Abstract
Introduction
Endometriosis can be difficult to diagnose clinically and models that use symptoms to predict whether the disease is present or not are based on limited patient populations. Endometriosis also influences health-related quality of life, but little is known about its impact across the world. We therefore initiated two integrated multicentre studies to collect prospective, standardised, epidemiological data, to 1) examine the global impact of endometriosis and relative effect of risk-factors, and 2) develop a symptom-based diagnostic tool.
Methods
The Global Study of Women’s Health (GSWH) and the Women’s Health Symptom Survey (WHSS) prospectively recruit 18-45 year old women having a laparoscopy across 23 and 19 centres, respectively, worldwide. Women with a previous surgical diagnosis of endometriosis are excluded. Multi-lingual patient questionnaires and a surgical questionnaire, incorporating validated instruments, are used to collect the data. The GSWH aims to recruit >2,000 women by December 2009; the WHSS to recruit 1,000 women in each of the two model-generating and validation stages.
Results
A six-week pilot study in Oxford, UK, established the feasibility of the study protocols. Of 32 eligible women, 27 participated (response rate - 84.4%); 26% completed the questionnaire online. Endometriosis was found in 47.4%. Extrapolating the recruitment rates from the pilot study, the target sample sizes for the GWSH and WHSS were deemed feasible.
Conclusions
Using standardised data collection, the GSWH and WHSS will provide insight into the global impact of endometriosis and develop a validated, symptom-based, diagnostic tool. They have the potential to provide the basis for future, longitudinal, follow-up studies and a collaborative Endometriosis Biobank implementing standardised collection of DNA and tissue samples.
Keywords: Endometriosis, Global, Quality of life, Pelvic pain, Infertility, Symptom, Diagnostic tool, Epidemiology
INTRODUCTION
Endometriosis is an oestrogen-dependent, chronic inflammatory condition characterised by benign proliferation of endometrial-like tissue in the peritoneal cavity resulting in inflammation and scarring, with associated symptoms of pelvic pain and infertility.1 Although exact prevalence rates in the general population are unknown - due to the need for surgery to establish a diagnosis - estimates indicate that endometriosis affects up to 10% of premenopausal women, rising to 30-50% of women presenting with pelvic pain and/or infertility.2,3
Women with endometriosis experience greater health-related quality of life (HRQOL) impairment than the general population. In a recent review, three of six included studies used generic instruments for descriptive assessment of health status (principally the General Health Questionnaire4 and the Modified Social Adjustment Scale5) to allow comparison of HRQOL in women experiencing chronic pelvic pain (CPP) due to endometriosis versus CPP due to other causes.6 Women with endometriosis suffered greater quality of life impairment in the domains of pain, distress, anxiety and social adjustment compared to women with CPP without a diagnosis.7-9 Other included studies using similar instruments highlighted the impact of endometriosis on work, intercourse and social relationships.10 However, the studies principally involved UK and USA populations and the potential for significant bias (e.g. due to failure to account for the impact of comorbidities) may have impaired the reliability and generalisability of the findings. Other important questions regarding the impact of endometriosis on women’s lives remain unanswered: for instance, to what extent demographic, life-style, and health-care seeking factors influence HRQOL, and whether patterns are generalisable across different countries.
The illness experience and care-seeking trajectory of women who suffer symptoms of endometriosis is characterised by long delays in obtaining a surgical diagnosis, typically averaging 8-12 years.11,12 In one UK study, a third of women had consulted their family physician six or more times before being diagnosed.13 Thus, there has been an increasing interest in developing either a non-invasive diagnostic test or a screening tool, for instance by utilising a combination of symptoms and risk factors to predict the likelihood of disease being present.14-17 However, the global utility of clinical prediction is hampered by inconsistencies in the definition of endometriosis and associated symptoms across studies and populations. To ensure validity and generalisability across clinical populations, diagnostic models should be based on large, multi-centre studies that include incident (newly diagnosed) endometriosis cases and appropriately sampled controls, representative of the underlying population.
