Abstract
Setting
We conducted a retrospective study among HIV-infected adult (≥18 years) pulmonary tuberculosis (TB) suspects who underwent Xpert MTB/RIF (Xpert) testing at McCord Hospital and its adjoining HIV clinic in Durban, South Africa.
Objective
To determine if Xpert testing performed at a centralized laboratory accelerated time to TB diagnosis.
Design
We obtained data on sputum smear microscopy (AFB), Xpert and the rationale for treatment initiation from medical records. The primary outcome was “total diagnostic time,” defined as time from sputum collection to clinicians’ receipt of results. A linear mixed-effects model compared the duration of steps in the diagnostic pathway across testing modalities.
Results
Among 403 participants, the median “total diagnostic time” for AFB and Xpert was 3.3 and 6.4 days, respectively (P <0.001). When compared to AFB, the median delay for Xpert “laboratory processing” was 1.4 days (P<0.001) and “result transfer to clinic” was 1.7 days (P<0.001). Among 86 Xpert-positive participants who initiated treatment, 49 (57%) started treatment based on clinical suspicion or AFB-positive results, while only 32 (37%) started treatment based on Xpert-positive results.
Conclusion
In our setting, Xpert results took twice as long as AFB results to reach clinicians. Replacing AFB with centralized Xpert may delay TB diagnoses in some settings.
Keywords: Tuberculosis, HIV/AIDS, Xpert MTB/RIF assay, diagnostic testing, South Africa
INTRODUCTION
Tuberculosis (TB) is the leading cause of death among HIV-infected South Africans, yet diagnosing TB in HIV-infected adults remains a challenge.1 Sputum smear microscopy for acid fast bacilli (AFB), the most widely available diagnostic test, achieves poor sensitivity (9-28%) in studies of HIV-infected South Africans.2–4 This has prompted calls for improved TB diagnostics, particularly among HIV-infected individuals.5 The Xpert MTB/RIF assay (Xpert) is a novel nucleic acid amplification test that has been shown to be more sensitive than AFB for diagnosing pulmonary TB.6,7 Following the World Health Organization (WHO) endorsement of Xpert,8 the South African Department of Health and National Health Laboratory Service (NHLS) initiated a nationwide roll-out of Xpert as the first-line diagnostic test for pulmonary TB.9
Since Xpert does not rely on laboratory-trained personnel and provides results in as little as 120 minutes, it could potentially be used at the clinical point-of-care to accelerate the time to diagnosis and treatment initiation.10 Instead, due to operational and financial concerns, the NHLS has placed 203 Xpert machines in centralized laboratories throughout South Africa.11 Consequently, sputum samples and test results are couriered between a healthcare facility and off-site laboratories. Thus, centralized use of Xpert may cause unexpected diagnostic delays, which might have clinical consequences for initiation of TB treatment.12 We sought to determine if sputum Xpert testing performed at a centralized laboratory accelerated time to pulmonary TB diagnosis for a hospital and outpatient clinic in Durban, South Africa.
METHODS
Study Design
We conducted a retrospective study of HIV-infected adult TB suspects at McCord Hospital and Sinikithemba Clinic in Durban, South Africa. The hospital is a state-aided general hospital that serves an urban population. The high-volume HIV clinic provided ART beginning in 2001, and scaled up activities from 2004 – 2012 with support from President's Emergency Plan for AIDS Relief (PEPFAR). ART was initiated according to South African HIV guidelines.13 Both the hospital and clinic began using Xpert in April 2011. Hospital guidelines suggested that all TB suspects, defined as individuals with signs and symptoms suggestive of TB, initially receive both AFB and Xpert as first line diagnostic tests. We included consecutive HIV-infected participants ≥18 years old with at least one sputum Xpert test performed between April 2011 and March 2012, and not taking TB medications at the time of diagnostic testing. This study was approved by the McCord Research Ethics Committee in Durban (reference number 070212/3.1 rf pd) and the Partners Human Research Committee in Boston (2012-P-000746/1; MGH).
