Abstract
BACKGROUND
The pathogenesis of Duchenne muscular dystrophy starts prior to birth. Despite this, clinical trials exclude young boys because traditional outcome measures rely on cooperation. We recently used the Bayley-III Scales of Infant and Toddler Development to study 24 infants and boys with Duchenne muscular dystrophy. Clinical evaluators at six centers were trained and certified to perform the Bayley-III. Here we report six and twelve month follow-up of two subsets of these boys.
PATIENTS
Nineteen boys (1.9 ± 0.8 years) were assessed at baseline and six months. Twelve boys (1.5 ± 0.8 years) were assessed at baseline, six, and twelve months.
RESULTS
Gross motor scores were lower at baseline compared to published controls (6.2 ± 1.7; normal 10 ± 3; p<.0001), and showed a further declining trend to 5.7 ± 1.7 (p =.20) at six months. Repeated measures analysis of the 12 boys followed for 12 months showed that gross motor scores, again low at baseline (6.6 ± 1.7; p<.0001), declined at six months (5.9 ± 1.8) and further at 12 months (5.3 ± 2.0) (p=0.11). Cognitive and language scores were lower at baseline compared to normal children (range p=.002 to p<0.0001) and did not change significantly at 6 or 12 months (range p=.89 to p=.09). Fine motor skills, also low at baseline, improved over one year (p=.05).
CONCLUSION
Development can reliably be measured in infants and young boys with DMD across time using the Bayley-III. Power calculations using these data show that motor development may be used as an outcome measure.
Keywords: Duchenne Muscular Dystrophy, Bayley-III, Infant development, Clinical trial outcomes
Introduction
Few clinical trials have included younger boys or infants with Duchenne Muscular dystrophy(DMD) because traditional Medical Research Council (MRC) testing and quantitative strength testing rely on cooperation. Both manual muscle and quantitative strength testing have been shown to be less reliable in children younger than age six years1,2. Prior studies of untreated younger boys demonstrate effectively the “honeymoon” period in DMD when absolute function and strength may improve but does not allow them to catch up with normal children3–7. This absolute improvement can complicate interpretation of functional gains during clinical trials in younger boys with DMD. The locomotor quotient of the Griffith’s scales decreases in young boys with DMD8. Recently, the Griffith’s motor scales and the Bayley-III have been shown to have a negative correlation with age in young boys with DMD9,10. Pane et al also demonstrated that those boys whose dystrophin mutations disrupt the promoter of the predominant dystrophin brain isoform were more likely to have deficits in language and cognition9.
It has been known for many years that boys with DMD gain motor skills and function over the first 6–7 years (the “honeymoon” period). We recently demonstrated that Bayley-III gross motor scaled scores correlated negatively with age of the infants and young boys10. Our work shows that infants and young boys with DMD lose ground and show less maturational improvement with age relative to their healthy peers. Here we followed two subsets of these boys to determine how performance changes over time relative to healthy peers. This provides the basis for a clinical trial outcome tool with power calculations through comparison of gross motor scaled scores of the Bayley-III.
Methods
Participants
In our original study, we recruited 24 boys with DMD who were less than three years of age (1.9 ± 0.7 years)10. Nineteen of these boys (age 1.9 ± 0.8 years) returned for the 6 month evaluation (Table 1). Fourteen boys were less than 30 months at baseline and 12 of these (1.5 ± 0.8 years) returned to be tested both at 6 and 12 months using the Bayley-III that is validated through age 42 months. All participating sites received human studies approval and informed consent was obtained from a parent prior to enrollment in the study. All six sites enrolled at least two infants or young boys who were followed through 6 or 12 months; Washington University (N=4), Nationwide Children’s (N=5) University of California-Davis (N=4), University of Minnesota (N=2), Harvard University (N=2), Newcastle (N=2). Mutations in the DMD gene were defined for all boys or for a primary relative with DMD (Table1). Ten had a primary relative (brother or uncle) with DMD. Three boys were identified through newborn screening11. None were taking corticosteroids or any other medications.
Table 1. Age and DMD mutations.
Asterisks mark subject in whom mutation analysis from an affected brother was accepted. Implications of reading frame are discussed in the text. The last two columns describe predicted effect of the mutation on expression of other relevant DMD isoforms by deletion of either the appropriate promoter/exon 1 or by downstream domains. * Indicates those followed at both 6 and 12 months.
