Abstract
Background/objectives:
Inflammation is a central process responsible for health outcomes among surgical patients. Immunonutrition has been investigated as a promising modifying factor; however, inflammatory properties of habitual diet have not yet been investigated. The purpose of this study was to describe inflammatory properties of diet measured by the dietary inflammatory index (DII) among surgical patients treated for colorectal cancer and to link inflammatory properties of habitual diet with a duration of hospitalization.
Subjects/methods:
A follow-up study among colorectal cancer patients treated surgically was performed in Krakow, Poland. In total, 689 patients were recruited for the study. Habitual diet was assessed using a standardized semiquantitative food frequency questionnaire. Overall, 23 dietary items (including macro-and micronutrients) were used to calculate individuals' DII. Gender, age, marital status, body mass index, smoking status, lifetime physical activity, taking vitamin supplements, number of chronic diseases, cancer site, Duke's staging and surgery type were considered as potential covariates.
Results:
Participants were aged 58 years, with the average hospitalization time of 11 days. Higher DII (meaning diet with higher anti-inflammatory properties) was negatively associated with the duration of hospitalization (univariable linear regression: b=−0.59; P=0.005). Multivariable logistic regression has shown the decrease of the risk of longer stays (>7 days) among patients with the DII >−4.25, but only among younger (⩽60 years) patients, irrespective of Duke's staging.
Conclusions:
The DII might be used as a potential predictor of longer hospitalization among colorectal cancer patients treated surgically. The study provides evidence for the role of dietary-related low-grade inflammation among surgical patients.
Introduction
It has been observed that inflammation is one of the important properties of several diseases. First, inflammation may be an image of the presence of acute injury or damage to tissue. Second, during the past decades, an increasing interest in inflammation as an underlying factor responsible for the development of the disease has been observed.1 There are several inflammatory biomarkers, such as, interleukin-1β (IL-1β), IL-4, IL-6, IL-10, tumor necrosis factor-α and C-reactive protein (CRP), involved in the process, and their increased concentration is an effect of the nonspecific immune response. In some disorders, inflammatory process, which under normal conditions is self-limiting, becomes continuous and chronic—being the underlying cause of chronic inflammatory diseases.2 Increased levels of CRP and some other inflammatory cytokines have been noticed as risk factors for lung cancer3 and colon cancer.4 Additionally, more specific high-sensitivity CRP has been observed and linked with the development of atherosclerosis, and a number of cardiovascular disease end points.5,6 Some proinflammatory cytokines, such as IL-6 and tumor necrosis factor-α, have also been associated with obesity and insulin resistance.7
Nowadays, there is an increasing evidence linking diet with inflammation. Dietary patterns have been investigated as one of the key components in the regulation of chronic inflammation.8 It has been discovered that the Western-type diet is associated with higher levels of inflammatory cytokines,9 whereas that of the Mediterranean diet with lower levels.10,11 The same has been noticed for some other dietary items and nutrients, such as fruits and vegetables, polyunsaturated fatty acids and vitamins.12, 13, 14
It should be mentioned that diet also has a key role in immunonutrition (meaning the effects of nutrients—including macronutrients, vitamins, minerals and trace elements—on inflammation, providing a means of modulating the inflammatory response to injury and infection, and thus improves clinical outcome). There is an increasing body of evidence showing a beneficial effect of modified diets (rich in vitamins C and E, arginine, glutamine, nucleotides and n-3 fatty acids), especially among critically ill patients in terms of surgical outcomes in both malnourished and well-nourished patients.15,16
All these observations suggest the need to create a system to score and assess general inflammatory properties of diet, considering both the role of pro- and anti-inflammatory dietary items. Therefore, Cavicchia et al.17 created the dietary inflammatory index (DII). As described by its authors, the DII was the first literature-based index to focus primarily on the inflammatory properties of diet. The benefit of the index is that it assesses diet as a whole and thus it seems to preponderate other scores as the alternate healthy eating index or the alternate Mediterranean diet index. Moreover, the DII is not dependent on the population means or on the recommendations of intake, and it is not only limited to macronutrients and micronutrients but also incorporates commonly consumed components of the diet, including spices, tea and others.17
Inflammation is a central process observed in surgical patients and is a key component—after underlying disease and general health condition, accompanying wound healing18 and responsible for the time of recovery.19 Modifying factors associated with inflammatory properties may have a role in surgical outcomes; therefore, considering diet as one of the determinants of low-grade inflammation possibly associated with individual's vulnerability to develop health-related outcomes, we decided to test the effect of habitual diet among surgically treated patients.
Purpose
The purpose of the study was to describe inflammatory properties of diet measured by the dietary inflammatory index (DII) developed by Cavecchia et al.17 among surgical patients treated for colorectal cancer and to link inflammatory properties of habitual diet with a short-term surgical outcome as a duration of hospitalization in this group of patients.
