vitamin C are the only compounds that had drug-like properties. However, traditionally most anti-cancerous drugs deviate from the Lipinski rule with many having a higher molecular weight than common drugs. An example is paclitaxel, which has a molecular weight of 853.9 Da, with eleven hydrogen acceptors and four hydrogen donors. It is highly lipophilic and cannot be administered orally[26],[27]. TPSA and the number of rotatable bonds for zeaxanthin and vitamin C were also determined since these parameters account for oral bioavailability.
Simulation studies carried out through AutoDock showed quercetin 3-glucoside 7-rhamnoside is a probable anticancerous candidate, confirming the results shown by Discovery Studio. Singh and Konwar also reported previously molecular docking studies of quercetin and its analogues as anticancerous agents[28]. Though all the compounds occupied the similar active site of H-Ras, the evident difference in binding energies may be due to wide structural variations and based on specificity of these natural compounds. Our previous studies showed the anti-diabetic prospects of these natural compounds present in Hippophae species [12].
Toxicity prediction deals with predicting the adverse effects of the synthesized or extracted chemicals on a living organism. Aqueous solubility model of DS predicts the solubility of compound in water at 25°C against a dataset of 775 drugs[29],[30]. The solubility levels of the four compounds were in the range 1–4, indicating good solubility. BBB penetration (Table 4) of all the compounds is 4, representing low penetration. With the exception of isorhamnetin-7-rhamnoside, the other three ligands are not hepatotoxic. All the ligands exhibited in silco cytochrome p4502D6 inhibition. In principle, cytochrome p4502D6 is involved in the metabolism of a variety of substrates in the liver and its inhibition by a drug constitutes major cases of drug-drug interactions[31],[32].
The partial plasma binding ability of the compounds influences the efficacy of the drug, since the bound fraction is temporarily shielded from metabolism and only the unbound fraction exhibits pharmacological effects[33].
Toxicity of the compounds may also be due to poor intestinal absorption levels. Intestinal absorption is defined as a percentage absorbed rather than as the ratio of concentrations (cf. blood brain penetration)[34],[35]. Since most of the anticancerous drugs are toxic in nature, the test compounds were subjected to Ames mutagenicity test of DS. The Ames test is a widely accepted biological assay to access the mutagenic potential of chemical compounds[36]. Zeaxanthin differs from lutein only in one double bond present on one of the hydroxyl groups. Hence, it has a poor absorption level which could be modified to obtain a compound with better absorption values.
In conclusion, currently most anti-cancer drugs being developed are derived from plant sources. This eliminates the risk of side effects, to an extent, when compared to synthetic drugs. H. salicifolia and H. rhamnoides are two plants that offer immense potential in the development of a new cancer drug that targets the Ras pathway. Many compounds present in these actinorhizal plants are currently being used as drugs in treatment of various diseases. Attempts to develop drugs that target oncogenic Ras proteins have been unsuccessful[27]. Hence, tumors having these mutations remain the most difficult to treat. This study showed that these two plant species contain a few compounds that are capable of binding to and inhibiting the Ras protein and thereby preventing cell proliferation in an uncontrolled manner. As determined by Discovery Studio 3.5 and Autodock 4.0, zeaxanthin, translutein, quercetin 3-glucoside 7-rhamnoside and isorhamnetin 7-rhamnoside show promising association to the binding site of the Ras protein. From the ADMET studies, we found that quercetin 3-glucoside 7-rhamnoside is a better drug candidate and shows the least adverse effects. Hence, quercetin 3-glucoside 7-rhamnoside could prove to be a probable anti-cancer drug. However, this molecular docking study is only one way of predicting the activity of the molecules involved. Therefore, in vitro and in vivo studies need to be performed on animal models to confirm the anti-cancerous activity of these compounds. The role of some important amino acids involved in the appropriate binding of inhibitors with the active site of H-Ras can be helpful for designing better drugs to combat cancer.