During the study period, sputum specimens were couriered twice daily to off-site laboratories located within 20 kilometers of the hospital and clinic. Specimens for AFB were couriered to either the private-sector Global Viral Clinical Laboratories (GLOBAL) or the public-sector NHLS laboratory at Addington Hospital. All Xpert specimens were first couriered to Addington Hospital and then to Prince Mshiyeni Memorial Hospital (PMMH), which has a high-volume Xpert machine capable of performing up to 400 tests per day.9 Results from PMMH were electronically reported to Addington Hospital, where reports were printed and couriered to McCord Hospital daily. Results from GLOBAL were electronically reported back to McCord Hospital. Upon receipt of results at Sinikithemba Clinic, a clinician entered results into a patient's electronic medical record (EMR).
Study Procedures
We identified participants using the hospital's TB suspect register, TB treatment register, and an electronic record of Xpert tests ordered during the study period. For eligible participants, we extracted data on age, gender, TB history, CD4 count, AFB results and Xpert results.
We obtained time interval data for TB diagnostics from the EMR and the NHLS TrakCare Laboratory Information System (LIS). We extracted the time of specimen collection, specimen arrival at the laboratory, and the time test results were authorized from the NHLS test result form. We documented a clinician's receipt of test results by analyzing clinician notes in the McCord Hospital EMR. Since the EMR did not record a time stamp for clinicians’ notes, we assumed all notes were entered at 5 PM on the date of entry for time interval calculations. We defined “total diagnostic time” as the time from sputum collection to a clinician's documented receipt of test results. “Total diagnostic time” was comprised of the following three discrete time intervals: “specimen transport to lab,” “laboratory processing,” and “result transfer to clinic.” We defined “specimen transport to lab” as the time between sputum collection and sputum arrival at the laboratory. We defined “laboratory processing” as the time between sputum arrival at the laboratory and result entry into the laboratory database. We defined “result transfer to clinic” as the time between result entry into the laboratory database and a clinician's documented receipt of test results.
We defined a diagnosis of “laboratory-confirmed pulmonary TB” as a positive Xpert or AFB result. We defined “clinical TB” as participants who had negative diagnostic sputum tests but were initiated on TB treatment by a clinician. We categorized the reason for initiation of TB treatment by analyzing clinicians’ notes. Participants were grouped into the following treatment initiation categories: “clinical”, “AFB”, “Xpert”, “AFB or Xpert”, or “other.” We assigned “clinical” when a diagnosis was based on diagnostic modalities other than sputum AFB or Xpert such as clinical signs, symptoms or radiographic evidence. We assigned “AFB or Xpert” when we could not distinguish which sputum test result was used for treatment initiation.
Statistical Analysis
We fit a linear mixed-effects model14 to investigate differences in the “total diagnostic time” for sputum AFB and Xpert while accounting for the correlation between repeated tests on the same patients. Having found a significant difference in “total diagnostic time” by test, we then fit similar models to “specimen transport to lab,” “laboratory processing,” and “result transfer to clinic” times. To examine whether test results had an impact on any of the measured time intervals, we conducted similar analyses among participants with only laboratory-confirmed TB. We conducted sensitivity analyses including only participants for whom all time intervals were available and for participants in whom AFB and Xpert specimens were collected on the same day. Since AFB was performed at one of two laboratories, we compared “total diagnostic time” for AFB between GLOBAL and NHLS laboratories. To evaluate whether the initial roll-out and implementation of Xpert affected “total diagnostic time,” we compared “total diagnostic time” between those patients who received sputum Xpert testing during the first three months of the study period against those who received the Xpert testing after the first three months. We managed study data using REDCap (Research Electronic Data Capture) 15 and used the statistical software R (R Foundation for Statistical Computing, Vienna, Austria) for data analyses.16
RESULTS
Cohort Characteristics
Four hundred three HIV-infected adults met the study criteria and were included in this analysis, of whom 190 (47.1%) were male, and mean age was 38.5 years (Table 1). At the time of TB diagnostic testing, 85 (21.1%) participants were receiving ART, the median CD4 count was 183/mm3 [Interquartile Range (IQR) 78 – 313/mm3], and 76 (18.9%) had a history of TB. Laboratory-confirmed pulmonary TB was diagnosed in 156 participants (38.7%), clinical TB was diagnosed in 39 (9.7%) participants, and active TB was not diagnosed in 207 (51.4%) participants. Of the 195 participants for whom TB treatment was indicated, 180 (92.3%) initiated treatment. Of the 403 participants who received an Xpert test, 288 (71.5%) had an AFB result. There were 141 Xpert-positive and 59 AFB-positive participants. There were 10 inconclusive tests (4 Xpert, 6 AFB).