Materials and methods
Study design and sample
A follow-up study was performed among patients admitted to the I Chair of General Surgery and Department of Gastroenterological Surgery, Jagiellonian University Medical College, Krakow, Poland to be treated for colorectal cancer. Patients eligible for the study were cases recruited for a case–control study conducted between 2000 and 2013. The study design of a primary research has been described elsewhere.20,21 In brief, participants were newly diagnosed incident cases of sporadic adenocarcinomas either of the colon or rectum. Inclusion criteria were as follows: age of up to 75 years, Caucasian, being a native Polish and referred for a surgical treatment of colorectal cancer. Exclusion criteria were as follows: presence of communication (verbal contact) problems and/or cognitive limitations, and diagnosis of any of the following: hereditary non-polyposis colorectal cancer, familiar adenomatous polyposis, attenuated familial adenomatous polyposis, mixed polyposis syndrome, Ashkenazi colon cancer, hereditary breast and colorectal cancer or any of hamartomatous polyposis syndrome, and also diagnosis of secondary cancer (its distant metastasis in large bowel), diagnosis of primary cancer other than colorectal, recurrent cancer, underwent surgery (before recruitment) of gastrointestinal tract, present or past diagnosis of chronic disease of gastrointestinal tract (diverticulitis, irritable bowel syndrome, acute or chronic gastric ulcer, acute or chronic pancreatitis), diabetes (any type), renal failure, hepatic insufficiency or the presence of prolonged gastrointestinal symptoms. The diagnosis of either colon cancer or rectal cancer in all study participants was confirmed histopathologically.
Tools and data collection
Study participants were interviewed, after the admission to the hospital but before surgery, about their dietary habits, lifetime recreational physical activity, some demographic characteristics (age, gender, marital status, etc.), presence of medical diagnosis and/or being treated for chronic conditions and some other potential covariates. Dietary habits were assessed by a semiquantitative food frequency questionnaire (SFFQ) developed in cooperation with the German Cancer Research Centre in Potsdam, where an introductory part of the European Prospective Investigation into Cancer and Nutrition (EPIC-Potsdam) project had been performed. In total, 148 dietary items were used, including questions about consumption of cereals, dairy products, bread, type and cuts of meat and fish (including preparation technique), fresh fruits (summer and winter time), salads and fresh and cooked vegetables, rice or pasta, soups, sweets, baked goods, drinks and others. For each food or beverage item, a commonly consumed portion size was specified by standardized photographs. Next, respondents were asked to provide information about frequency of consumption. For the research, information about usual (habitual) consumption over the period of 1 year by calendar seasons was gathered by trained interviewers. Patient-cases were asked about their dietary patterns at a time of 5 years before the onset of gastrointestinal symptoms (if present) or before the beginning of the diagnosis process. Next, dietary data were recalculated using Polish food composition tables to obtain information about an average consumption of dietary macro- and micronutrients.22,23 The validity and reproducibility of the questionnaire was assessed and published elsewhere.24,25
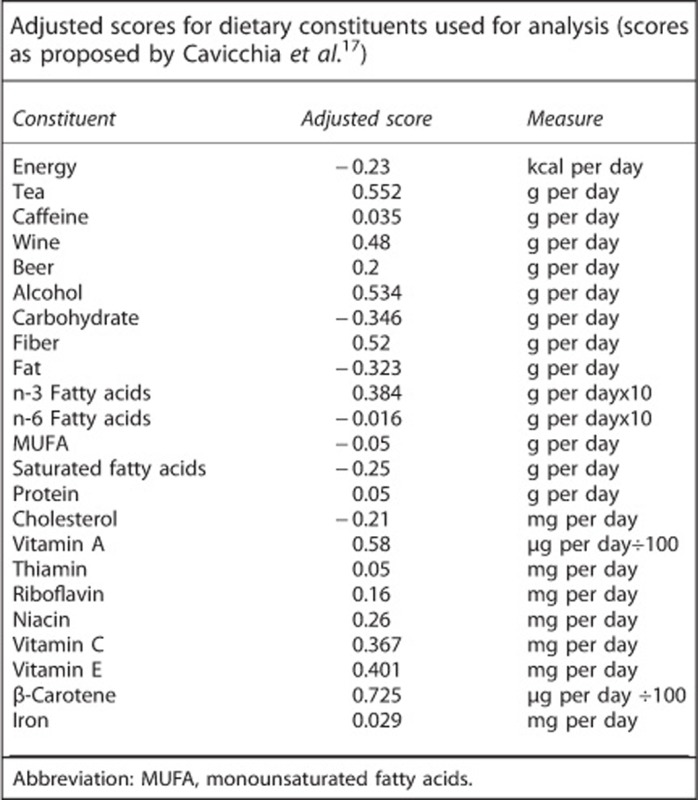
Inflammatory properties of diet have been assessed by the DII developed by Cavicchia et al.17 The DII considers different nutritional data including caloric intake, consumption of different beverages and alcoholic drinks and intake of macro- and micronutrients such as vitamins, microelements and others. Dietary data collected in the current investigation allow calculation of the following: an average intake of energy, drinking of tea, consumption of caffeine, different alcoholic drinks such as wine, beer and liquor (for the purpose of the study, the consumption of wine and beer was considered directly, and other sources of alcohol such as as fruit wine, sparking wine and vodka were recalculated according to their content of pure alcohol, and thus they were used in the calculation of the DII), an intake of carbohydrates, fiber, fat, n-3 and n-6 fatty acids, monounsaturated fatty acids, saturated fatty acids, protein, cholesterol, vitamin A, thiamin, riboflavin, niacin, vitamin C, vitamin E, β-carotene and iron. In this study, therefore, it was possible to obtain the DII based on the 23 different dietary items. The DII measure was calculated as a sum of adjusted scores as they were published by Cavicchia (see Annex). Finally, the DII related to habitual diet was obtained for every individual.