Acknowledgments
The authors wish to thank the Deanship of Life Science Research, DBT-Bioinformatics Infrastructure facility, Govt. of India and BT-Finishing School, Govt. of Karnataka at Maharani Lakshmi Ammanni College for Women for providing infrastructure and the licensed software to carry out the project.
References
- 1.Dikmen M, Ozturk N, Ozturk Y. The Antioxidant Potency of Punica granatum L. Fruit Peel Reduces Cell Proliferation and Induces Apoptosis on Breast Cancer. J Med Food. 2011;14:1638–46. doi: 10.1089/jmf.2011.0062. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Medarde A, Santos E. Ras in Cancer and Developmental Diseases. Genes Cancer. 2011;2:344–58. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 4.Rousi A. The genus Hippophae L: A taxonomic study. Annales Botanici Fennici. 1971;8:177–227. [Google Scholar]
- 5.Singh V. Sea-buckthorn a wonder plant of dry temperate Himalayas. Ind J Hort. 1998;43:6–8. [Google Scholar]
- 6.Beveridge T, Li TS, Oomah BD, Smith A. Sea-buckthorn products: manufacture and composition. J Agri Food Chem. 1999;47:3480–8. doi: 10.1021/jf981331m. [DOI] [PubMed] [Google Scholar]
- 7.Basistha BC, Sharma NP, Lepcha L, Arrawatia A, Sen A. Ecology of Hippophae salicifolia D. Don of temperate and subalpine forests of North Sikkim Himalayas – a case study. Symbiosis. 2010;50:87–95. [Google Scholar]
- 8.Goyal AK, Basistha BC, Sen A, Middha SK. Antioxidant profiling of Hippophae salicifolia growing in sacred forests of Sikkim, India. Funct Plant Biol. 2011;38:697–701. doi: 10.1071/FP11016. [DOI] [PubMed] [Google Scholar]
- 9.Xing J, Yang B, Dong Y, Wang B, Wang J, Kallio HP. Effects of sea buckthorn seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia. 2002;73:644–50. doi: 10.1016/s0367-326x(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Kallio HP. Fatty acid composition of lipids in sea-buckthorn (Hippophae rhamnoides L.) berries of different origins. J Agri Food Chem. 2001;49:1939–47. doi: 10.1021/jf001059s. [DOI] [PubMed] [Google Scholar]
- 11.Zeb A. Anti Carcinogenic Potential of Lipids from Hippophae – Evidence from the Recent Literature. Asian Pac J Cancer Prev. 2006;7:32–5. [PubMed] [Google Scholar]
- 12.Middha SK, Goyal AK, Faizan S, Sanghmithra N, Basistha BC, Usha T. In Silico based Combinatorial Pharmacophore Modeling and Docking Studies of GSK-3β and GK Inhibitors of Hippophae. J Bio Sci. 2013;38:805–14. doi: 10.1007/s12038-013-9367-y. [DOI] [PubMed] [Google Scholar]
- 13.Middha SK, Usha T, Ravikiran T. Influence of Punica granatum L. on region specific responses in rat brain during Alloxan-Induced diabetes. Asian Pac J Trop Biomed. 2012;2:S905–9. [Google Scholar]
- 14.Cox PA, Balick M. The ethnobotanical approach to drug discovery. Sci Am. 1994;270:82–7. [PubMed] [Google Scholar]
- 15. “Marvin was used for drawing, displaying and characterizing chemical structures, substructures and reactions, Marvin 5.12.3, ChemAxon (http://www.chemaxon.com),” 2010. [Google Scholar]
- 16.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 17.Usha T, Tripathi P, Pande V, Middha SK. Molecular docking and quantum mechanical studies on Pelargonidin-3-Glucoside as renoprotective ACE inhibitor. ISRN Comput Biol. 2013 Article ID 428378. [Google Scholar]
- 18.Selick HE, Beresford AP, Tarbit MH. The emerging importance of predictive ADME simulation in drug discovery. Drug Discover Today. 2002;7:109–16. doi: 10.1016/s1359-6446(01)02100-6. [DOI] [PubMed] [Google Scholar]