There is strong evidence that endometriosis is a complex disease, caused by the interplay between environmental and genetic factors18,19, but the mechanisms of disease onset and progression remain largely unclear. It is generally accepted that the initial step in pathogenesis involves retrograde menstruation, the reflux of menstrual debris containing viable endometrial cells into the pelvic cavity.20 This hypothesis is supported by the observation that endometriosis occurs spontaneously only in species that menstruate 21, and that women with increased exposure to menstruation (longer, heavier flow; shorter cycle length, earlier age at menarche) are at increased risk of endometriosis.22-24 However, retrograde menstruation has been observed to varying degrees in up to 90% of women 25 yet only a proportion of these go on to develop endometriosis. Thus, the underlying mechanisms responsible for the survival, proliferation and neo-vascularisation of endometriotic deposits have been an important focus of aetiological research. Indeed, increasing understanding of the molecular basis of endometriosis is leading some researchers to consider it to be a chronic systemic disorder.26 Other than menstrual characteristics, few other risk-factors (low body mass index27 and birthweight28 being possible exceptions) have been robustly associated with endometriosis in well-designed epidemiological studies. These should ideally enrol surgically confirmed cases, provide clear criteria for control selection and adjust for potential confounding variables, but only a few studies to date have met these requirements.29,30
The lack of robust information on the global impact of endometriosis, and the difficulty in interpreting the relative impact of risk-factors from different studies, led us to initiate a large-scale, multicentre research effort to gather prospective, standardised, epidemiological data on endometriosis. This initiative is in line with the Priorities for Endometriosis Research, which outlined the need for ‘Collaborations to be established between research groups with sufficient participant numbers and appropriate standardisation of sample and information collection’.3 To this end, we designed two integrated epidemiological studies of endometriosis - the Global Study of Women’s Health (GSWH) and the Women’s Health Symptom Survey (WHSS) - that enable the collaborative investigation of key research questions at an unprecedented global scale.
Following a standardised protocol, centres across a number of countries in Africa, Asia, Europe, and North and South America, are collecting comprehensive and robust epidemiological information from prospectively recruited women aged 18-45, with and without symptoms, who are undergoing a laparoscopy. The studies will provide novel insights into the effects of endometriosis and its associated symptoms on women’s lives across different countries, as well as explore (differences in) the effects of various putative risk-factors, and the predictive value of clinical symptoms in establishing a diagnosis. It is hoped that the studies will contribute to a clearer understanding of the aetiology, inform preventive efforts and draw attention to the public health importance and quality of life impact of endometriosis in women with the condition. The resulting data registries also have the potential to serve as a framework for future studies, such as the longitudinal assessment of the effectiveness of different treatment options for endometriosis and the investigation of biological influences and diagnostic markers.
This paper outlines the aims and methods of the studies, which use tailored web-based patient and surgical outcome questionnaires for data collection, along with the results from the pilot study that assessed feasibility.
MATERIALS AND METHODS
Aims
The GSWH aims to:
-
(a)
assess the relative impact of endometriosis and associated symptoms on HRQOL among >2,000 women aged 18-45 across different countries;
-
(b)
determine the effect of previously investigated risk-factors for endometriosis, using large-scale, prospective, recruitment of >1,000 incident cases and matched controls.
The WHSS aims to:
-
(a)
develop a symptom-based assessment tool to predict the presence of endometriosis at laparoscopy in 1,000 women, aged 18-45, who are being investigated for endometriosis-associated pain and/or infertility
-
(b)
identify the sensitivity and specificity of the assessment tool in predicting the likelihood of a diagnosis of endometriosis in a separate validation set of 1,000 women.
Study design
Data collection for GSWH and WHSS involves the prospective, clinic-based recruitment of all women aged 18-45, who are undergoing a laparoscopy for symptoms suggestive of endometriosis, or for tubal sterilization. To reduce the potential bias from differential recall of information, women complete the relevant questionnaires prior to undergoing surgery (and therefore identification of case/control status); those who have had a previous surgical diagnosis of endometriosis are excluded.
GSWH
The GSWH is a study of prospectively recruited women attending for a diagnostic laparoscopy or for tubal sterilisation.
Exclusion criteria:
-
-
Previous surgical diagnosis of endometriosis
-
-
Age <18 or >45
-
-
Post-menopausal status
The recruitment strategy will enable both descriptive cross-sectional and nested case-control analyses to be conducted. The cross-sectional evaluation will compare the cumulative diagnostic incidence of endometriosis and prevalence of symptoms (e.g. pelvic pain and infertility) with general population estimates and across different centres and countries. In the case-control analyses, incident cases will comprise women diagnosed with endometriosis at laparoscopy. Controls will be women in whom no endometriosis is found at laparoscopy, matched on recruitment centre and other potential confounders (such as age). The case- control analyses will compare HRQOL across diagnostic categories (i.e. cases vs. symptomatic controls) and symptom groups (e.g. cases with CPP vs. cases without CPP), and examine the effect of risk-factors for endometriosis. The categories of putative risk factors to be assessed include sociodemographic, reproductive, contraceptive, lifestyle and anthropometric characteristics.