| # | Age (mos) |
Family History |
Mutation | Exon(s) | Frame | Disruption of Dp140 isoform |
Disruption of Dp71 isoform |
|---|---|---|---|---|---|---|---|
| 1* | 4.4 | Yes | Deletion | 3–32 | In | No | No |
| 2* | 4.9 | No | Deletion | 3–41 | In | No | No |
| 3* | 7.6 | Yes | Deletion | 45 | Out | Yes | No |
| 4* | 14.6 | Yes | Duplication | 2 | Out | No | No |
| 5* | 14.8 | Yes | Deletion | 45–50 | Out | Yes | No |
| 6* | 17.0 | Yes | Deletion | 46–50 | Out | Yes | No |
| 7* | 20.6 | Yes | Deletion* | 46 | Out | Yes | No |
| 8* | 21.7 | Yes | Deletion | 45–50 | Out | Yes | Yes |
| 9 | 26.5 | Yes | Nonsense (c.2353C>T; p.Gln785X) | 19 | Out | No | No |
| 10* | 26.9 | No | Deletion | 51–57 | Out | Yes | Yes |
| 11* | 28.9 | No | Deletion | 53–55 | Out | Yes | No |
| 12 | 28.9 | No | Deletion | 45 | Out | Yes | No |
| 13* | 29.2 | No | Deletion | 49–52 | Out | Yes | No |
| 14* | 29.3 | No | Deletion | 58–64 | Out | Yes | Yes |
| 15 | 31.3 | Yes | Deletion | 18–25 | In | Yes | No |
| 16 | 32.5 | No | Deletion | 46–52 | Out | Yes | No |
| 17 | 33.6 | No | Nonsense (c.2791G>T; p.Glu931X) | 21 | Out | No | No |
| 18 | 33.8 | No | Deletion | 45 | Out | Yes | No |
| 19 | 34.2 | Yes | Deletion | 12–44 | Out | Unknown | No |
Measures
Bayley-III Scales of Infant Development-Third Edition (Bayley-III)
1) Cognitive, Language and Motor Scales
The Bayley-III includes assessment of cognition, language (receptive and expressive) and motor function (gross and fine) in infants and young children from 0 to 42 months and provides a measureable and validated cognitive quotient12. Bayley-III language assessment is divided into receptive and expressive subtests. When these two language subtests are combined a composite score is determined. Bayley-III motor assessment includes scaled scores for fine motor and gross motor as well as a composite score.
2) Adaptive Behavioral Subtest of Bayley (ABS)
The ABS is a detailed, validated parental questionnaire which allows calculation of social emotional and adaptive behavioral assessment scores12. Subscales include Communication, Community Use, Functional Pre-Academic, Home Living, Health and Safety, Leisure, Self- Care, Self-Direction, Social, and Motor. Subscale scores in normal children are standardized to a mean of 10 ± 3. Based on these subscales, four composite scores are calculated which include the general adaptive composite (GAC), conceptual adaptive domain (CON), social adaptive domain (SO) and practical adaptive domain (PR). The general adaptive composite (GAC) reflects all ten subscales. The conceptual adaptive domain (CON) is derived from the communication, functional pre-academics, and self-direction subscale scores. The social adaptive (SO) composite score considers the leisure and the social subscale scores. Finally, the practical adaptive domain reflects community use, health and safety, home living, and self-care subscales.
3) Social-Emotional (SE) Scale
This questionnaire, completed by the child’s parent, rates a child’s social emotional competence and sensory processing and is based on earlier work by Greenspan13.
For all of the Bayley-III indices, composite scores have a mean of 100 ± 15. Subscale scores have a mean of 10 ± 3.
Training
Training required all clinical evaluators (CE’s) to attend a three-day session at Washington University in Saint Louis. For the Bayley-III, CE’s received didactic and healthy infant training (by MMC, Washington University). Each CE was then required to recruit at least two additional well infants (under and over age 18 months) and perform a videotaped practice Bayley-III. These tapes were then also reviewed and scored by a single investigator (MMC) and if needed, additional well infants were evaluated by the CE. Upon completion, CEs at each site were certified (prior to recruitment of DMD children). Retraining was performed twelve months into the study.
Statistical analyses
Descriptive statistics and paired statistical comparisons were performed with GraphPad Prism (GraphPad Software, La Jolla, CA). P-values are all 2-tailed. Power calculations were made using G*Power 3.114.
Results
Retention and Cooperation for Bayley-III and Adaptive Behavioral Assessment (ABS)
Twenty of the original 24 infants returned for the 6 month visit (83%). However, one boy did not cooperate with the testing at the 6 month visit so 19 (mean age 1.9 ± 0.8 years; range=0.37–2.99) were able to complete both baseline and 6 month evaluations (80%). The ages, family history and dystrophin mutation analysis are captured in table 1.
All 19 completed the gross motor, language, and cognitive assessments of the Bayley-III at baseline and 6 month follow-up. Eighteen were able to complete the fine motor component at baseline. One boy (age 2.4 years) became irritable and did not complete the fine motor component at the baseline visit. Fourteen boys were less than 30 months at baseline and therefore qualified to have testing both 6 and 12 month later. Of these, 12 boys (1.5 ± 0.8 years) returned and completed both of these evaluations (86%).
The ABS was completed by a parent of each of the 19 boys at baseline and 6 months. It was completed by one parent of 11 of 12 boys at baseline and 12 months (91%).