Length of hospitalization
Information about a length of hospitalization was collected from hospital registry. The duration of hospitalization was determined as the number of hospital days after the day of surgery.
Covariates
The following characteristics were considered as potential covariates: (1) gender; (2) age (continuous); (3) marital status (married, other); (4) body mass index (<25 kg/m2, ⩾25 kg/m2); (5) current smoking status (active smoker, non-smoker); (6) an average adult lifetime physical activity (as an average MET-h per day, continuous); (7) taking vitamin supplements (yes/no); (8) number of chronic diseases (non, 1, 2 or more): patients were questioned if they had been diagnosed with myocardial infarction, angina pectoris, stroke, an episode of ischemia of central nervous system, hypertension, diabetes, hyperlipidaemia or cholelithiasis; (9) cancer site (colon, rectum); (10) Duke's staging; (11) surgery type (radical, palliative).
Statistical analysis
As a first step of investigation, the DII for every individual was calculated as described above. A linear regression was used to assess an association between DII and the length of hospitalization. This analysis was performed for the whole sample and also after exclusion of individuals who might be outliers, meaning that these with the values below 5th percentile or over 95th percentile of the DII distribution were excluded.
Next, two groups were created depending on a length of hospitalization. The cutoff value was decided primarily on the value of 7 days. This decision was based considering a typical duration of hospitalization observed among patients treated surgically on the gastrointestinal surgery ward, that is, patients without complications who were treated in a standard way. The main determinants related to the length of hospitalization have been provided across groups. Characteristics expressed as categorical were compared across groups by the χ2 test, because every expected count was higher than 5. Continuous variables were tested by Shapiro–Wilk test to be normally distributed. The analyses had shown skewed distributions, and the Mann–Whitney U-test was subsequently used. Finally, different cutoffs of the DII were tested to assess usefulness of the DII in terms of prediction of longer (meaning >7 days) duration of hospitalization. The values of the first tertile, the first quartile and the first quintile of the DII distribution were considered and analyzed in the multivariable logistic regression models. The aforementioned covariates, as potentially associated with the health condition and the duration of hospitalization were used.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Bioethical Committee of Jagiellonian University (number KBET/115/B/2011).
Results
In total, during the period 2000–2013, 703 cases were recruited for the study and interviewed. Overall 14 individuals were excluded from the analyses owing to the lack of information about lifestyle factors (not willing to finish an interview), refusals to undergo surgery treatment, no access to detailed information about hospitalization or being a patient admitted for reoperation. Finally, the remaining 689 cases were analyzed.
Basic characteristics of the study sample are presented in Table 1. Participants were more frequently males, at an average age of 58 years. They were mainly married (86.5%), overweight or obese (69.5%), and one-fourth were active smokers. Patients reported an average adult lifetime physical activity at a level of 17.4 MET-h per day and in total less than 20% reported taking vitamin supplements. Overall 83% did not report being diagnosed with other chronic diseases. Considering clinical status, there were similar proportions of colon and rectal cancers, patients were mainly in Duke's B stage and over 90% of cancers were radically removed during surgery. The mean hospitalization time was 10.9 days (s.d.=9.4), with an interquartile range from 6 to 12 days.
