Abstract
Cardiovascular morbidity imposes a high degree of disability and mortality, with limited therapeutic options available in end-stage disease. Integral to standard-of-care, cardiac rehabilitation aims on improving quality of life and prolonging survival. The recent advent of regenerative technologies paves the way for a transformative era in rehabilitation medicine whereby, beyond controlling risk factors and disease progression, the prospect of curative solutions is increasingly tangible. To date, the spectrum of clinical experience in cardiac regenerative medicine relies on stem cell-based therapies delivered to the diseased myocardium either acutely/subacutely, following a coronary event, or in the setting of chronic heart failure. Application of autologous/allogeneic stem cell platforms has established safety and feasibility, with encouraging signals of efficacy. Newer protocols aim to purify cell populations in an attempt to eliminate non-regenerative and enrich for regenerative cell types prior to use. Most advanced technologies have been developed to isolate resident cell populations directly from the heart, or alternatively condition cells from non-cardiac sources to attain a disease targeted lineage-specified phenotype for optimized outcome. As a multiplicity of cell-based technologies has undergone Phase I/II evaluation, pivotal trials are currently underway in larger patient populations. Translation of regenerative principles into clinical practice will increasingly involve rehabilitation providers across the continuum of patient care. Regenerative rehabilitation is thus an emerging multidisciplinary field, full of opportunities and ready to be explored.
“We cannot solve problems with the same thinking that we used when we created them.”
Albert Einstein
Introduction
The evolving regenerative toolkit is the newest addition to the medical armamentarium, poised to offer transformative solutions for tissue and organ repair. Regenerative technologies enable strategies targeted to restitute function in patients with disability, extending thereby the reach of traditional rehabilitation. Addressing unmet needs, implementation of regenerative medicine paradigms drives a major innovation in modern patient management.1 A case in point is the anticipated impact of regenerative medicine in the context of cardiovascular disease, the leading cause of global morbidity and mortality.2
In conjunction with rehabilitation programs, pharmacological therapies have shown significant clinical and mortality benefit with impact on disease progression and quality of life.3-5 Furthermore, individuals who have progressive heart disease attain benefit from electrical resynchronization, as afforded by bi-ventricular pacing, or augmented cardiac output with implantation of a left-ventricular assist device (LVAD).6-8 However, current standard-of-care falls short in reversing the course of disease. Since the only definitive treatment for end-stage heart disease is transplantation, regenerative biotherapies are of significant interest as a potential new way of addressing the management of patients with limited options.
Over the last decade, regenerative medicine applied to cardiovascular disease has increasingly gained significant interest. Careful design and execution of clinical trials have demonstrated a favorable safety profile and documented feasibility of delivering stem cell-based therapy.9 Although initial efficacy data has shown rather limited signs of clinical benefit, the significant need for curative therapies in cardiovascular medicine has continued to drive innovation and refinement of cell-based technologies.10-12 Initially envisioned as a stand-alone technology, most recently stem cell-based therapies have been assessed for their ability to add to the benefit attained with standard-of-care. In this regard, cardiovascular regeneration has focused on two main avenues, namely protection of heart muscle at the time of injury (e.g., acute myocardial infarction) and regeneration of damaged or scarred myocardium in the chronically injured/failing heart.13
Here, we provide an overview of clinical trials utilizing various iterations of stem cell platforms, including the most recent progress in optimizing stem cell-based technology. Furthermore, we will look at the role of cardiac rehabilitation, specifically exercise therapy, as a partner therapy to stem cell-based regeneration and highlight the evolution of regenerative medicine from a cell-centric platform to one with the potential to engage a community of multispecialty practice.