Bayley-III baseline assessment of infants and boys with DMD (Table 2)
Table 2. Bayley-III scores for two cohorts of boys with DMD compared to normal children.
The second group is the 12 boys of the 19 who were followed 12 months. (Average score ± Std. Dev.)
| Age at Entry |
Cognitive (n=19) |
Language Receptive (n=19) |
Language Expressive (n=19) |
Gross Motor (n=19) |
Fine Motor (n=18) |
|
|---|---|---|---|---|---|---|
| Normal | NA | 100±15 | 10±3 | 10±3 | 10±3 | 10±3 |
| Baseline for 19 boys | 1.9±0.8 | 87.6 ±10.7 | 6.7±2.4 | 7.5±3.1 | 6.2±1.7 | 7.7±2.1 |
| t and p values vs. to normal | NA | t=−5.0 p<.0001 | t=−5.9 p<.0001 | t=−3.5 p=.002 | t=−3.7 p<.0001 | t=−4.7 p<.0001 |
| Baseline for 12 boys | 1.5±0.8 | 86.2 ±8.0 | 6.1±1.6 | 6.1±1.6 | 6.6±1.7 | 7.7±2.1 |
| t and p values vs. to normal | NA | t=−5.94 p<.0001 | t=−8.6 p<.0001 | t=−5.2 p=.0003 | t=−6.8 p<.0001 | t=−3.5 p=.005 |
As previously reported10, the Bayley-III demonstrated clear statistically and clinically meaningful differences between the boys with DMD and typically developing children at baseline in all domains (p values range from .002 to P<.0001) (Table 2). This deviation from normal was consistent for the group followed 6 months and the group followed 12 months.
Bayley-III follow-up at 6 and 12 months (Table 3)
Table 3. Bayley-III scores for two cohorts of boys with DMD compared across time (Correlated Samples).
The second group is comprised of the 12 boys who were young enough to be followed 12 months. (Average score ± Std. Dev.)
| Scale | Baseline 19 boys with DMD |
6 month Follow-up 19 boys with DMD |
Baseline vs. 6 months |
Baseline 12 boys with DMD |
12 mo. Follow-up 12 boys with DMD |
Baseline vs. 12 months |
|---|---|---|---|---|---|---|
| Cognitive | 87.6 ±10.7 | 87.4±11.2 | p=.89 | 86.2±8.0 | 88.7±8.6 | p=.35 |
| Receptive Language | 6.7±2.4 | 7.1±2.3 | p=.09 | 6.1±1.6 | 6.7±1.8 | p=.23 |
| Expressive Language | 7.5±3.1 | 8.1±2.9 | p=.25 | 6.9±2.1 | 7.4±2.4 | p=.48 |
| Gross Motor | 6.2±1.7 | 5.7±1.7 | p=.20 | 6.6±1.7 | 5.3±2.0 | p=.11 |
| Fine Motor | 7.7±2.1 | 8.1±1.6 | p=.17 | 7.7±2.1 | 8.8±2.4 | p=.05 |
While average gross motor scaled scores were lower at 6 months compared to baseline(5.7±1.7 vs. 6.2±1.7) (p=.20) and lower still for the group followed 12 months (5.3±2.0 vs. 6.6 ±1.7) (p=.11), neither follow up measurement was statistically different from baseline (p=.20 and p=.11). Fine motor scores showed a trend toward increase by 6 months (p=0.17) and this became significant in the group followed 12 months (p=0.05). Cognitive composite scores, albeit very low compared to normal children remained stable across both 6 month and 12 months (Table 3). Receptive language improved marginally in follow up scores at 6 and 12 months (p=.09 and .23). Expressive language remained low. While modest increases in-group means occurred, these were not significant at either 6 or 12 months.