Table 1. Basic characteristic of the study group.
| Total (n=689) | Hospitalized ⩽7 days (n=274) | Hospitalized >7 days (n=415) | P-value | |
|---|---|---|---|---|
| Males (n (%)) | 391 (56.7%) | 145 (52.9%) | 246 (59.3%) | d.f.=1 Pχ=0.099 |
| Age (years) | ||||
| Mean (s.d.) | 58.0 (8.9) | 58.1 (8.9) | 57.9 (9.0) | |
| Median, Q1–Q3 | 59 (52–65) | 59 (52–65) | 59 (52–65) | PMW=0.967 |
| Marital status (n (%)) | ||||
| Married | 596 (86.5%) | 232 (84.7%) | 364 (87.7%) | |
| Single/widowed/divorced | 93 (13.5%) | 42 (15.3%) | 51 (12.3%) | d.f.=1 Pχ=0.253 |
| BMI (n (%)) | ||||
| <25 kg/m2 | 210 (30.5%) | 94 (34.4%) | 116 (28.0%) | |
| ⩾25 kg/m2 | 478 (69.5%) | 179 (65.6%) | 299 (72.0%) | d.f.=1 Pχ=0.071 |
| Current smoking (n (%)) | 173 (25.1%) | 68 (24.8%) | 105 (25.3%) | d.f.=1 Pχ=0.886 |
| Average adult lifetime physical activity (MET-h per day) | ||||
| Mean (s.d.) | 17.4 (16.1) | 15.3 (13.5) | 18.8 (17.4) | |
| Median, Q1–Q3 | 13.0 (5.6–24.6) | 11.7 (5.7–20.4) | 13.8 (5.6–27.0) | PMW=0.039 |
| Taking vitamin supplements (n (%)) | 127 (18.4%) | 38 (13.9%) | 89 (21.5%) | d.f.=1 Pχ=0.012 |
| Dietary inflammatory index | ||||
| Mean (s.d.) | −2.66 (2.43) | −2.37 (2.29) | −2.85 (2.51) | |
| Median, Q1–Q3 | −2.42 (−3.91 to −1.15) | −2.24 (−3.59 to −1.11) | −2.60 (−4.09 to −1.19) | PMW=0.020 |
| Chronic diseases (n (%)) | ||||
| Non | 572 (83.0%) | 235 (85.7%) | 337 (81.2%) | |
| 1 | 103 (15.0%) | 33 (12.0%) | 70 (16.9%) | |
| 2+ | 14 (2.0%) | 6 (2.2%) | 8 (1.9%) | d.f.=2 Pχ=0.219 |
| Cancer site (n (%)) | ||||
| Colon | 332 (48.2%) | 147 (53.6%) | 185 (44.6%) | |
| Rectum | 357 (51.8%) | 127 (46.4%) | 230 (55.4%) | d.f.=1 Pχ=0.020 |
| Duke's staging (n (%)) | ||||
| A | 45 (6.9%) | 30 (11.5%) | 15 (3.8%) | |
| B | 263 (40.3%) | 113 (43.1%) | 150 (38.5%) | |
| C | 166 (25.5%) | 69 (26.3%) | 97 (24.9%) | |
| D | 178 (27.3%) | 50 (19.1%) | 128 (32.8%) | d.f.=3 Pχ<0.001 |
| Surgery type (n (%)) | ||||
| Radical | 628 (91.1%) | 259 (94.5%) | 369 (88.9%) | |
| Palliative | 61 (8.9%) | 15 (5.5%) | 46 (11.1%) | d.f.=1 Pχ=0.011 |
| Hospitalization time (days) | ||||
| Mean (s.d.) | 10.9 (9.4) | 5.7 (1.3) | 14.4 (10.8) | |
| Median, Q1–Q3 | 8 (6–12) | 6 (5–7) | 11 (9–15) | PMW<0.001 |
Abbreviations: BMI, body mass index; chi, chi-squared test; MW, Mann–Whitney U-test.