Bone marrow mononuclear cells: A first generation platform
The bone marrow is a readily accessible source whose use has been well established for the treatment of hematologic malignancies. Initial indication that bone marrow mononuclear cells could also be utilized for the treatment of heart disease was reported in pre-clinical models of ischemic cardiomyopathy14-16 Established protocols to harvest and process marrow-derived cells have expedited the evaluation of this “first generation” cardioregenerative platform. Accordingly, bone marrow mononuclear cells (BMMNC) were delivered to patients following myocardial infarction initially via intracoronary infusion (Figure 1).17-19 Within these studies, the largest trial showing efficacy was the European REPAIR-AMI, which showed in 101 cell-treated individuals (versus 103 placebo) an overall 5% improvement in left ventricular ejection fraction (EF).19 Long-term follow-up of this trial has also indicated a mortality benefit.20 However, when REPAIR-AMI was compared with other trials, in particular the ASTAMI, inter-trial variability was noted and primarily ascribed to differences in the number of cells delivered and the methodology implemented for cell purification.21,22 Recent US-based trials coordinated by the Cardiovascular Cell Therapy Network (CCTRN) attempted to overcome this issue by carrying out trials that evaluated timing of cell delivery in tandem with imposition of rigorous uniformity in cellular processing.23-25 In two trials, TIME and Late-TIME, the impact of “first generation” BMMNC was evaluated and found to have no evidence of functional benefit.23,24,26 However, an inherent pitfall with imposing a uniformity standard in the trials carried out is that a specific protocol had to be selected. To avoid the need for a central processing facility, the CCTRN coordinated trials employed a “GMP in-a-box” clinical grade manufacturing system. This approach provided significant inter-trial uniformity in the cell population assessed, but did not necessarily select for the cellular phenotype associated with repair.27,28 Accordingly, a pivotal trial has now been set into motion in Europe termed BAMI that will mimic the cell processing and delivery utilized in REPAIR-AMI to conclusively evaluate its therapeutic utility.
Figure 1. Differences in harvest, processing and delivery of First Generation, Purified and Next Generation progenitors.
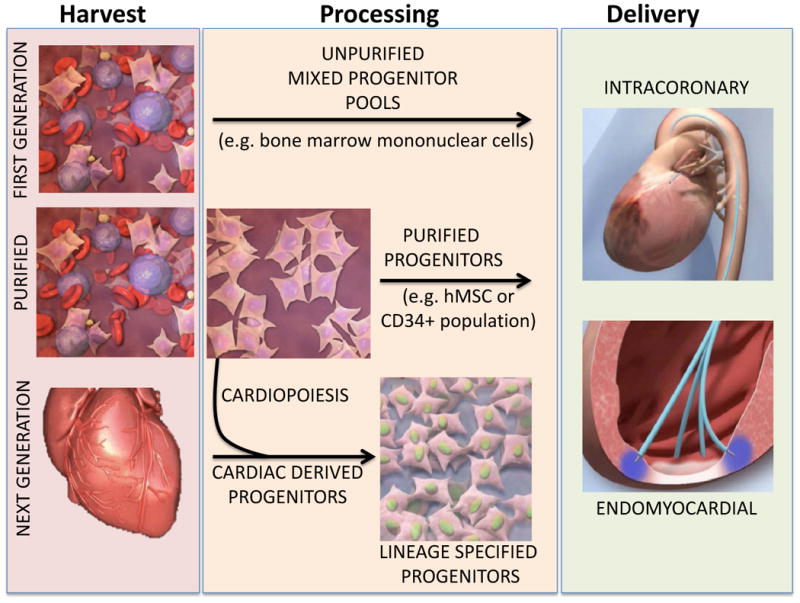
First Generation stem cells are harvested from source tissue and delivered without significant processing as mixed progenitor pools. In contrast, Purified biologics utilize a cell purification step to isolate specific cell phenotypes from source tissue, eliminating cells devoid of regenerative benefit. Next Generation stem cell therapy introduces the concept of lineage specification by either harvesting regenerative stem cells from the heart, or by guiding them with a process termed cardiopoiesis to yield a pure population of progenitors guided for maximal regenerative potency.
Purification of stem cells from the bone marrow
With advancement in cell biology and pre-clinical models, individual cell populations extractable from the bone marrow have been evaluated for myocardial regeneration, identifying specific populations associated with therapeutic benefit. As such, the advent of cell purification initiated a new paradigm in regenerative medicine, moving it away from the “first generation” mixed population therapies to a more selective approach (Figure 1). As the true basis for the benefit acquired with BMMNC continues to be uncertain, the isolation and use of purified cell populations affords the opportunity to evaluate therapeutic impact from a particular cell phenotype without the complexity of having to decipher the influence of competing cell populations.29 Accordingly, two populations have been identified as having therapeutic potential and are currently under extensive trial-based investigation. The CD34+ population from the bone marrow, also termed endothelial progenitor cells, has been shown both in vitro and in pre-clinical studies to have a pro-vasculogenic action on the myocardium.30 Accordingly, early application of this cell population was made in patients with critical limb ischemia and was shown to impart benefit on the risk of amputation.31,32 Furthermore, in patients with angina refractory to medical therapy, the CD34+ population has been implanted, providing initial evidence for diminished frequency of angina noted.33-35 This was more formally evaluated in the ACT34-CMI trial that demonstrated evidence for improvement in exercise performance.33 Human mesenchymal stem cells (hMSC) are the second cell population from the bone marrow recently evaluated for their therapeutic benefit. These cells came into consideration after initial studies suggested significant capacity for multipotent differentiation.36-38 The cells were formally assessed in the POSEIDON trial to ascertain whether the impact on chronic ischemic cardiomyopathy was altered when cells were delivered in autologous or allogeneic form. No difference in outcomes was noted, albeit evidence for benefit was limited.39 The TACT-HFT clinical study evaluated “first generation” BMMNC versus the purified hMSC phenotype to establish their safety and provide initial evidence for efficacy.40 With isolation of pure cell populations, the notion of identifying/generating a reparative cell population was born.