Comparison of DMD boys’ performance of Bayley-III across time
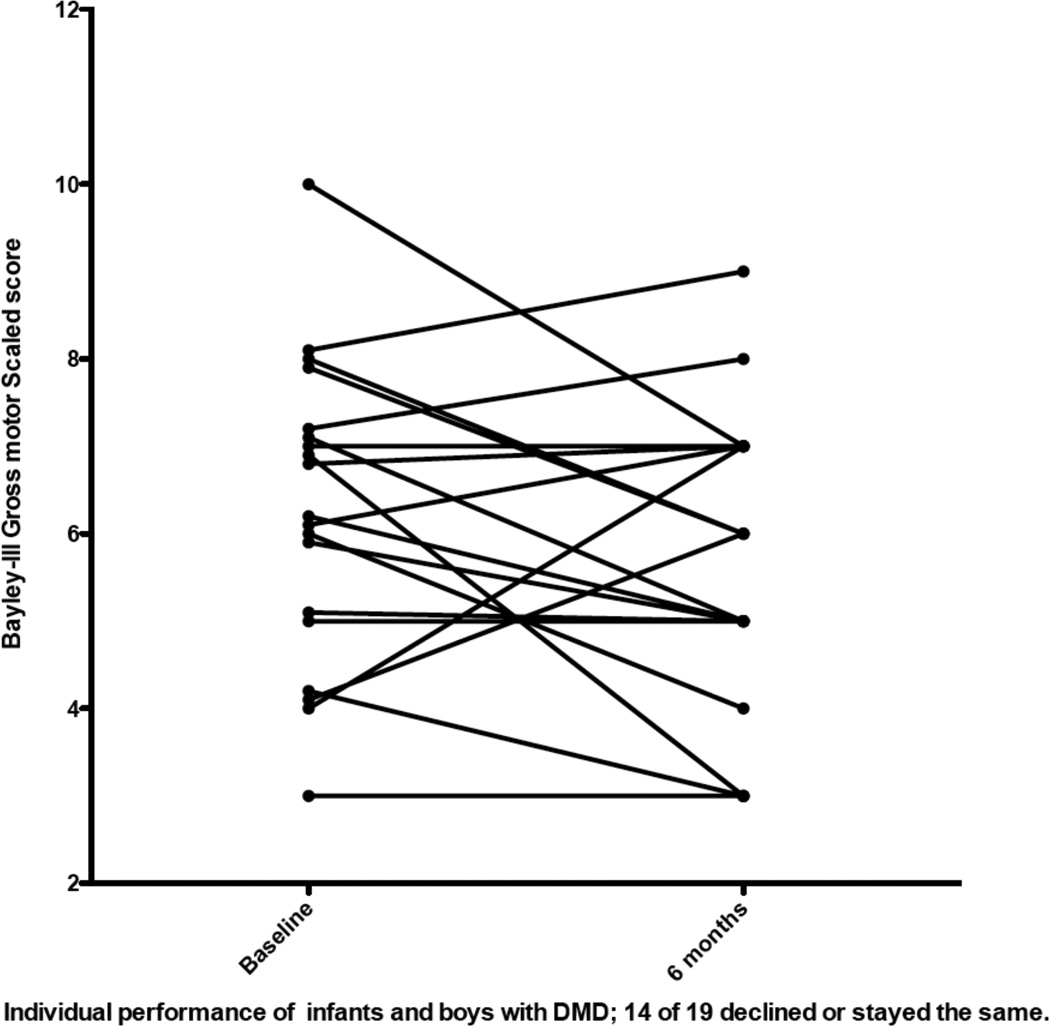
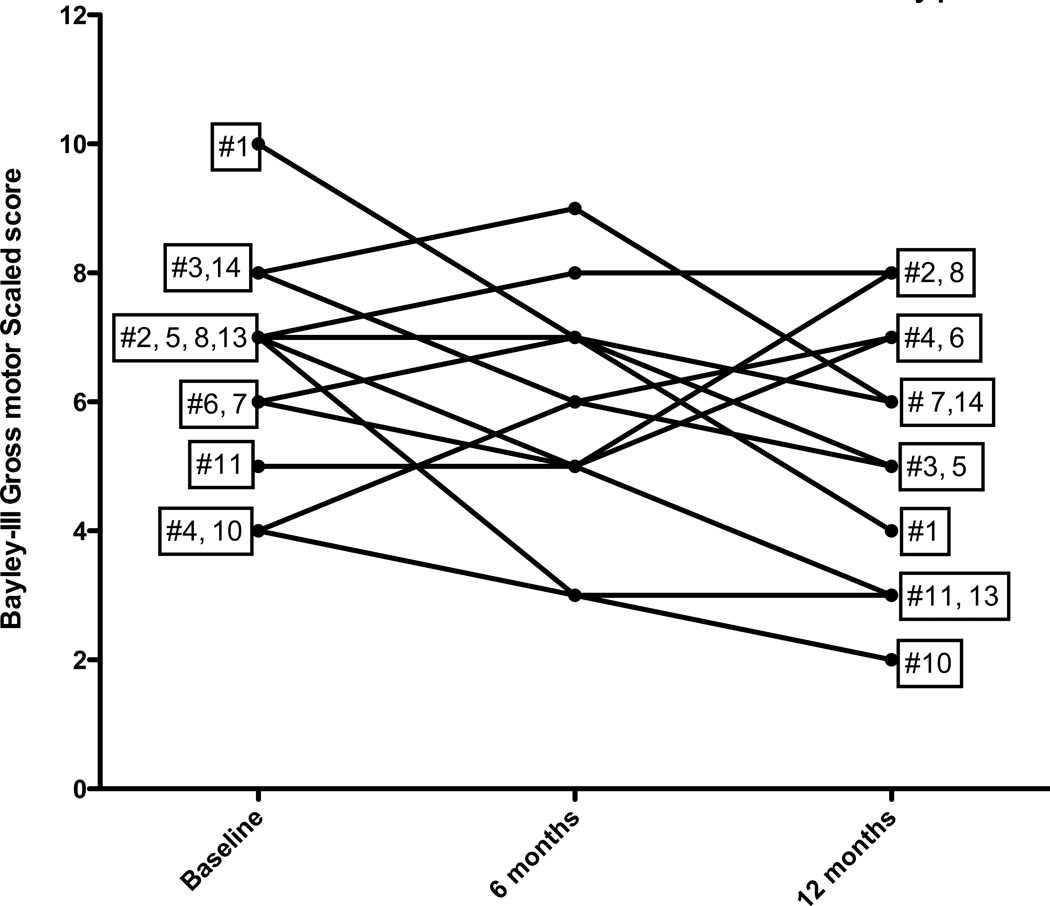
As tables 2 and 3 demonstrate, the change across time did not meet statistical significance for any subtest of the Bayley-III for either the 6 months subset of 19 boys or the 6 and 12 month subset for 12 boys. However, analysis of individual performances showed the variability between subjects (Figures 1 and 2). Of the 19 boys who had gross motor skills assessed at baseline and 6 months, 5 improved, 5 remained stable and 9 decreased their performance relative to age norms. Of the 12 children followed one year for gross motor skills, 4 improved, one remained stable and 7 declined (Figure 2).