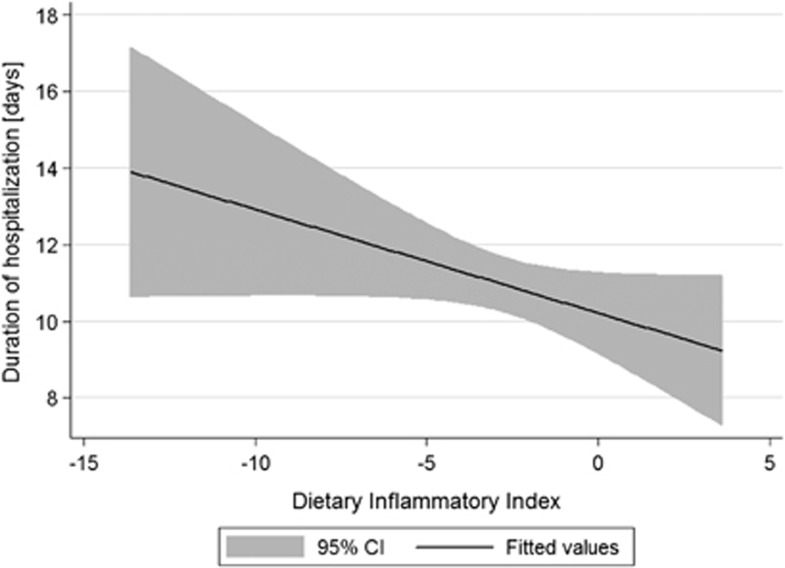
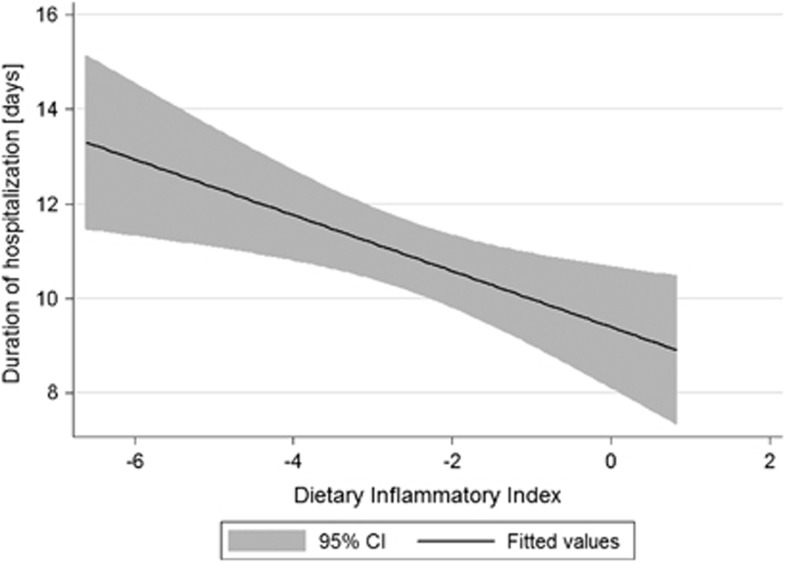
As the DII is a new index to measure inflammatory properties of diet—as a first step of investigation, the relationship between DII and the time of hospitalization was measured by a simple linear regression. The analysis considering all available subjects has shown negative association, but insignificant (b=−0.27; P=0.067) (Figure 1). As linear regression is very sensitive for outliers, the same regression was performed after exclusion of subjects with the DII values below 5th or over 95th percentile, and subsequently the observed negative association was statistically significant (b=−0.59; P=0.005) (Figure 2).
Figure 1.
Univariable linear regression between the DII and duration of hospitalization after surgery among patients treated for colorectal cancer (whole sample n=689).
Figure 2.
Univariable linear regression between the DII and duration of hospitalization after surgery among patients treated for colorectal cancer (after exclusion of subjects with the DII <5th percentile and with the DII >95th percentile n=620).
In the second part of the analysis, patients were divided into two subgroups: those hospitalized no longer than 7 days and others. Basic characteristics have been compared between these groups, and differences have been noticed for DII, an average adult lifetime physical activity, taking vitamin supplements, cancer location, Duke's staging and surgery type (Table 1). Next, we decided to investigate different cutoffs to test the relationship between the DII and duration of hospitalization in the multivariable logistic regression (Table 2). The decrease in the risk was observed, but results were not significant. A significant effect was noticed if patients were divided according to the Duke's staging considering separately those with local or distant metastases (Duke's C or D) and those without (Duke's A or B), and then patients with the DII over the first tertile (>−3.41) or over the first quartile (>−3.91) presented significant decrease in the risk of hospitalization of longer than 7 days. As the last step, differences in the effect of the DII across different age groups were analyzed. This decision was driven by some previous results showing differences in the outcomes related to the age category of patients.26 The current investigation has shown that younger (⩽60 years) patients presented, consequently, the decrease in the risk for the cutoff of the first quartile (>−3.91) and first quintile (>−4.25) for both Duke's subgroups, whereas the effect was not observed among older patients.
Table 2. ORs of prolonged hospitalization (>7 days) in different Duke's staging groups.