Table 1.
Participant Characteristics and Test Results (N=403)
| N (%) | |
|---|---|
| Age - mean ± SD | 38.5 ± 9.9 |
| Gender | |
| Male | 190 (47.1) |
| Female | 213 (52.9) |
| Median CD4+ (IQR) - cells/mm3 * | 183 (78 – 313) |
| On ART at time of TB diagnostic test † | |
| Yes | 85 (21.1) |
| No | 193 (47.9) |
| History of TB ‡ | |
| Yes | 76 (18.9) |
| No | 174 (43.2) |
| Final Diagnosis | |
| Laboratory-confirmed pulmonary TB | 156 (38.7) |
| Clinical pulmonary TB | 39 (9.7) |
| Not pulmonary TB | 207 (51.4) |
| All tests unsuccessful/contaminated | 1 (0.2) |
| TB treatment for confirmed or clinical TB § | |
| Yes | 180 (92.3) |
| No | 2 (1.0) |
| Missing Data ∥ | 13 (6.7) |
Abbreviations: SD (Standard Deviation); IQR (Interquartile Range)
Median CD4 was collected on 346 patients
ART history of collected for 278 patients
TB history was collected for 250 patients
TB treatment was confirmed for 195 patients
TB treatment was not confirmed for 13 patients who were lost-to-follow-up.
Time to Test Result
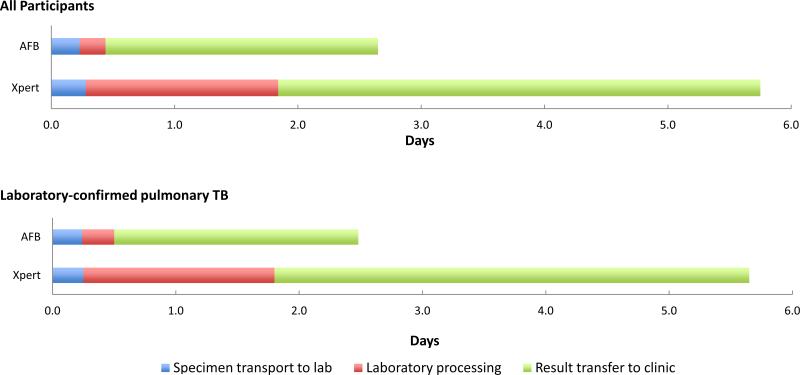
Time interval data was collected for 101 participants with AFB results and 327 participants with Xpert results (Table 2). The median “total diagnostic time” for Xpert was 3.1 days longer than that for AFB (6.4 vs. 3.3, P<0.001). “Specimen transport to lab” times were similar for the two testing modalities (P=0.26), despite the fact that specimens for the different tests were sent to different labs. When compared to AFB, the median delay for Xpert “laboratory processing” was 1.4 days (1.6 vs. 0.2, P<0.001) and “result transfer to clinic” was 1.7 days (3.9 vs. 2.2, P<0.001) (Figure 1). We obtained similar results in sensitivity analyses of participants with complete time interval data and among participants for whom AFB and Xpert specimens were collected on the same day. There was no significant difference in “total diagnostic time” for AFB results between laboratory sites (P=0.15). There was also no significant difference in “total diagnostic time” for Xpert testing during in the first three months, as compared to the remainder of the study period (P=0.95).
Table 2.