Figure 1.
Individual performance of gross motor scales scores in 19 boys followed 6 months. A score of 10 is the normative mean for healthy peers
Figure 2.
Individual performance of gross motor scales scores in 12 boys followed 12 months. A score of 10 is the normative mean for healthy peers.
Fine motor skills tended to remain stable or improve (p=.17 at 6 months and p=.14 at 12 months). Of the 18 boys who had fine motor skills assessed both at baseline and 6 months, 8 improved their score, 5 remained stable, and 5 decreased their score. In the 11 boys followed for 12 months, seven improved, one remained stable, and three decreased their score.
Adaptive Behavior Subtest
The results for each ABS are shown in table 4. While the mean of all subtests was less than typically developing children, significant deficits were demonstrated in Functional pre-academic, Health and safety, Leisure, Self care, Social, and Motor subtests. There were no significant differences in this parent reported measure between baseline and six months or at 12 months.
Table 4.
Adaptive Behavior Subtest for infants and young boys with DMD at baseline, 6 and 12 months.
| Sub- scale |
Com- muni- cation |
Com- munity Use |
Functi onal Pre- Acade mic |
Home Living |
Health and Safety |
Leisure | Self Care |
Self Direc- tion |
Social | Motor |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean ± s.d. | n=19 8.8± 2.0 |
n=16 8.7± 2.9 |
n=16 7.9± 2.5 |
n=16 8.6± 2.6 |
n=19 8.0± 2.0 |
n=19 8.4± 2.3 |
n=19 6.4± 2.3 |
n=19 8.9± 3.2 |
n=19 8.0± 3.1 |
n=19 7.9± 4.1 |
| 6 months Mean ± s.d. | n=19 8.8± 1.9 |
n=17 8.7± 2.9 |
n=17 7.9± 2.5 |
n=17 8.6± 2.6 |
n=19 8.0± 2.0 |
n=19 8.4± 2.3 |
n=19 6.4± 2.3 |
n=19 8.9± 2.1 |
n=19 8.0± 2.3 |
n=19 7.9± 3.2 |
| 12 months Mean ± s.d. | n=11 9.0± 2.2 |
n=11 8.7± 2.4 |
n=11 8.2± 2.8 |
n=11 9.0± 3.8 |
n=11 8.7± 3.7 |
n=11 7.7± 2.9 |
n=11 6.0 ± 3.0 |
n=11 7.7 ± 3.6 |
n=11 9.3± 4.0 |
n=11 7.5± 4.1 |
| Baseline vs. normal children | t=−1.8 p=.08 |
t=−1.4 p=.17 |
t=−2.5 p=.02 |
t=−1.6 p=.12 |
t=−3.2 p=.005 |
t=−2.3 p=.03 |
t=−5.2 p<.001 |
t=−1.5 p=.15 |
t=−2.7 p=.01 |
t=−2.2 p=.04 |
| Baseline vs. 6 months | n=19 p=.46 |
n=16 p=0.54 |
n=16 p=.49 |
n=16 p=.52 |
n=19 p=.53 |
n=19 p=.71 |
n=19 p=.87 |
n=19 p=.82 |
n=19 p=.74 |
n=19 p=.70 |
| Baseline vs. 12 months | n=11 p=.59 |
n=10 p=.91 |
n=10 p=.74 |
n=10 p=.46 |
n=11 p=.64 |
n=11 p=.11 |
n=11 p=.29 |
n=11 p=.12 |
n=11 p=.74 |
n=11 p=.72 |
Four Composite scores for the ABS are captured in table 5. The general adaptive composite (GAC) reflects all subscales and showed a mean composite score of 86 ± 19 for boys followed 6 months and 86 ± 22 for the 11 boys whose parents completed the ABS at baseline, 6 and 12 months. The mean conceptual adaptive domain (CON), social adaptive (SO) domain and practical adaptive (PR) domain scores were all low compared to typically developing children but did not vary significantly across time (table 5). The level and pattern of scores based on parent report on the ABS questionnaire is similar to those achieved on the Bayley-III. This provides evidence that the parents of boys with DMD are aware of these deficits.