| DII | Total ORs (95% CI) | Duke's A or B | Duke's C or D |
|---|---|---|---|
| All agesa | |||
| ⩽−3.411 | 1b | 1b | 1b |
| >−3.41 | 0.76 (0.53–1.09) | 0.66 (0.44–0.996) | 0.79 (0.38–1.66) |
| ⩽−3.912 | 1b | 1b | 1b |
| >−3.91 | 0.69 (0.46–1.03) | 0.63 (0.40–0.99) | 0.56 (0.24–1.32) |
| ⩽−4.253 | 1b | 1b | 1b |
| >−4.25 | 0.69 (0.45–1.07) | 0.64 (0.39–1.03) | 0.53 (0.21–1.38) |
| ⩽60 | |||
| ⩽−3.411 | 1b | 1b | 1b |
| >−3.41 | 0.68 (0.43–1.08) | 0.69 (0.40–1.19) | 0.51 (0.21–1.28) |
| ⩽−3.912 | 1b | 1b | 1b |
| >−3.91 | 0.46 (0.27–0.78) | 0.51 (0.28–0.93) | 0.23 (0.07–0.75) |
| ⩽−4.253 | 1b | 1b | 1b |
| >−4.25 | 0.43 (0.24–0.77) | 0.44 (0.23–0.85) | 0.26 (0.07–0.97) |
| >60 | |||
| ⩽−3.411 | 1b | 1b | 1b |
| >−3.41 | 0.88 (0.49–1.60) | 0.62 (0.33–1.17) | 2.59 (0.48–13.90) |
| ⩽−3.912 | 1b | 1b | 1b |
| >−3.91 | 1.25 (0.66–2.37) | 0.83 (0.42–1.63) | 4.73 (0.81–27.45) |
| ⩽−4.253 | 1b | 1b | 1b |
| >−4.25 | 1.44 (0.72–2.92) | 1.09 (0.51–2.31) | 3.11 (0.52–18.62) |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for gender, age (years), total number of chronic diseases (non, 1, 2+), current smoking status (smoker, non-smoker), surgery type (palliative/radical), cancer site (colon/rectum), taking vitamin supplements (yes/no), level of adult lifetime physical activity (MET-h per day), overweight or obesity (yes/no) and marital status (currently married, other).
Reference group: 1, first tertile; 2, first quartile; 3, first quintile.
Discussion
The study presents an evidence of the relationship between inflammatory properties of diet and a short-term clinical outcome as a duration of hospitalization among surgically treated colorectal cancer patients. Moreover, it supports a usefulness of the DII in the prediction of longer (>7 days) hospitalization. In the current investigation, it has been shown in a modern way that habitual diet associated with inflammatory biomarkers might be an additional predictor of recovery time among surgical patients. Although the results suggest a linear relationship between the DII and the duration of hospitalization, it was decided to test and to find a cutoff that might be used for predictive purposes. This decision was also supported by previously published results showing that the DII, fitted as a categorical variable, was able to predict some interval changes in CRP.17 Additionally, the knowledge of the cutoffs may be useful for both clinical purposes to define patients being at higher risk and for research, as the results suggest the importance of the inflammatory properties of habitual diet.
The role of inflammation among surgical patients was tested in several ways. It was found that inflammation which complicates the course of a clinical treatment is one of the key component of hospital morbidity and mortality.27, 28, 29, 30 It was observed that within hours of physical injury, large number of arginase-1 (ARG1) expressing immature myeloid cells accumulate in the spleen and other lymphoid tissue.31 These cells (also called myeloid-derived suppressor cells) inhibit T-lymphocyte growth and function, resulting in the impairment of T-cell proliferative response.32 It was found that T helper type 2 cytokines, catecholamines and prostaglandin E2 induce the expression of myeloid-derived suppressor cells and act synergistically to increase the expression of ARG1.33 Thus, the stress response with the release of cortisol and catecholamines together with the release of prostaglandin E2 results in further impairment of T-cell proliferative responses. The preventive aim in this situation is to minimize the immunosuppression to reduce the risk of secondary infections, and to promote healing and tissue repair. Recently, increased attention has been focused on an immunomodulating diet, and it has been suggested a beneficial effect of pre- and postoperative immunonutrition,16,34,35 but published results are not consistent.36,37 Diet as a determinant of low-grade inflammation seems to be directly associated with the ARG1 pathway. Prolonged, low-grade inflammation may lead to the lack of reserves on the subclinical level, and in the case of surgery, it may be a risk factor for unfavorable outcomes among patients.
In our study, it was possible to investigate the role of habitual diet as a potential predictor of a length of hospitalization. There are some weaknesses and strengths of our findings. The strength comes from the possibility to assess the role of habitual diet as a long-term lifestyle determinant. Several studies have shown that the presence or the development of different health outcomes is determined not only by the concurrent clinical factors but also by the accumulation of ‘good and bad experiences over the lifetime'.38 This is nowadays the key concept of the life-course approach in medicine, meaning that our past exposures are responsible for the outcomes observed in a present day. Thus, the role of the DII in the duration of hospitalization should be considered rather as a measure of vulnerability related to the general condition of the human body—which is also a result of a prolonged exposure to dietary factors. The role of diet as a determinant of low-grade inflammation in terms of life-course approach for clinical outcomes has not been investigated so far. Other strengths of our study include considering of several important covariates. The results have been accounted for age and number of diagnosed chronic diseases—main determinants of health outcomes. It has been decided to adjust for marital status as it is a proxy measure of social circumstances, which may influence some decisions regarding the timing of discharge. Other important covariates considered were smoking status, adult lifetime physical activity and, more importantly, clinical features as cancer site, Duke's staging and surgery type. Regarding staging, the results have shown the benefit of ‘better' (meaning with lower proinflammatory potential, that is, higher the DII) diet for both Duke's A to B stage and C to D stage patients, but only among younger (⩽60 years) patients. This has not been observed in older patients. Some possible explanations are that older patients share some additional features related to the response to the presence of a disease and surgery that may, more importantly than among younger patients, determine the duration of hospitalization. In our study, patients at Duke's A and B have been investigated together. If they were considered separately, the odds ratios were 0.53 and 0.52 for the 1st quartile cutoff (for Duke's A and Duke's B, respectively), and 0.49 and 0.42 for the 1st quintile cutoff; however, these results (although similar to the results observed for the whole A plus B group) were not statistically significant owing to a decrease in sample size.