WHSS
The WHSS is a two-stage, clinic-based study of prospectively recruited women attending for a diagnostic laparoscopy because of at least one of the following symptoms: dysmenorrhoea, dyspareunia, non-menstrual pelvic pain, dyschezia, infertility.
Exclusion criteria:
-
-
Previous surgical diagnosis of endometriosis
-
-
Age <18 or >45
-
-
Post-menopausal status
-
-
Amenorrhoea or current pregnancy
-
-
Currently taking hormonal medication, including combined oral contraception.
During Stage 1, 1,000 participants will complete, prior to their scheduled surgery, a comprehensive questionnaire, which includes questions from traditional medical history taking and previously validated symptom surveys. Using standard methods of predictive regression modelling and principal component analysis31, models will be built to identify a set of symptom-based questions with maximum predictive value of an endometriosis diagnosis. In Stage 2, the sensitivity and specificity of the symptom-based assessment tool will be tested in a second cohort of 1,000 prospectively recruited women.
Recruitment
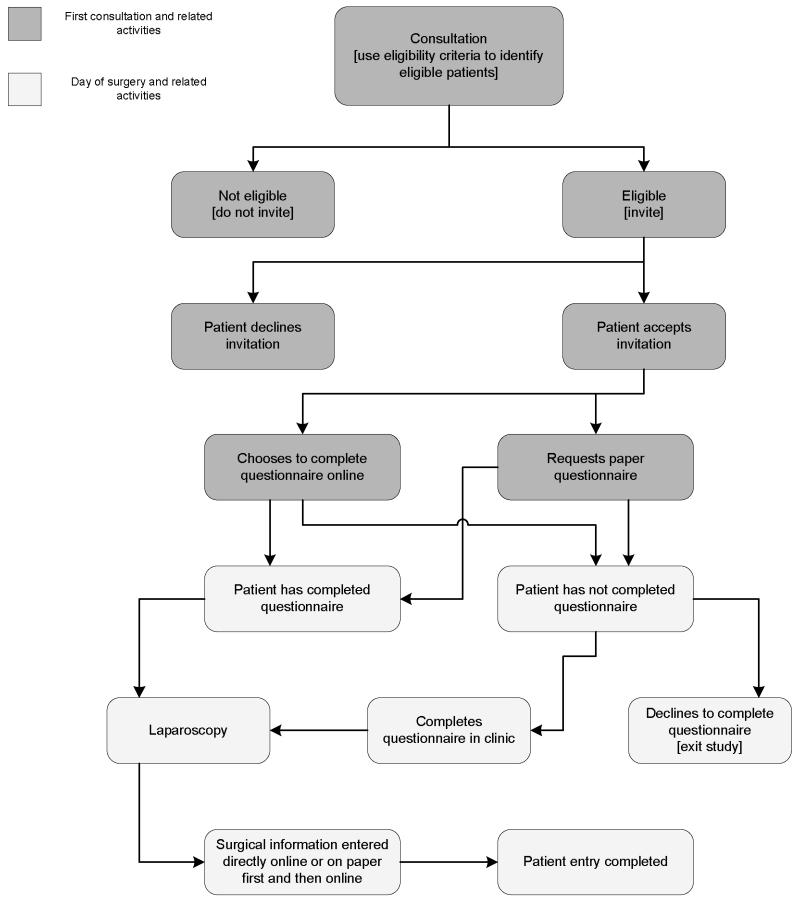
The clinical centres that are taking part in GSWH and WHSS as of 1 February 2009 (19 and 23 centres, respectively) are shown in Table 1. The flow chart summarising the protocol for recruitment is shown in Figure 1. At each centre, women meeting the inclusion criteria are invited to participate in the study. Those agreeing to participate are asked to complete either an online or paper-copy questionnaire seeking retrospective information about symptoms, use of health care resources, quality of life and medical, family and life-style history. Questionnaires are completed before surgery; women who do not complete the questionnaire before laparoscopy are not asked to do so post-operatively. Following surgery, the surgeon completes a questionnaire detailing operative findings, either online or on paper. At each centre, a local research assistant liaises with patients and surgeons, organises recruitment, and - where paper copies of patient or surgeon questionnaires were completed - enters the information into the online system. The coordinating centre at the University of Oxford monitors recruitment levels electronically and liaises with the centres in case of problems.