Table 5.
Adaptive Behavior Subtest Composite scores for two cohorts of boys with DMD compared across time (Correlated Samples).
| Baseline n=19 |
6 mos. FU n=19 |
Baseline vs. 6 mos. |
Baseline n=11 |
12 mos. FU n=11 |
Baseline vs. 12 mos. |
|
|---|---|---|---|---|---|---|
| General Adaptive composite | 86 ±19 | 89.7±25 | p=.52 | 87±22 | 84±27 | p=.54 |
| Conceptual adaptive Domain | 91±17 | 7.1±2.3 | p=.93 | 91±20 | 87±22 | p=.25 |
| Social Adaptive Domain | 86±13 | 85±27 | p=.91 | 86±15 | 88±26 | p=.82 |
| Practical Domain | 85±17 | 81±18 | p=.57 | 88±19 | 87±26 | p=.93 |
Social Emotional (SE) Scale of the Bayley-III
Eighteen parents rated their children on the SE Scale of the Bayley-III at baseline and 6 months. The social emotional composite scores ranged from 55–135 (mean 96 ± 15) at baseline and this did not change significantly after 6 months (range 50–120; mean 92 ± 12;p=.29). In the 11 children who were again measured at 12 months the SE score at baseline (range 65–145; mean 94 ± 18) did not significantly change at 12 months (range 65–145 mean 94 ± 13; p=.92).
Variability and Power calculation for theoretical trial using gross motor scaled scores
Over 12 months, the average change in gross motor scaled score was 1.25 lower with a standard deviation of 2.5 points. While this did not reach statistical significance (p=.11), it is possible to use this data to power a clinical study if the therapy used increases that scaled score. If a therapy causes the boys to improve by 0.75 points on average (an effect size of +2.0 points), then 12 boys would be required to detect this change (1-tailed alpha = 0.05) for a single-sample test against a historical control or 20 boys in each of two groups for a randomized trial against placebo.
We carefully reviewed the mutations and the family information in the four boys who improved their gross motor scaled score over one year. These boys were subjects #2 (0.41years), # 4 (age 1.22 years), #6 (age 1.42 years) and #8 (1.81 years). Subject #2, who improved one point, had a very large in-frame deletion (exon 3–41) but had no family history to predict ultimate clinical outcome. This large in-frame deletion has been previously reported to the Leiden database (www.dmd.nl) to be associated with DMD. Interestingly, the two other subjects with in-frame dystrophin mutations (Subjects #1 and #15) did not improve. Subject #4 who improved his gross motor scaled score from 4 to 7, had an outof- frame duplication of exon 2, which is usually associated with DMD but has also been associated with milder intermediate and Becker muscular dystrophies15. His family history included two maternal great uncles who died in late teens or early twenties. A maternal uncle, who was walking well without corticosteroids at age nine, may be an outlier but is too young to be certain. Both subject #6 and subject # 8 improved one point; both had out-of-frame deletions of exons 46–50, and positive family histories of typical DMD.
Discussion
The overall goal of this research was to determine whether standardized measures of infant and young child development can be used to measure changes over time in children with DMD. Trials of therapies that target the genetic defect in DMD are well underway16–20. Other therapeutic strategies aim to ameliorate downstream pathogenic mechanisms including signaling pathways which may impact fibrosis or blood flow21–23. To date, none of these clinical trials have included boys under age three years. Here we demonstrate in a multicenter natural history trial that young boys, including infants can be assessed with a validated measure.
Relative to normative information, our results show trends of worsening gross motor performance over one year although variability within group performance was also noted. Variability in boys with DMD has been recognized for many years and this was also true in our work. Some variability likely relates to the site of the mutation15. Some of the variability likely relates to other genetic factors24,25. Only a single boy (subject # 4) improved more than one point over 12 months and his score remained well below the normative mean.
Gross motor scores were shown to correlate negatively with age in our first baseline study of 24 infants (r = −0.45; p = .03)10. In this study we used repeated measure testing with a smaller cohort and showed a trend for the gross motor scaled score to decrease both in the 6 month and 12 month groups. This difference indicates that, as expected, our sample failed to make expected progress in the intervening months.