- 19.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suri C, Naik PK. Elucidating the precise interaction of reduced and oxidized states of Neuroglobin with Ubc12 and Cop9 using molecular mechanics studies. Int J Fund Appl Sci. 2012;1:4, 74–7. [Google Scholar]
- 21.Kalim M, Khan A, Baig MH, Arif JM, Lohani M, Jamal F. Efficacy of natural inhibitors against pkc: an in silico approach to combat cancer. Recent Res Sci Technol. 2011;3:90–3. [Google Scholar]
- 22.Rao SN, Head MS, Kulkarni A, LaLonde JM. Validation studies of the site-directed docking program LibDock. J Chem Inf Model. 2007;47:2159–71. doi: 10.1021/ci6004299. [DOI] [PubMed] [Google Scholar]
- 23.Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, et al. CHARMM: The Bio-molecular simulation Program. Computational Chem. 2009;30:1545–615. doi: 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J Compt Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madeswaran A, Umamaheswari M, Asokkumar K, Sivashanmugam T, Subhadradevi V, Jagannath P. Computational drug discovery of potential phosphodiesterase inhibitors using in silico studies. Asian Pac J Trop Dis. 2012;2:S822–6. [Google Scholar]
- 26.Sharma M, Naik PK. To study the mode and mechanism of interaction of Angiopoietin II with receptor tyrosine kinase Tie-2 using molecular mechanics and molecular dynamics approach. Int J Fundamental Appl Sci. 2013;2:8–11. [Google Scholar]
- 27.Neidle S, Thurston DE. Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer. 2005;5:285–96. doi: 10.1038/nrc1587. [DOI] [PubMed] [Google Scholar]
- 28.Singh SP, Konwar BP. Molecular docking studies of quercetin and its analogues against human inducible nitric oxide synthase. SpringerPlus. 2012;1:69. doi: 10.1186/2193-1801-1-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gysin S, Salt M, Young A, McCormick F. Therapeutic Strategies for Targeting Ras Proteins. Genes Cancer. 2011;2:359–72. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laskowski RA, Hutchinson EG, Michie AD, Wallace AC, Jones ML, Thornton J. PDBsum: a Web-based database of summaries and analyses of all PDB structures. Trends Biochem Sci. 1997;22:488–90. doi: 10.1016/s0968-0004(97)01140-7. [DOI] [PubMed] [Google Scholar]
- 31.Cheng A, Merz K., Jr Prediction of aqueous solubility of a diverse set of compounds using quantitative structure-property relationships. J Med Chem. 2003;46:3572–80. doi: 10.1021/jm020266b. [DOI] [PubMed] [Google Scholar]
- 32.Badyal DK, Dadhich AP. Cytochrome P450 and Drug Interactions. Ind J Pharmacol. 2001;33:248–59. [Google Scholar]
- 33.Susnow RG, Dixon SL. Use of robust classification techniques for the prediction of human cytochrome P450 2D6 inhibition. J Chem Inform Compr Sci. 2003;43:1308–15. doi: 10.1021/ci030283p. [DOI] [PubMed] [Google Scholar]
- 34.Dixon SL, Merz KM., jr One-Dimensional Molecular Representations and Similarity Calculations: Methodology and Validation. J Med Chem. 2001;44:3795–809. doi: 10.1021/jm010137f. [DOI] [PubMed] [Google Scholar]
- 35.Egan WJ, Merz KM, Baldwin JJ. Prediction of Drug Absorption Using Multivariate Statistics. J Med Chem. 2000;43:3867–77. doi: 10.1021/jm000292e. [DOI] [PubMed] [Google Scholar]
- 36.Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29–60. doi: 10.1016/s0027-5107(00)00064-6. [DOI] [PubMed] [Google Scholar]