Table 1. Centres participating in the GSWH and WHSS studies.
| Country: Centre | Contributing to: |
|
|---|---|---|
| GSWH | WHSS | |
|
| ||
| Argentina: CGR, Buenos Aires | ✓ | ✓ |
| Belgium: University of Leuven | ✓ | ✓ |
| Brazil: University of Campinas | ✓ | ✓ |
| Brazil: University of São Paulo | ✓ | ✓ |
| China: Guangdong Provincial Hospital of TCM | ✓ | ✓ |
| China: Shanghai Obstetrics and Gynaecology Hospital | ✓ | ✓ |
| Egypt: Assiut University Hospital | ✓ | |
| France: CHU Montpellier-Nîmes | ✓ | ✓ |
| Germany: Endometriosis Research Center Berlin | ✓ | ✓ |
| Greece: Aristotle University of Thessaloniki | ✓ | ✓ |
| Guatemala: CRC, Guatemala City | ✓ | |
| Ireland: National Maternity Hospital, Dublin | ✓ | ✓ |
| Italy: Universita’ Cattolica del Sacro Cuore, Rome | ✓ | ✓ |
| Italy: University of Siena | ✓ | ✓ |
| Nigeria: University of Ibadan | ✓ | |
| South Africa: University of Witwatersrand, Johannesburg | ✓ | ✓ |
| Spain: University of Barcelona | ✓ | ✓ |
| UK: Women’s Centre, University of Oxford | ✓ | ✓ |
| USA: Brigham & Women’s Hospital, Boston | ✓ | ✓ |
| USA: National Institutes of Health, Washington | ✓ | ✓ |
| USA: University of California SF, San Francisco | ✓ | ✓ |
| USA: Fertility Physicians of Northern California, Palo Alto | ✓ | ✓ |
| Thailand: Khon Kaen University | ✓ | |
Figure 1. Recruitment flowchart.
Questionnaire design and data collection
To collect comprehensive exposure information, we designed a sixty-seven item quantitative questionnaire for the GSWH. The questionnaire (an unlinked copy of which is available at http://www.well.ox.ac.uk/~krinaz/GSWH) incorporates standardised questions and instruments previously validated in either women with pelvic pain or other clinical symptom groups. These instruments included:
The Short Form 36 version 2 (SF-36 v2) to assess HRQOL32
The Work Productivity and Activity Impairment (WPAI) questionnaire to assess the impact of symptoms on work productivity and general non-work-related activity33
The IBS Rome III questionnaire to identify women with pelvic pain symptoms due to Irritable Bowel Syndrome34
Standardised pelvic pain symptom assessment used in earlier studies in Oxford35
Questionnaire items assessing educational background and ethnicity included comprehensive categories relevant to an international cohort of patients. The questionnaire was translated by native speakers into Chinese, Dutch, French, German, Italian, Portuguese and Spanish, and translations were verified independently by clinical collaborators in the respective centres. For the SF-36 version 2, validated language versions were used (http://www.sf-36.org).
For the WHSS, we designed a 25-item questionnaire, incorporating standardised questions previously validated in either women with pelvic pain or other clinical symptom groups (http://www.well.ox.ac.uk/~krinaz/WHSS). These instruments included:
The IBS Rome III questionnaire to identify women with pelvic pain symptoms due to Irritable Bowel Syndrome
Standardised pelvic pain symptom assessment used in earlier studies in Oxford
The questionnaire also incorporates items to women’s past medical, obstetric and family histories.
In addition to the patient questionnaires, a standardised surgical questionnaire was designed incorporating the American Society for Reproductive Medicine (ASRM) classification of endometriosis36 supplemented by questions relating to bladder, rectovaginal, and bowel involvement, as well as additional pathological findings that could explain pelvic pain or infertility (http://www.well.ox.ac.uk/~krinaz/surgquest). Both patient and surgical questionnaires were implemented as online instruments.
To ensure secure online data-collection, we employed a three-tier internet-based network system comprising (a) remote web browsers running on client computers; (b) a Web Enterprise application server (Microsoft.net), and (c) a secure database server (SQL server 2000) based at the Oxford University Computing Service. Key elements of online data collection are data security and confidentiality (information disclosed only to authorised users), integrity (information entered only by authorised users) and availability (information accessed only by authorised users).37 These were given careful consideration in the design of the online questionnaire and database. Data security and confidentiality are ensured through encrypted transmissions and firewall protection. Data integrity is ensured by respondents registering a username and password to access the questionnaire, allowing them to return to it as often as they wish until completion. Data availability is regulated by password protected access of centre-specific patient information (user ID, name, date of birth and status of questionnaires) only to local research assistants, after informed consent has been obtained. The study administrator at Oxford has access to encrypted questionnaire information once completed, but no access to named patient information. Informed consent is provided electronically. All collaborating centres obtained local Ethics Committee approval.