“Next generation” stem cell technology
There have been two main avenues of investigation pursuing this next generation approach. The first focuses on the harvest and application of resident cardiac stem cells (CSC) from the heart itself. This technique has been tested and reported in two separate trials using different methods for cell harvest and processing.41,42 The SCIPIO trial utilized ckit+ cells isolated from right atrial appendage samples obtained as part of coronary artery bypass grafting. Although the final report anticipated to be published soon, early reports of this Phase I trial indicated positive efficacy signals following autologous administration of 1,000,000 cells down the coronary vessel supplying the area of transmural scar.41,43 CSCs were also utilized in the randomized phase I CADUCEUS trial42 which isolated cells from endomyocardial biopsies obtained from the right ventricular septum. Cellular outgrowths underwent a cardiosphere processing step prior to being isolated as cardiosphere derived cells (CDC) and delivered, at different doses, into patients 65 days after myocardial infarction. Safety and feasibility in tandem with impact on LV scar dynamics were documented, but no impact on left ventricular systolic function was noted.42,44-46 Newer studies, such as ALCADIA, implement a hybrid approach, combining growth factors with CSCs.47 Furthermore, the CDC population will be additionally tested in Phase II studies to evaluate the efficacy of the autologous (RECONSTRUCT – NCT01496209) or allogeneic (ALLSTAR – NCT01458405) derivation paradigms in the treatment of ischemic heart disease.
The concept of lineage specification was introduced as an alternative to the use of myocardial tissue through cardiac biopsy, where cells are initially obtained from an abundant and more readily accessible tissue source (e.g., bone marrow).37,48,49. Following isolation, cells (e.g., hMSCs) are subject to a guiding environment during cell expansion that specifies a cardiovascular fate in a process termed cardiopoiesis (Figure 1).50-52 In contrast to hybrid approaches that combine cell therapy with growth factors at the time of therapy, this technology preemptively guides patient-derived cells with a growth factor cocktail to prime their regenerative potential. Clinical application of this technology was recently reported in the phase II C-CURE trial demonstrating safety and feasibility of using the cardiopoietic stem cell population in patients with chronic ischemic cardiomyopathy. Robust efficacy signals were documented with improvement in left ventricular ejection fraction, in tandem with global benefit, such as the 6-minute walk test.48 Pivotal phase III evaluation of this newest technology is currently underway in the CHART-1 trial.
Cardiac Rehabilitation and Stem Cells
Cardiac rehabilitation integrates a systematic approach to exercise training and management of cardiovascular risk factors through regular patient evaluation, monitoring and support of compliance and adherence to life styles changes. Patients who participated in cardiac rehabilitation programs have reduced all-cause and cardiac mortality, as well as a mitigated recurrence of acute cardiac events and blunted need for invasive procedures.53-55 As stem cell-based platforms emerge as an option in the treatment of cardiac disease, recent interest aims at establishing the putative impact of cardiac rehabilitation, specifically exercise programs in support of stem cell therapies. Stem cells are mobilized form the bone marrow and the number of circulating cells is demonstrated to be dynamic, responding to different factors and conditions, including cytokines in the setting of infraction as well as co-morbidities such as diabetes.56,57 Recent studies have evaluated the impact of exercise, with high intensity prolonged exercise programs associated with higher number of circulating stem cells.58,59. To date, however, only limited data sets exist to define the precise effect of different exercise training programs on stem cell numbers and function. It has been proposed that exercise may exert a liberating action freeing stem cells from their niche, inducing proliferation and/or differentiation, and by improving homing and paracrine action by the host organ.60,61 Exercise modulates different signaling pathways known to affect stem cell biology. In addition to exercise, recent studies show that the number of circulating stem cells is inversely associated with cardiovascular risk factors. In this regard, patients with coronary artery disease who had a strict control of lipid profile with use of statins, displayed a higher number of circulating progenitor cells.62,63 Taken together, cardiac rehabilitation - in patients undergoing stem cell therapy - is of importance as it may enhance outcome not only by establishing the adequate exercise regimen but also by controlling cardiovascular risk factors.