Time Interval Analysis
| AFB (N=101) |
Xpert (N=327) |
P-value | |||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||
| All participants | |||||
| Specimen transport to lab | 76 | 0.2 (0.2 – 0.4) | 306 | 0.3 (0.2 – 0.9) | 0.26 |
| Laboratory processing | 83 | 0.2 (0.2 – 0.3) | 327 | 1.6 (1.3 – 2.6) | < 0.001 |
| Result transfer to clinic | 95 | 2.2 (1.1 – 4.1) | 172 | 3.9 (2.4 – 5.5) | < 0.001 |
| Total diagnostic time* | 90 | 3.3 (2.1 – 5.2) | 156 | 6.4 (5.3 – 8.1) | < 0.001 |
| Laboratory-confirmed pulmonary TB | |||||
| Specimen transport to lab | 19 | 0.2 (0.2 – 0.4) | 117 | 0.3 (0.2 – 0.9) | 0.33 |
| Laboratory processing | 20 | 0.3 (0.2 – 0.5) | 124 | 1.6 (1.4 – 2.1) | < 0.001 |
| Result transfer to clinic | 26 | 2.0 (1.2 – 3.7) | 82 | 3.9 (2.6 – 5.6) | 0.03 |
| Total diagnostic time* | 26 | 2.4 (2.1 – 4.0) | 77 | 6.3 (5.2 – 8.2) | <0.001 |
| Sensitivity Analyses | |||||
| Total diagnostic time* (All time intervals) | 72 | 3.3 (1.4 – 5.2) | 156 | 6.4 (5.3 – 8.1) | <0.001 |
| Total diagnostic time* (Same day AFB/Xpert) | 56 | 3.2 (1.9 – 4.6) | 74 | 6.3 (5.2 – 7.2) | <0.001 |
Total diagnostic time was only calculated for participants who had data on the time of sputum specimen collection and the time a clinician received a test result.
Figure 1.
Median time between consecutive events from sputum specimen collection to a clinician's receipt of test results, for AFB and Xpert tests.
Among participants with laboratory-confirmed pulmonary TB (N=156), the median “total diagnostic time” for a positive AFB and positive Xpert test was 2.4 (IQR 2.1 – 4.0) and 6.3 (IQR 5.2 – 9.2) days, respectively (P<0.001) (Table 2). There was no difference in “specimen transport to lab” for either test (P=0.33). Similar to all participants, Xpert took a median of 1.3 and 1.9 days longer than AFB for “laboratory processing” (P<0.001) and “specimen transport to lab” (P=0.03), respectively.
Rationale for TB Treatment Initiation
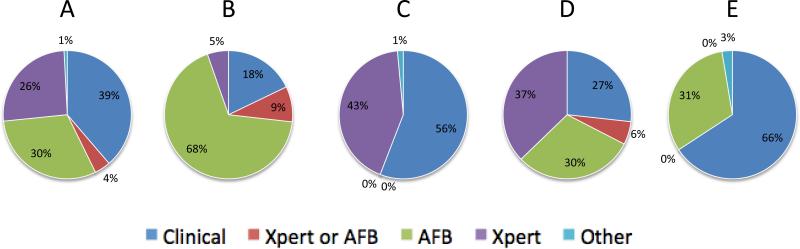
Among 180 participants who initiated TB treatment, 124 (69%) had both AFB and Xpert testing. Clinical suspicion, AFB, and Xpert results prompted TB treatment initiation in 48 (39%), 38 (30%), and 32 (26%) participants, respectively (Figure 2A). Five participants initiated TB treatment for either AFB or Xpert results, and one participant started TB treatment based on pre-treatment protocol for a lung resection.
Figure 2.
The rationale for TB treatment initiation among participants with both AFB and Xpert results (A, N=124), AFB-positive results (B, N=56) AFB-negative results (C, N=68), Xpert-positive results (D, N=86), or Xpert-negative results (E, N=38).
The rationale for TB treatment initiation differed between the 56 AFB-positive and 68 AFB-negative participants who initiated TB treatment. Among the AFB-positive participants, AFB results and clinical suspicion prompted TB treatment initiation in 38 (68%) and 10 (18%) participants, respectively (Figure 2B). While only 3 (5%) AFB-positive participants started TB treatment based on Xpert results, there were 29 (43%) AFB-negative participants for whom Xpert results prompted treatment. The remaining 38 (56%) AFB-negative participants started TB treatment based on clinical suspicion (Figure 2C).
Whereas AFB results prompted treatment in the majority of AFB-positive participants, Xpert results were relevant for treatment initiation for only a minority of Xpert-positive participants. Among Xpert-positive participants who initiated TB treatment (N=86), 49 (57%) were based on clinical suspicion or faster AFB results, while 32 (37%) were based on Xpert results (Figure 2D). There were 38 Xpert-negative participants, 12 (31%) of whom were initiated on TB treatment based on AFB results (Figure 2E).