Power and sample size calculations
The calculation of power and required sample sizes to detect risk-factors for disease involve assumptions on (a) the likely prevalence rate of exposure variables of interest in controls; (b) the effect sizes to be detected; (c) the significance level with which the effect size is to be detected, and (d) minimum and maximum sample sizes likely to be achieved in the study
Table 2 shows sample sizes required to detect associations between endometriosis and several putative risk, or predictive, factors with a power of 80% and at a significance level of 5%. For the WHSS, the Stage 1 sample size of 1,000 women will enable the detection of a symptom such as migraine as a predictive factor for endometriosis. For the GSWH, the target sample size >2,000 will enable the detection of a risk-factor with a prevalence of ~0.2 among controls, with relative risk down to 1.3 (e.g. early age at menarche).
Table 2. Indicative required sample sizes for the GSWH and WHSS based on different relative risk estimates and prevalence in controls.
| Risk/Predictive factor | Relative risk of endometriosis * | Prevalence in controls | Required sample size per group | Total required sample size |
|---|---|---|---|---|
| Smoking | 0.5 (37) | 0.24 | 223 | 446 |
| Long duration of menstrual flow | 2.4 (2) | 0.06 | 257 | 514 |
| Short menstrual cycle length | 2.1(2) | 0.06 | 376 | 752 |
| History of oral contraception use | 1.6 (38) | 0.75 | 434 | 868 |
| Migraine | 1.6 (39) | 0.18 | 459 | 918 |
| Early age at menarche | 1.3 (40) | 0.21 | 1280 | 2560 |
Literature references on which effect size estimates were based are provided in brackets.
Pilot study
The GSWH and WHSS studies were pre-piloted from April to June 2008 in volunteer women at the Nuffield Department of Obstetrics and Gynaecology (NDOG)/Women’s Centre, John Radcliffe Hospital, Oxford, UK, as well as in healthy volunteers. Following the pre-pilots, minor revisions were made to the questionnaire to improve comprehensibility and ensure face validity. From 30 June to 8 August 2008, a full pilot study was conducted at the Women’s Centre, Oxford, to assess the feasibility of the studies.
Over the period of the pilot study, 32 consecutive eligible women scheduled for laparoscopic surgery were invited to participate. All 32 were undergoing surgery for gynaecological symptoms (none for tubal sterilisation). Of these, 27 took part in the study, yielding a response rate of 84.4% and recruitment rate of ~5 women per week. Of the five women who did not take part, one felt unable to participate as she neither spoke English nor any of the languages in which the questionnaire was available. The other four women gave no specific reasons for not participating. Of the 27 women who completed questionnaires, seven (26%) completed the questionnaire online.
Table 3 shows the breakdown of the 27 participating women in terms of demographic characteristics. Most women were 30-34 years old (51.9%), were university-educated (55.6%), married (59.2%) and of white ethnicity (81.5%). The commonest indications for laparoscopy were to investigate pelvic pain (29.6%) or infertility (59.3%), with 30% reporting both. At the time of pilot data analysis, 19 of the 27 women had undergone their surgery. Although based on an extremely small sample, the prevalence of endometriosis found at surgery was 47.4%.
Table 3. Demographic characteristics of 27 participants in the Oxford GSWH/WHSS pilot study.
| Variable | n | % |
|---|---|---|
| Age | ||
| 25-29 | 2 | 7.4 |
| 30-34 | 14 | 51.9 |
| 35-39 | 7 | 25.9 |
| 40+ | 4 | 14.8 |
| Ethnicity | ||
| Asian/oriental | 1 | 3.7 |
| Black African/American/Caribbean | 2 | 7.4 |
| White | 22 | 81.5 |
| Mixed race | 1 | 3.7 |
| Other | 1 | 3.7 |
| Education | ||
| Primary | 1 | 3.7 |
| Lower/Upper secondary school | 2 | 7.4 |
| Post-secondary school training/College | 9 | 33.3 |
| University/Postgraduate | 15 | 55.6 |
| Marital status | ||
| Single | 4 | 14.8 |
| Married/Living with partner | 16 | 59.2 |
| Divorced/separated | 7 | 25.9 |
The pilot study established the feasibility of the study protocol and - assuming recruitment rates of ≥3 women per week in other centres - of the sample sizes to be achieved by the end of the studies in December 2009. Following the pilot, only minor changes were implemented to the patient questionnaire.