Summary
As “first” and “next generation” cell therapy nears pivotal evaluation for therapeutic efficacy, the field of cardio-regenerative medicine is poised to yield real impact on the prognosis of cardiovascular disease. Initial pioneering work established the safety and feasibility of cell delivery into diseased patients' hearts. More recent cell purification and optimization steps have been associated with significant improvement in efficacy signals with consistent safety. 41,42,48 Furthermore, the appreciation for the heart's innate propensity for regeneration highlights the importance of cross-talk between the host organ and the implanted stem cells underscoring the complexity of the regenerative response.64,65 Indeed, regenerative biotherapies are no longer seen as a standalone magic bullet, but rather an inductive active ingredient that can promote a regenerative response within the injured tissue. As such, a combinatorial approach leveraging traditional approaches, such as rehabilitation, with modern regenerative strategies, such as newest stem cell biologics, provides a complementary armamentarium to reimagine clinical problems and in so doing usher curative therapies for cardiovascular disease.
References
- 1.Terzic A, Harper CM, Jr, Gores GJ, Pfenning MA. Regenerative medicine blueprint. Stem cells and development. 2013;22(Suppl 1):20–24. doi: 10.1089/scd.2013.0448. [DOI] [PubMed] [Google Scholar]
- 2.Waldman SA, Terzic A. Cardiovascular health: the global challenge. Clinical pharmacology and therapeutics. 2011;90:483–485. doi: 10.1038/clpt.2011.213. [DOI] [PubMed] [Google Scholar]
- 3.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. The New England journal of medicine. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 4.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail. 2003;9:354–363. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Pitt B. Digitalis Investigation Group (DIG) trial: a stimulus for further research. American heart journal. 1997;134:3–12. doi: 10.1016/s0002-8703(97)70100-5. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Feldman AM, Saxon LA. Heart failure management using implantable devices for ventricular resynchronization: Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial. COMPANION Steering Committee and COMPANION Clinical Investigators. J Card Fail. 2000;6:276–285. doi: 10.1054/jcaf.2000.9501. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. The New England journal of medicine. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 8.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 9.Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: insights and lessons from clinical trials. Journal of the American Heart Association. 2013;2:e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohde LE, Bertoldi EG, Goldraich L, Polanczyk CA. Cost-effectiveness of heart failure therapies. Nat Rev Cardiol. 2013;10:338–354. doi: 10.1038/nrcardio.2013.60. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 12.Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace. 2011;13(Suppl 2):ii13–17. doi: 10.1093/europace/eur081. [DOI] [PubMed] [Google Scholar]
- 13.Gerczuk PZ, Kloner RA. An update on cardioprotection: a review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. Journal of the American College of Cardiology. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 15.Deb A, Wang S, Skelding KA, Miller D, Simper D, Caplice NM. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 16.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of clinical investigation. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 18.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 19.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England journal of medicine. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 20.Assmus B, Rolf A, Erbs S, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circulation Heart failure. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 21.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 22.Egeland T, Brinchmann JE. Cell quality in the ASTAMI study. European heart journal. 2007;28:2172. doi: 10.1093/eurheartj/ehm125. author reply 2173-2174. [DOI] [PubMed] [Google Scholar]
- 23.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA : the journal of the American Medical Association. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA : the journal of the American Medical Association. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traverse JH, Henry TD, Vaughan DE, et al. LateTIME: a phase-II, randomized, double-blinded, placebo-controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Texas Heart Institute journal/from the Texas Heart Institute of St Luke's Episcopal Hospital, Texas Children's Hospital. 2010;37:412–420. [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 28.Seeger FH, Rasper T, Fischer A, et al. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circulation research. 2012;111:854–862. doi: 10.1161/CIRCRESAHA.112.265678. [DOI] [PubMed] [Google Scholar]
- 29.Perin EC, Willerson JT. CD34+ autologous human stem cells in treating refractory angina. Circulation research. 2011;109:351–352. doi: 10.1161/CIRCRESAHA.111.250696. [DOI] [PubMed] [Google Scholar]