DISCUSSION
HIV-associated TB continues to be a diagnostic challenge in sub-Saharan Africa. Failure to rapidly diagnose and treat TB puts patients at risk for increased morbidity (i.e. drug resistance, ongoing transmission) and mortality.17–19 Improving patient outcomes will require a low-cost, point-of-care test that is highly accurate. South Africa has invested in Xpert to meet this need. We have demonstrated, however, that sputum Xpert results from a high-volume Xpert machine placed in a centralized laboratory took almost twice as long as sputum AFB results to reach a clinician. The primary delays for Xpert results were laboratory processing time and the time required to transfer results from the laboratory to the clinic. Time intervals were not affected by positive or negative test results. Delays with Xpert results had direct implications for clinical decisions regarding treatment initiation. Over half of the Xpert-positive participants were initiated on treatment based on either clinical suspicion or a faster positive sputum AFB result. Therefore, replacing AFB with centralized Xpert testing without first addressing operational issues would delay diagnoses for some people with pulmonary TB in this setting. These delays may prolong the time to TB treatment initiation, adversely affecting patient outcomes.
Our findings suggest that centralized Xpert testing may take longer than previously reported. In an HIV clinic in Cape Town, the median time between sputum collection and results being available to the clinic for AFB and Xpert were 3 and 4 days, respectively.3,12 A large multi-center study with testing sites in South Africa and Uganda found that sputum AFB and Xpert results took 2 and 1 days to return results to clinicians when testing was performed at district and sub-district levels.7 Both of these studies followed patients prospectively in highly coordinated research settings, and clinical specimens were either tested on-site or couriered between one lab and the clinical setting. In this study, the Xpert delays were due to longer processing times and inefficient transport of test results from centralized laboratories to the clinic in a “real-world,” non-research setting.
Several studies have demonstrated that Xpert could be used at the clinical point-of-care. A clinic in Johannesburg had same-day treatment initiation in over 80% of new TB cases.20 Similarly, Yoon et al. found a significant reduction in the median days to TB detection, with most patients receiving same-day diagnoses, when performing clinic-based Xpert testing on TB suspects in a Ugandan hospital.21 One South African study decreased the time to treatment initiation from 13 days to 0 days when using Xpert at the clinical point-of-care.22 A large, multicenter study across five primary-care health-care facilities in South Africa, Zimbabwe, Zambia, and Tanzania recently documented similar findings regarding the improvement in same-day treatment initiation between patients receiving AFB and those receiving Xpert.23 These studies suggest that if Xpert had been used at the clinical point-of-care, observed testing delays could have been reduced and Xpert might have been more beneficial for TB treatment initiation.
The WHO recommends Xpert machines be placed at the “health facility level (ideally district or sub-district level),”24 despite initial reports suggesting Xpert could be implemented at the clinical point-of-care.7,25,26 In South Africa, Xpert has been placed in centralized laboratories, instead of peripheral health facilities, based on concerns about the test's cost and infrastructure requirements, such as air conditioning and stable electricity.24,27 As HIV care continues to decentralize in Africa, there is a concurrent need for decentralized TB diagnostic capacity.28 Decentralized care, including the use of nucleic acid-based diagnostic tests for TB, has been shown to improve early treatment outcomes for MDR-TB patients.29–31 Although Xpert can increase TB case detection by up to 45%,3 our results suggest that centralized placement of Xpert diminishes the clinical benefits of the Xpert test. If South Africa phases out AFB testing,9 treatment delays would primarily occur among sputum smear-positive patients, which has additional public health implications. Others have suggested replacing quality-assured AFB in peripheral health facilities without first demonstrating the feasibility, cost-effectiveness, and timeliness of Xpert testing is “irresponsible”,32 and our study supports the need for operational assessments in the transition to Xpert testing.33
It is important to note several features of our study design. We did not include Mycobacterium tuberculosis culture data in this analysis, since very few participants had results available. AFB was not available for all patients, which was likely a consequence of the laboratory prioritizing Xpert if inadequate specimen was available for both tests. Time interval data was collected for the study population with available data, and a reporting bias may be present. In addition, we were unable to report on overall time to TB treatment initiation, since pharmacy data with the exact times that prescriptions were filled was not available for the majority of study participants. Nevertheless, we were able to show that Xpert's relevance in a “real-world” setting is hindered by centralized implementation. This finding is important because multiple clinics in Durban are already using this implementation model. Future research might include qualitative analyses to characterize the operational challenges faced by clinicians and laboratory staff that could be used to further streamline the process and reduce diagnostic delays and improve patient care.