DISCUSSION
The two prospective studies of endometriosis described in this paper, the GSWH and WHSS, aim to collect standardised, and comprehensive epidemiological information on endometriosis and its associated symptoms on an unprecedented global scale. The design of the studies attempts to address methodological limitations of previous epidemiological studies, particularly in relation to the application of consistent case definitions, appropriate selection of controls sampled from the same source population as cases, and collection of comprehensive information on risk-factor exposure to allow control of confounding factors. The design allows for cross sectional, case-control and prospective analyses that will provide novel insights into the effects of endometriosis on women’s lives and test the association of the disease with a number of sociodemographic, reproductive, contraceptive, lifestyle and personal characteristics, as well as the predictive value of clinical symptoms.
In the GSWH and WHSS, incident (i.e. newly diagnosed) rather than prevalent endometriosis cases are recruited. Well-designed case-control studies of non-genetic risk-factors for endometriosis should enrol incident cases, allowing for the collection of unbiased exposure information pre-dating disease onset and an accurate approximation of relative risk by the odds ratio.30 If prevalent rather than incident cases were studied, any association found between the outcome (endometriosis) and the exposure would strictly not refer to the exposure itself but to its interaction with disease duration. However, in the case of endometriosis, the interval between risk-factor exposure and onset of disease is unknown as the interval between symptom onset and surgical diagnosis varies widely. Consequently, recruiting incident cases may not guarantee exclusion of the influence of disease duration on both exposure and the reporting of exposure, especially in the context of long-standing symptoms. In our prospective studies, adjustment for symptom duration will be undertaken to address this issue. Furthermore, any case definition based on a surgical diagnosis is likely to introduce selection bias, as women who more readily seek medical advice (often of higher socioeconomic status), those who are more readily referred, and those with more aggressive or severe disease may be more likely to be identified as cases.29 One of the strengths of the GSWH and WHSS is that, because of their multi-centre nature, they are recruiting women across diverse health systems, which should enable comparisons across these settings.
Another important element of a well-designed epidemiological study is the need to select controls representative of the population from which cases arose, ensuring that any difference in the outcome between cases and controls is not due to differential levels of the exposure of interest in the control group due to selection bias.30 For endometriosis, selecting appropriate controls is difficult, due to the often unknown trajectory through which cases have come to be diagnosed. Previous studies of endometriosis have mainly adopted the following approaches: a) selection of symptomatic members of the study cohort (with CPP and/or infertility) who were free of endometriosis on laparoscopy and b) selection of asymptomatic, fertile women who were undergoing laparoscopy for tubal sterilisation.18 The GSWH and WHSS allow for the analysis of multiple control groups, thereby diluting the potential bias of including a specific control group that is not representative of the source population.30 Moreover, matching controls to cases on symptom profile will allow the investigation of hypotheses related to endometriosis specifically, rather than to its associated symptoms.
The use of web-based questionnaires assumes that participating patients have access to the internet and are relatively competent in using a personal computer. Indeed, substantial selection bias is likely to occur if participants would require access to a personal computer to participate in the studies. In the design of both the GSWH and WHSS, therefore, women are equally encouraged to complete a paper copy or an online version of the questionnaire, which should reduce the potential of this bias occurring.
In summary, the GSWH and WHSS are a response to a call by the international endometriosis research community for better designed, adequately powered multi-centre epidemiological studies conducted in collaboration across global research groups, using standardised protocols for information collection. Initial findings will be reported in early 2010. The study results are expected to provide major improvements in the insight into the impact of endometriosis in different countries worldwide, as well as the potential for symptom subsets to act as a diagnostic tool. If successful, the studies will be an ideal framework for longitudinal follow-up studies, and in particular the establishment of a collaborative Endometriosis Biobank implementing standardised collection of DNA and tissue samples. Such an initiative would allow much-needed integrated investigations of environmental and biological hypotheses and the discovery of biomarkers for endometriosis.