- 30.Ruifrok WP, de Boer RA, Iwakura A, et al. Estradiol-induced, endothelial progenitor cell-mediated neovascularization in male mice with hind-limb ischemia. Vasc Med. 2009;14:29–36. doi: 10.1177/1358863X08096666. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Lee SH, Yoo SY, Asahara T, Kwon SM. CD34 hybrid cells promote endothelial colony-forming cell bioactivity and therapeutic potential for ischemic diseases. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1622–1634. doi: 10.1161/ATVBAHA.112.301052. [DOI] [PubMed] [Google Scholar]
- 32.Losordo DW, Kibbe MR, Mendelsohn F, et al. A randomized, controlled pilot study of autologous CD34+ cell therapy for critical limb ischemia. Circulation Cardiovascular interventions. 2012;5:821–830. doi: 10.1161/CIRCINTERVENTIONS.112.968321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 35.Losordo DW, Schatz RA, White CJ, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 36.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nature medicine. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 37.Behfar A, Terzic A. Derivation of a cardiopoietic population from human mesenchymal stem cells yields cardiac progeny. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S78–82. doi: 10.1038/ncpcardio0429. [DOI] [PubMed] [Google Scholar]
- 38.Crespo-Diaz R, Behfar A, Butler GW, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- 39.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA : the journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heldman AW, Difede DL, Fishman JE, et al. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy: The TAC-HFT Randomized Trial. JAMA : the journal of the American Medical Association. 2013 doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heusch G. SCIPIO brings new momentum to cardiac cell therapy. Lancet. 2011;378:1827–1828. doi: 10.1016/S0140-6736(11)61648-6. [DOI] [PubMed] [Google Scholar]
- 44.Chimenti I, Smith RR, Li TS, et al. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. Circulation research. 2010;106:971–U304. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 46.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation research. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 47.Fujita J. Report of the American Heart Association (AHA) Scientific Sessions 2012, Los Angeles. Circulation journal : official journal of the Japanese Circulation Society. 2013;77:35–40. doi: 10.1253/circj.cj-12-1490. [DOI] [PubMed] [Google Scholar]
- 48.Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 49.Behfar A, Yamada S, Crespo-Diaz R, et al. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. Journal of the American College of Cardiology. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 52.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goel K, Pack QR, Lahr B, et al. Cardiac rehabilitation is associated with reduced long-term mortality in patients undergoing combined heart valve and CABG surgery. European journal of preventive cardiology. 2013 doi: 10.1177/2047487313512219. [DOI] [PubMed] [Google Scholar]
- 54.Pack QR, Goel K, Lahr BD, et al. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–597. doi: 10.1161/CIRCULATIONAHA.112.001365. [DOI] [PubMed] [Google Scholar]
- 55.Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/CIRCULATIONAHA.110.983536. [DOI] [PubMed] [Google Scholar]
- 56.Suresh R, Chiriac A, Goel K, et al. CXCR4+ and FLK-1+ identify circulating cells associated with improved cardiac function in patients following myocardial infarction. Journal of cardiovascular translational research. 2013;6:787–797. doi: 10.1007/s12265-013-9502-z. [DOI] [PubMed] [Google Scholar]
- 57.Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2012;26:23–33. doi: 10.1038/leu.2011.184. [DOI] [PubMed] [Google Scholar]
- 58.Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci (Lond) 2010;118:303–311. doi: 10.1042/CS20090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucia A, De La Rosa A, Silvan MA, et al. Mobilisation of mesenchymal cells in cardiac patients: is intense exercise necessary? British journal of sports medicine. 2009;43:221–223. doi: 10.1136/bjsm.2007.044693. [DOI] [PubMed] [Google Scholar]
- 60.Witkowski S, Jenkins NT, Hagberg JM. Enhancing treatment for cardiovascular disease: exercise and circulating angiogenic cells. Exercise and sport sciences reviews. 2011;39:93–101. doi: 10.1097/JES.0b013e31820a595e. [DOI] [PubMed] [Google Scholar]
- 61.Ribeiro F, Ribeiro IP, Alves AJ, et al. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2013;92:1020–1030. doi: 10.1097/PHM.0b013e31829b4c4f. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt-Lucke C, Fichtlscherer S, Aicher A, et al. Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PloS one. 2010;5:e13790. doi: 10.1371/journal.pone.0013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fadini GP, Maruyama S, Ozaki T, et al. Circulating progenitor cell count for cardiovascular risk stratification: a pooled analysis. PloS one. 2010;5:e11488. doi: 10.1371/journal.pone.0011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajstura J, Rota M, Cappetta D, et al. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