In conclusion, this study demonstrates that implementation of Xpert testing at a centralized laboratory causes operational delays that limit the test's clinical utility for diagnosing pulmonary TB. A clinician's assessment and a faster sputum AFB result remained central to a timely diagnosis of pulmonary TB in our setting. While placing Xpert at the clinical point-of-care may reduce diagnostic delays and improve clinical outcomes, benefits must be weighed against increased technical and operational costs.34 In the meantime, centralized processing and reporting of Xpert testing should be streamlined to provide faster results to clinicians, and there continues to be a role for sputum AFB testing. There is also a need for development of new point-of-care tests that are rapid and inexpensive. Like Xpert, the lateral flow test for urinary lipoarabinomannan (LAM) has shown promise as a point-of-care test for TB screening.4 Its utility, however, is restricted to a subset of HIV-positive patients with low CD4 counts.4 To overcome the limitations of tests like Xpert and urinary LAM, new diagnostics, such as the loop-mediated isothermal amplification (LAMP) sputum assay,35 will need to be broadly applicable and easy to implement. With these technologic advances, it will be possible to identify TB early, start treatment promptly, and reduce TB associated morbidity and mortality.
ACKNOWLEDGEMENTS
The authors thank the health care workers and staff of McCord Hospital and Sinikithemba Clinic. GC and PKD were supported by the Fogarty International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988). The Centre for the AIDS Programme of Research in South Africa (CAPRISA) in Durban, South Africa served as the site for GC's clinical research training year. PKD was also supported by the Harvard Global Health Institute and The Program for AIDS Clinical Research Training (T32 AI007433). IVB was supported by the National Institute of Mental Health (R01 MH090326). Access to REDCap was made available through the Vanderbilt Institute for Clinical and Translational Research grant support (UL1TR000011 from NCATS/NIH).
Footnotes
Conferences: Presented as Poster Presentation at The Conference on Retroviruses and Opportunistic Infections (CROI) March 2013, Atlanta, Georgia, Abstract No. S-138.
REFERENCES
- 1.World Health Organization (WHO) Global Tuberculosis Control 2011. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 2.Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin. Infect. Dis. 2010 Oct 1;51(7):823–9. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med. 2011 Jul;8(7):e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect. Dis. 2012 Mar 17;12(3):201–9. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J. Infect. Dis. 2011 Nov;204(Suppl):S1159–67. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 2010 Sep 9;363(11):1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011 Apr 30;377(9776):1495–505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) Policy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system. WHO; Geneva, Switzerland: 2011. [PubMed] [Google Scholar]
- 9.Smart T. [2012 Sep 20];GeneXpert to be rolled out as first-line diagnostic for TB in South Africa [Internet]. aidsmap. 2011 Available from: http://www.aidsmap.com/genexpert-to-be-rolled-out-as-first-line-diagnostic-for-TB-in-South-Africa/page/1746803.