ACKNOWLEDGEMENTS
We thank all women participating in the GSWH and WHSS for their valuable contributions. We gratefully acknowledge our clinical collaborators and their assistants involved in recruitment of women: Hany Abdel-Aleem (Assiut University Hospital, Assiut, Egypt); Mauricio Abrão (University of São Paulo, São Paulo, Brazil); David Adamson (Fertility Physicians of Northern California, Palo Alto, California, USA); Francisco Carmona (University of Barcelona, Barcelona, Spain); Thomas D’Hooghe (University of Leuven, Leuven, Belgium); Carlo De Cicco (Università Cattolica del Sacro Cuore, Rome, Italy); Fiorenzo De Cicco (Università Cattolica del Sacro Cuore, Rome, Italy); Hervé Dechaud (CHU Montpellier-Nîmes, Nîmes, France); Andreas Ebert (Endometriosis Research Centre, Berlin, Germany); Bukola Fawole (University of Ibadan, Ibadan, Nigeria); Linda Giudice (University of California San Francisco, San Francisco, California, USA); Bhaskar Goolab (University of Witwatersrand, Johannesburg, South Africa); Bernard Hédon (CHU Montpellier-Nîmes, Nîmes, France); Mark Hornstein (Brigham & Women’s Hospital, Boston, Massachusetts, USA); Stephen Kennedy (University of Oxford, Oxford, UK); Xishi Liu (Shanghai Obstetrics and Gynaecology Hospital, Shanghai, China); Luis Lombardi (Clinical Research Centres Guatemala, Guatemala City, Gutemala); Xu Min (Guangdong Provincial Hospital of Traditional Chinese Medicine, Guangzhou, China); Stacey Missmer (Brigham & Women’s Hospital, Boston, Massachusetts, USA); Felice Petraglia (University of Siena, Siena, Italy); Carlos Petta (State University of Campinas, Campinas, Brazil); Kanok Seejorn (Khon Kaen University, Khon Kaen, Thailand); Pamela Stratton (National Institutes of Health, Washington, USA); Carlos Sueldo (Center for Gynecology and Reproduction, Buenos Aires, Argentina); Basil Tarlatzis (Aristotle University of Thessaloniki, Thessaloniki, Greece); Mary Wingfield (National Maternity Hospital, Dublin, Ireland); Menelaos Zafrakas (Aristotle University of Thessaloniki, Thessaloniki, Greece).
The GSWH and WHSS are funded by the World Endometriosis Research Foundation, through donations from Bayer Schering Pharma AG, and Abbott Endocrine Inc. Three of the European centres, Leuven, Oxford and Rome, are part-funded by the EU Public Health Programme through their involvement in the European Network on Endometriosis. SS is an NCCRCD funded Academic Clinical Fellow. SK is supported by by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. KTZ is supported by a Wellcome Trust Career Development Fellowship.
References
- 1.Kennedy S, Bergqvist A, Chapron C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 2.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 3.Rogers PA, D’Hooghe TM, Fazleabas A, et al. Priorities for Endometriosis Research: Recommendations From an International Consensus Workshop. Reprod Sci. 2009 Feb;5 doi: 10.1177/1933719108330568. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldberg DP, Blackwell B. Psychiatric illness in general practice. A detailed study using a new method of case identification. BMJ. 1970;1(5707):439–43. doi: 10.1136/bmj.2.5707.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper P, Osborn M, Gath D, Feggetter G. Evaluation of a modified self-report measure of social adjustment. Br J Psychiatry. 1982;141:68–75. doi: 10.1192/bjp.141.1.68. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Yeh YC, Outley J, Simon J, Botteman M, Spalding J. Health-related quality of life burden of women with endometriosis: a literature review. Curr Med Res Opin. 2006;22(9):1787–97. doi: 10.1185/030079906X121084. [DOI] [PubMed] [Google Scholar]
- 7.Low WY, Edelmann RJ, Sutton C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J Psychosom Res. 1993;37(2):111–6. doi: 10.1016/0022-3999(93)90077-s. [DOI] [PubMed] [Google Scholar]
- 8.Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–7. doi: 10.1016/0029-7844(95)00458-0. [DOI] [PubMed] [Google Scholar]
- 9.Peveler R, Edwards J, Daddow J, Thomas E. Psychosocial factors and chronic pelvic pain: a comparison of women with endometriosis and with unexplained pain. J Psychosom Res. 1996;40(3):305–15. doi: 10.1016/0022-3999(95)00521-8. [DOI] [PubMed] [Google Scholar]
- 10.Denny E. Women’s experience of endometriosis. J Adv Nurs. 2004;46(6):641–8. doi: 10.1111/j.1365-2648.2004.03055.x. [DOI] [PubMed] [Google Scholar]
- 11.Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod. 1996;11(4):878–80. doi: 10.1093/oxfordjournals.humrep.a019270. [DOI] [PubMed] [Google Scholar]