While these changes did not meet statistical significance, it was possible to use them to calculate a sample size for a one-year trial using these infants and young boys. If a therapy improves gross motor function, this will be reflected with an improved score. If a therapy causes the boys to improve by 0.75 points on average (an effect size of +2.0 points), then 12 boys would be required to detect this change (1-tailed alpha = 0.05) for a clinical trial a single-sample test against a historical control. In the case of a placebo-controlled trial, 20 boys in each of two groups for a randomized trial against placebo would be required to detect this change (alpha error 0.05). We have shown it is possible to recruit this many infants in a 6 site multicenter trial. Recruitment in the first study took place over nine months. Most boys in our study satisfactorily completed the Bayley-III assessments without difficulty. Although retention in this observation, longitudinal study did not reach 80%, we anticipate higher retention in a treatment trial due to increased family motivation for the offered intervention.
In our baseline study, we showed that cognitive and language skills did not vary with age10. In this study, with correlated samples, cognitive and language skills remained stable albeit low on average at both the six month and 12 month time points.
Interestingly, fine motor skills showed a trend toward improvement at six months and this improvement reached statistical significance at 12 months. While the mean scores for boys with DMD remained below the normative mean, this improvement suggests that early fine motor delays in children with DMD may improve in these early years. It is interesting to speculate that the young boys with DMD may choose to spend more time on activities that do not involve walking and running.
Two subtests of the Bayley-III are designed to reflect parent’s observation of the development of the child. The first subtest is the SE Scale, and the second, the ABS. As in our previous study,10 the adaptive measures were consistent with the results obtained on the Bayley cognitive, language and motor domains. These findings suggest that parent report via these measures may also be useful in assessing and following these skills in the first years of life. Scores were stable over time. These both show similar deficits that can be identified in the first years of life. Only scores of motor, leisure activities and self direction showed trends to decline over time, possibly reflecting an impact of progressive, muscular weakness on these activities.
It is widely held that most therapies will work best early in life before severe fibrosis has developed, although research is still needed to better identify particular modalities of therapy matched with disease stage. Therefore it is very important to develop outcome assessments that include these very young boys. This work also shows that while boys gain gross motor skills most do not do so at a rate that parallels typically developing boys. The “honeymoon” phase of DMD, when the rate of development is expected to be typical of other children, does not exist for most boys. Even the boys who improved over one year did not approach the gross motor skills expected for typically developing boys of the same age. As effective therapeutic trials become a reality, this young group should be identified and included. While most of our boys were identified through affected family members, some were identified through newborn screening. This work supports the importance of early diagnosis through newborn screening26. Even in the absence of a clinical trial, early identification of language and social deficits in young boys with DMD is very important, as it will lead to early interventions of speech and language therapy. These services likely have their biggest impact in the first few years of life27,28.
Acknowledgements
This work was funded by the Muscular Dystrophy Association DMD-center grants to AMC, JDM, CMM, JWD, and BTD. This publication was also made possible by Grant No. UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Acknowledgement of other members of the MDA/DMD network team Jeanine R. Schierbecker PT, MHSa, Catherine A. Siener, PT, MHSa, Charlie O. Wulf BAa, Pallavi Anand MBBSa, Linda P Lowes PT, PhDd, Lindsay N. Alfano, PT, DPT, PCSd, Laurence Viollet-Callendret, PhDd, Erica Goude, BAe, Linda Johnson, PTe, Alina Nicorici, BSe, John W. Day MD, PhDf, Joline C. Dalton MS, CGCf, Janey M. Farber DPTf, Karen K. Buser MSf, Peter B. Kang MDg Susan O. Riley PT, MS, DPT, PCSg, Elizabeth Shriber BAg, Anna G. Mayhew PhDh, Rebecca Parad BAg,
Department of Neurology, Washington University School of Medicine in Saint Louis, Saint Louis Children’s Hospital, Missouri USA
Department of Pediatrics, Washington University School of Medicine in Saint Louis, Saint Louis Children’s Hospital, Missouri, USA
Division of Biostatistics, Washington University School of Medicine in Saint Louis, Missouri, USA
Department of Pediatrics, Ohio State University, and the Center for Gene Therapy, Nationwide Children’s Hospital, Columbus Ohio, USA
Department Physical Medicine and Rehabilitation, University of California, Davis Medical Center, Sacramento, California, USA
Department of Neurology, University of Minnesota, Minneapolis, Minnesota, USA
Department of Neurology, Harvard University, Boston Children’s Hospital, Boston Massachusetts, USA
Department of Neurology and Institute of Genetic Medicine, Newcastle upon Tyne, Newcastle, England
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Merlini L, Dell'Accio D, Granata C. Reliability of dynamic strength knee muscle testing in children. J Orthop Sports Phys Ther. 1995 Aug;22(2):73–76. doi: 10.2519/jospt.1995.22.2.73. [DOI] [PubMed] [Google Scholar]