- 10.Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011 Sep;6(9):1067–82. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Laboratory Service . GeneXpert MTB/RIF Progress Report: February 2013. South Africa Department of Health; South Africa: 2013. [Google Scholar]
- 12.Lawn SD, Kerkhoff AD, Wood R. Location of Xpert® MTB/RIF in centralised laboratories in South Africa undermines potential impact. Int. J. Tuberc. Lung Dis. 2012 May;16(5):701. doi: 10.5588/ijtld.12.0131. [DOI] [PubMed] [Google Scholar]
- 13.South Africa National Department of Health . The South African Antiretroviral Treatment Guidelines. South Africa: 2010. [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982 Dec;38(4):963–74. [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- 17.Farmer P, Bayona J, Becerra M, et al. The dilemma of MDR-TB in the global era. Int. J. Tuberc. Lung Dis. 1998 Nov;2(11):869–76. [PubMed] [Google Scholar]
- 18.Van Rie A, Enarson D. XDR tuberculosis: an indicator of public-health negligence. Lancet. 2006 Nov 4;368(9547):1554–6. doi: 10.1016/S0140-6736(06)69575-5. [DOI] [PubMed] [Google Scholar]
- 19.Millen SJ, Uys PW, Hargrove J, et al. The effect of diagnostic delays on the drop-out rate and the total delay to diagnosis of tuberculosis. PLoS One. 2008 Jan;3(4):e1933. doi: 10.1371/journal.pone.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouse K, Page-Shipp L, Dansey H, et al. Implementation of Xpert MTB/RIF for routine point-of-care diagnosis of tuberculosis at the primary care level. S. Afr. Med. J. 2012 Oct;102(10):805–7. doi: 10.7196/samj.5851. [DOI] [PubMed] [Google Scholar]
- 21.Yoon C, Cattamanchi A, Davis JL, et al. Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda. PLoS One. 2012 Jan;7(11):e48599. doi: 10.1371/journal.pone.0048599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Rie a, Page-Shipp L, Hanrahan CF, et al. Point-of-care Xpert® MTB/RIF for smear-negative tuberculosis suspects at a primary care clinic in South Africa. Int. J. Tuberc. Lung Dis. 2013 Mar;17(3):368–72. doi: 10.5588/ijtld.12.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014 Mar 1;383(9915):424–35. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) Rapid Implementation of the Xpert MTB/RIF diagnostic test: technical and operational “How-to”: practical considerations. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 25.Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J. Clin. Microbiol. 2010 Jan;48(1):229–37. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris K. Xpert TB diagnostic highlights gap in point-of-care pipeline. Lancet Infect. Dis. 2010 Nov;10(11):742–3. doi: 10.1016/s1473-3099(10)70231-0. [DOI] [PubMed] [Google Scholar]
- 27.Trébucq A, Enarson DA, Chiang CY, et al. Xpert(®) MTB/RIF for national tuberculosis programmes in low-income countries: when, where and how? Int. J. Tuberc. Lung Dis. 2011 Dec;15(12):1567–72. doi: 10.5588/ijtld.11.0392. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, Howard AA, Reid MJA, et al. TB diagnostic capacity in sub-Saharan African HIV care settings. J. Acquir. Immune Defic. Syndr. 2012 Oct 1;61(2):216–20. doi: 10.1097/QAI.0b013e3182638ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loveday M, Wallengren K, Voce A, et al. Comparing early treatment outcomes of MDR TB in decentralised and centralised settings in KwaZulu-Natal, South Africa. Int. J. Tuberc. Lung Dis. 2012 Feb;16(2):209–15. doi: 10.5588/ijtld.11.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson KR, Theron D, Kendall E a, et al. Implementation of GenoType MTBDRplus Reduces Time to Multidrug-Resistant Tuberculosis Therapy Initiation in South Africa. Clin. Infect. Dis. 2013 Feb;56(4):503–8. doi: 10.1093/cid/cis920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dheda K, Ruhwald M, Theron G, et al. Point-of-care diagnosis of tuberculosis - past, present and future. Respirology. 2012 Nov 27; doi: 10.1111/resp.12022. [DOI] [PubMed] [Google Scholar]
- 32.Trébucq A, Harries AD, Rieder HL. In reply to “Should Xpert® MTB/RIF be rolled out in low-income countries?”. Int. J. Tuberc. Lung Dis. 2012 May;16(5):703–4. doi: 10.5588/ijtld.12.0034-2. [DOI] [PubMed] [Google Scholar]
- 33.Kirwan DE, Cárdenas MK, Gilman RH. Rapid implementation of new TB diagnostic tests: is it too soon for a global roll-out of Xpert MTB/RIF? Am. J. Trop. Med. Hyg. 2012 Aug;87(2):197–201. doi: 10.4269/ajtmh.2012.12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trébucq A, Harries AD. In reply to “Location of Xpert® MTB/RIF in centralised laboratories in South Africa undermines potential impact.”. Int. J. Tuberc. Lung Dis. 2012 May 1;16(5):702. doi: 10.5588/ijtld.12.0131-2. [DOI] [PubMed] [Google Scholar]
- 35.Yuan L, Li Y, Wang M, et al. Rapid and effective diagnosis of pulmonary tuberculosis with novel and sensitive loop-mediated isothermal amplification (LAMP) assay in clinical samples: a meta-analysis. J. Infect. Chemother. 2014 Feb;20(2):86–92. doi: 10.1016/j.jiac.2013.07.003. [DOI] [PubMed] [Google Scholar]