- 12.Ballard K, Lowton K, Wright J. What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296–301. doi: 10.1016/j.fertnstert.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Pugsley Z, Ballard K. Management of endometriosis in general practice: the pathway to diagnosis. Br J Gen Pract. 2007;57(539):470–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study. Br J Obstet Gynaecol. 2008;115(11):1382–91. doi: 10.1111/j.1471-0528.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths AN, Koutsouridou RN, Penketh RJ. Predicting the presence of rectovaginal endometriosis from the clinical history: a retrospective observational study. J Obstet Gynaecol. 2007;27(5):493–5. doi: 10.1080/01443610701405721. [DOI] [PubMed] [Google Scholar]
- 16.Calhaz-Jorge C, Mol BW, Nunes J, Costa AP. Clinical predictive factors for endometriosis in a Portuguese infertile population. Hum Reprod. 2004;19(9):2126–31. doi: 10.1093/humrep/deh374. [DOI] [PubMed] [Google Scholar]
- 17.Chapron C, Barakat H, Fritel X, Dubuisson JB, Bréart G, Fauconnier A. Presurgical diagnosis of posterior deep infiltrating endometriosis based on a standardized questionnaire. Hum Reprod. 2005;20(2):507–13. doi: 10.1093/humrep/deh627. [DOI] [PubMed] [Google Scholar]
- 18.Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22. doi: 10.1111/j.1749-6632.2002.tb02761.x. discussion 34. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery GW, Nyholt DR, Zhao ZZ, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update. 2008;14(5):447–57. doi: 10.1093/humupd/dmn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 21.D’Hooghe TM, Kyama CM, Chai D, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–61. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 22.Missmer SA, Hankinson SE, Spiegelman D, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004;104(5 Pt 1):965–74. doi: 10.1097/01.AOG.0000142714.54857.f8. [DOI] [PubMed] [Google Scholar]
- 23.Rock JA, Zacur HA, Dlugi AM, Jones HW, TeLinde RW. Pregnancy success following surgical correction of imperforate hymen and complete transverse vaginal septum. Obstet Gynecol. 1982;59(4):448–51. [PubMed] [Google Scholar]
- 24.D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update. 2002;8(1):84–8. doi: 10.1093/humupd/8.1.84. [DOI] [PubMed] [Google Scholar]
- 25.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151–4. [PubMed] [Google Scholar]
- 26.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 27.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–96. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 28.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82(6):1501–8. doi: 10.1016/j.fertnstert.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 29.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30(1):1–9. vii. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 30.Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17(6):1415–23. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]
- 31.Marshall G, Grover FL, Henderson WG, Hammermeister KE. Assessment of predictive models for binary outcomes: an empirical approach using operative death from cardiac surgery. Stat Med. 1994;13(15):1501–11. doi: 10.1002/sim.4780131502. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 33.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 34.Gwee KA. Irritable bowel syndrome and the Rome III criteria: for better or for worse? Eur J Gastroenterol Hepatol. 2007;19(6):437–9. doi: 10.1097/MEG.0b013e328013c0fa. [DOI] [PubMed] [Google Scholar]
- 35.Zondervan KT, Yudkin PL, Vessey MP. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract. 2001;51(468):541–7. al. [PMC free article] [PubMed] [Google Scholar]
- 36.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and sterility. 1997;67(5):817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 37.Edworthy SM. World wide web: opportunities, challenges, and threats. Lupus. 1999;8(8):596–605. doi: 10.1191/096120399680411434. [DOI] [PubMed] [Google Scholar]
- 38.Parazzini F, Ferraroni M, Bocciolone L, Tozzi L, Rubessa S, La Vecchia C. Contraceptive methods and risk of pelvic endometriosis. Contraception. 1994;49(1):47–55. doi: 10.1016/0010-7824(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 39.Nyholt DR, Gillespie NG, Merikangas KR, Treloar SA, Martin NG, Montgomery GW. Common genetic influences underlie comorbidity of migraine and endometriosis. Genet Epidemiol. 2009;33(2):105–13. doi: 10.1002/gepi.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer DW, Wilson E, Stillman RJ, et al. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA. 1986;255(14):1904–8. [PubMed] [Google Scholar]