The study has also some limitations, as we investigated only 23 dietary items for the calculation of the DII. Although there are much more of them—those included in the analyses are the main constituents responsible for the effect of diet in the Polish population. In that study, there were no information about herbs available, but the use of herbs in the Polish population was rather rare in previous years, and it has become more popular over the past few years and more typically among younger population; therefore, the distorting effect of herbs, even though possible, was rather unlikely. In general, only patients with good health condition were referred to surgery, but there was still a possibility that some of them had additional (not considered in the analyses) factors associated with longer hospitalization. We had only a possibility to assess a duration of hospitalization as an outcome measure in the study. There are published papers showing the relationship between inflammatory status and some other outcomes as number of infectious complications or long-term survival.27,34,35 Although the data regarding the number of infectious complications and their severity were not available, the duration of hospitalization is also a very good proxy measure for these outcomes, because the development of any complication increases the duration of onward stay. The weakness is also the fact that inflammatory status of the patient is determined by much more factors other than diet, and, although the results have been adjusted for several covariates, there is still a possibility to have a residual confounder. The participants of our research had no measurements of serum inflammatory cytokines. The only inflammatory biomarker available for some of the study subject was the leukocyte count. To check weather inflammatory status at the admission to surgical ward was responsible for a longer hospitalization in our research, we compared shorter (⩽7 days) and longer (>7 days) hospitalization group, and we did not observe any statistically significant difference (white blood cells: 8.2 × 103/ml vs 7.1 × 103/ml in groups, respectively, P=0.312).
In summary, the investigation of inflammatory properties of diet using the DII as a predictor of some clinical outcomes is novel to our knowledge, as there are no published papers to date, and it provides a quite new insight in the role of diet. The results of the current research have shown the usefulness of the DII developed by Cavicchia as a potential predictor of longer hospitalization among patients treated surgically for colorectal cancer. Moreover, by more complex assessment of the role of diet considering its inflammatory properties, the study provides evidence for the role of dietary habits among surgically treated patients.
Acknowledgments
This research project was founded by the Polish National Science Centre in 2010–2013 (project no. N N404 034039)—the principal investigator is Aleksander Galas. Presented results were also partially supported by the data from Ministry of Science and Education projects (6P05D00220 and 2P05D05329)—the principal investigator is Professor Wieslaw Jedrychowski. We thank Dr Barbara Maziarz, the Head of the Department of Diagnostics, University Hospital in Krakow, Poland, for the availability to collect laboratory measurements.
ANNEX
The authors declare no conflict of interest.
References
- Prasad S, Sung B, Aggarwal BB. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54 (Suppl:S29–S37. doi: 10.1016/j.ypmed.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk of lung cancer. J Natl Cancer Inst. 2013;105:1871–1880. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cai Q, Li H, Cai H, Gao J, Yang G, et al. Circulating C-reactive protein and colorectal cancer risk: a report from the Shanghai Men's Health Study. Carcinogenesis. 2013;34:2799–2803. doi: 10.1093/carcin/bgt288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168:5126–5134. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring, MD) 2006;14:2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- Barbaresko J, Koch M, Schulze MB, Nothlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71:511–527. doi: 10.1111/nure.12035. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Estruch R. Anti-inflammatory effects of the Mediterranean diet: the experience of the PREDIMED study. Proc Nutr Soc. 2010;69:333–340. doi: 10.1017/S0029665110001539. [DOI] [PubMed] [Google Scholar]
- Root MM, McGinn MC, Nieman DC, Henson DA, Heinz SA, Shanely RA, et al. Combined fruit and vegetable intake is correlated with improved inflammatory and oxidant status from a cross-sectional study in a community setting. Nutrients. 2012;4:29–41. doi: 10.3390/nu4010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of omega-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013:743171. doi: 10.1155/2013/743171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herpen-Broekmans WM, Klöpping-Ketelaars IA, Bots ML, Kluft C, Princen H, Hendriks HF, et al. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–921. doi: 10.1007/s10654-004-5760-z. [DOI] [PubMed] [Google Scholar]
- Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. Perioperative nutrition in malnourished surgical cancer patients - a prospective, randomized, controlled clinical trial. Clin Nutr. 2011;30:708–713. doi: 10.1016/j.clnu.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. J Parenter Enteral Nutr. 2010;34:378–386. doi: 10.1177/0148607110362692. [DOI] [PubMed] [Google Scholar]
- Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res. 2012;304:589–597. doi: 10.1007/s00403-012-1278-5. [DOI] [PubMed] [Google Scholar]
- Modan-Moses D, Kanety H, Dagan O, Ehrlich S, Lotan D, Pariente C, et al. Leptin and the post-operative inflammatory response. More insights into the correlation with the clinical course and glucocorticoid administration. J Endocrinol Invest. 2010;33:701–706. doi: 10.1007/BF03346673. [DOI] [PubMed] [Google Scholar]
- Galas A, Augustyniak M, Sochacka-Tatara E. Does dietary calcium interact with dietary fiber against colorectal cancer? A case–control study in Central Europe. Nutr J. 2013;12:134. doi: 10.1186/1475-2891-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Maugeri U, Pac A, Sochacka-Tatara E, Galas A. Protective effect of fish consumption on colorectal cancer risk. Hospital-based case–control study in Eastern Europe. Ann Nutr Metab. 2008;53:295–302. doi: 10.1159/000195770. [DOI] [PubMed] [Google Scholar]
- Kunachowicz H, Nadolna I, Przygoda B, Iwanow K (eds)Food Composition Tables National Food and Nutrition Institute: Warszawa, Poland; 1998 [Google Scholar]
- Nadolna I, Przygoda B, Troszczyńska A, Kunachowicz H (eds)Food Composition Tables. Vitamins National Food and Nutrition Institute: Warszawa, Poland; 2000 [Google Scholar]
- Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26 (Suppl 1:S59–S70. doi: 10.1093/ije/26.suppl_1.s59. [DOI] [PubMed] [Google Scholar]
- Bohlscheid-Thomas S, Hoting I, Boeing H, Wahrendorf J. Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26 ( (Suppl 1:S71–S81. doi: 10.1093/ije/26.suppl_1.s71. [DOI] [PubMed] [Google Scholar]
- Galas A. Inflammatory effect of diet and hospital outcomes after colorectal cancer surgery—Do older patients vary. Eur Geriatr Med. 2013;4 (Suppl 1:S124. [Google Scholar]
- Andalib A, Ramana-Kumar AV, Bartlett G, Franco EL, Ferri LE. Influence of postoperative infectious complications on long-term survival of lung cancer patients: a population-based cohort study. J Thorac Oncol. 2013;8:554–561. doi: 10.1097/JTO.0b013e3182862e7e. [DOI] [PubMed] [Google Scholar]
- Fearon KC, Jenkins JT, Carli F, Lassen K. Patient optimization for gastrointestinal cancer surgery. Br J Surg. 2013;100:15–27. doi: 10.1002/bjs.8988. [DOI] [PubMed] [Google Scholar]
- Marik PE, Flemmer M. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg. 2012;73:801–808. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- Szczepanik AM, Scislo L, Scully T, Walewska E, Siedlar M, Kolodziejczyk P, et al. IL-6 serum levels predict postoperative morbidity in gastric cancer patients. Gastric Cancer. 2011;14:266–273. doi: 10.1007/s10120-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- Zhu X, Herrera G, Ochoa JB. Immunosupression and infection after major surgery: a nutritional deficiency. Crit Care Clin. 2010;26:491–500. doi: 10.1016/j.ccc.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LA, Gray C, Wilson L, Thomson BN, Shedda S, Crowe TC. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr. 2013;67:802–807. doi: 10.1038/ejcn.2013.117. [DOI] [PubMed] [Google Scholar]
- Klek S, Szybinski P, Szczepanek K. Perioperative immunonutrition in surgical cancer patients: a summary of a decade of research. World J Surg. 2013;38:803–812. doi: 10.1007/s00268-013-2323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner M, Cerantola Y, Grass F, Bertrand PC, Schafer M, Demartines N. Preoperative immunonutrition in patients at nutritional risk: results of a double-blinded randomized clinical trial. Eur J Clin Nutr. 2012;66:850–855. doi: 10.1038/ejcn.2012.53. [DOI] [PubMed] [Google Scholar]
- Turnock A, Calder PC, West AL, Izzard M, Morton RP, Plank LD. Perioperative immunonutrition in well-nourished patients undergoing surgery for head and neck cancer: evaluation of inflammatory and immunologic outcomes. Nutrients. 2013;5:1186–1199. doi: 10.3390/nu5041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offidani E, Tomba E, Linder MD. Two key concepts in the life course approach in medicine: allostatic load and cumulative life course impairment. Curr Probl Dermatol. 2013;44:17–32. doi: 10.1159/000350385. [DOI] [PubMed] [Google Scholar]