- 2.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 1995 Sep-Oct;74(5 Suppl):S70–S92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Brooke MH, Fenichel GM, Griggs RC, et al. Duchenne muscular dystrophy: Patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 4.Brooke M, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. determination of the "power" of theapeutic trials based on the natural history. Muscle & nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 5.Mayhew JE, Florence JM, Mayhew TP, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle & nerve. 2007 Jan;35(1):36–42. doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- 6.McDonald CM, Henricson EK, Han JJ, et al. The 6-minute walk test in Duchenne/Becker muscular dystrophy: longitudinal observations. Muscle & nerve. 2010 Dec;42(6):966–974. doi: 10.1002/mus.21808. [DOI] [PubMed] [Google Scholar]
- 7.Mazzone E, Vasco G, Sormani MP, et al. Functional changes in Duchenne muscular dystrophy: a 12-month longitudinal cohort study. Neurology. 2011 Jul 19;77(3):250–256. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, Newcombe RG, Sibert JR, Harper PS. Assessment of locomotor function in young boys with Duchenne muscular dystrophy. Muscle & nerve. 1991 May;14(5):462–469. doi: 10.1002/mus.880140513. [DOI] [PubMed] [Google Scholar]
- 9.Pane M, Scalise R, Berardinelli A, et al. Early neurodevelopmental assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2013 Jun;23(6):451–455. doi: 10.1016/j.nmd.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Connolly AM, Florence JM, Cradock MM, et al. Motor and cognitive assessment of infants and young boys with Duchenne Muscular Dystrophy: results from the Muscular Dystrophy Association DMD Clinical Research Network. Neuromuscul Disord. 2013 Jul;23(7):529–539. doi: 10.1016/j.nmd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of neurology. 2012 Mar;71(3):304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 12.Bayley N, et al. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio: Harcourt Assessment; 2005. [Google Scholar]
- 13.Greenspan SI. Clinical assessment of emotional milestones in infancy and early childhood. Pediatr Clin North Am. 1991 Dec;38(6):1371–1385. doi: 10.1016/s0031-3955(16)38225-6. [DOI] [PubMed] [Google Scholar]
- 14.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009 Nov;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 15.Flanigan KM, Dunn DM, von Niederhausern A, et al. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Human mutation. 2009 Dec;30(12):1657–1666. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govoni A, Magri F, Brajkovic S, et al. Ongoing therapeutic trials and outcome measures for Duchenne muscular dystrophy. Cell Mol Life Sci. 2013 Jun 18; doi: 10.1007/s00018-013-1396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo T, Wood MJ. Clinical trials using antisense oligonucleotides in duchenne muscular dystrophy. Human gene therapy. 2013 May;24(5):479–488. doi: 10.1089/hum.2012.234. [DOI] [PubMed] [Google Scholar]
- 18.McDonald CM, Henricson EK, Abresch RT, et al. The 6-minute walk test and other endpoints in Duchenne muscular dystrophy: longitudinal natural history observations over 48 weeks from a multicenter study. Muscle & nerve. 2013 Sep;48(3):343–356. doi: 10.1002/mus.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendell JR, Rodino-Klapac L, Sahenk Z, et al. Gene therapy for muscular dystrophy: Lessons learned and path forward. Neurosci Lett. 2012 May 17; doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel RS. Read-through strategies for suppression of nonsense mutations in Duchenne/ Becker muscular dystrophy: aminoglycosides and ataluren (PTC124) Journal of child neurology. 2010 Sep;25(9):1158–1164. doi: 10.1177/0883073810371129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huebner KD, Jassal DS, Halevy O, Pines M, Anderson JE. Functional resolution of fibrosis in mdx mouse dystrophic heart and skeletal muscle by halofuginone. Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1550–H1561. doi: 10.1152/ajpheart.01253.2007. [DOI] [PubMed] [Google Scholar]
- 22.Martin EA, Barresi R, Byrne BJ, et al. Tadalafil alleviates muscle ischemia in patients with Becker muscular dystrophy. Science translational medicine. 2012 Nov 28;4(162):162ra155. doi: 10.1126/scitranslmed.3004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PloS one. 2007;2(8):e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanigan KM, Ceco E, Lamar KM, et al. LTBP4 genotype predicts age of ambulatory loss in duchenne muscular dystrophy. Annals of neurology. 2012 Nov 26; doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegoraro E, Hoffman EP, Piva L, et al. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011 Jan 18;76(3):219–226. doi: 10.1212/WNL.0b013e318207afeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle & nerve. 2013 Jul;48(1):21–26. doi: 10.1002/mus.23810. [DOI] [PubMed] [Google Scholar]
- 27.Spittle A, Orton J, Anderson P, Boyd R, Doyle LW. Early developmental intervention programmes post-hospital discharge to prevent motor and cognitive impairments in preterm infants. The Cochrane database of systematic reviews. 2012;12:CD005495. doi: 10.1002/14651858.CD005495.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Blauw-Hospers CH, de Graaf-Peters VB, Dirks T, Bos AF, Hadders-Algra M. Does early intervention in infants at high risk for a developmental motor disorder improve motor and cognitive development? Neuroscience and biobehavioral reviews. 2007;31(8):1201–1212. doi: 10.1016/j.neubiorev.2007.04.010. [DOI] [PubMed] [Google Scholar]