Commentary on: Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014; 507(7492): 323–328. Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014; 507(7492): 376–380.
It is a well-established principle that the presence of an adequate vascular supply is absolutely required for bone formation. A close spatial–temporal association exists between the processes of angiogenesis and osteogenesis during skeletal development in embryogenesis, in postnatal growing bones, and during the healing of bone fractures.1 Bone homeostasis in the adult also involves a close physical relationship between blood vessels and osteoblasts at remodeling sites.2,3 Moreover, reduced blood flow has been linked to old age and low bone mass disorders such as osteoporosis and to impaired fracture healing, rendering angiogenic modulation an angle of considerable interest from osteo–anabolic therapeutic viewpoints.2,4
The intimate relationship between blood vessels and bone formation has been referred to as ‘angiogenic–osteogenic coupling'.5,6,7 Blood vessels near bone formation sites ensure an adequate supply of oxygen and nutrients during the energy expensive process of ossification and may also deliver osteo-regulatory factors to their target cells. More recent evidence suggests that blood vessels may even have a role in determining the site of bone formation by carrying osteoprogenitors or mesenchymal stem cells with osteoblastic differentiation potential in close association with the endothelial wall of skeletal blood vessels (see Figure 1).8,9,10
Figure 1.
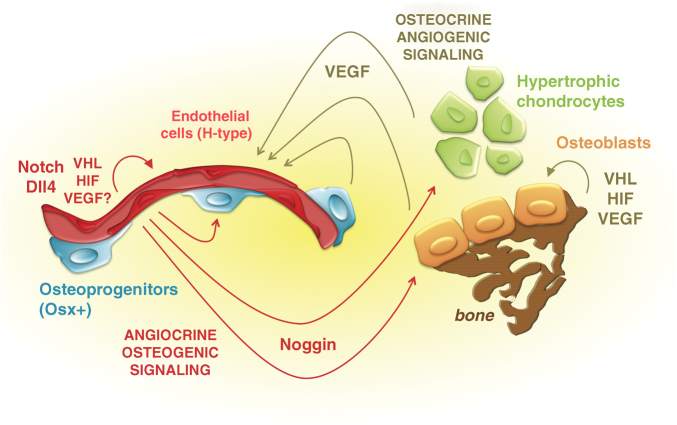
Angiogenic–osteogenic coupling mediated by bidirectional cross talk between chondro-/osteoblast lineage cells and skeletal endothelial cells. Schematic view of the dynamic and reciprocal interplay between the different cell types in the bone environment that couples angiogenesis and osteogenesis. (Right and upper part) Previous work implicated the VHL–HIF–VEGF signaling pathway in the osteogenic compartment of the long bones as a key driver of angiogenic–osteogenic coupling by effecting both (i) cell autonomous roles in chondrocytes, osteoprogenitors and osteoblasts and (ii) VEGF-mediated paracrine effects on the blood vessels in the bone environment, stimulating angiogenesis (brown arrows and notifications). (Left and lower part) New work from the group of Ralf Adams (red in the scheme) identifies a specialized subtype of endothelial cells (ECs), constituting type H vessels in the metaphysis and endosteum. These ECs mediate the growth of the blood vessels in bone, through a tissue-selective mechanism of angiogenesis involving positive regulation by the VHL/HIF and Notch/Dll4 signaling pathways. Coupling of angiogenesis back to osteogenesis is mediated by osteo-regulatory signals produced by ECs (angiocrine, osteogenic signaling). Among the angiocrine signals released from H-type vessels, Noggin stood out as the candidate most influential in mediating the osteogenic effects; addition of recombinant Noggin rescued the abundance of perivascular osteoprogenitors, the growth plate abnormalities and the ossification defect in mice carrying EC-specific loss-of-function mutations in the Notch pathway.
Conversely, the osteo-chondrocytic cellular compartments of the bones are known to determine the growth and integrity of the vascular bed. Studies using genetically altered mice and modulatory compounds have provided compelling evidence that the angiogenic growth factor VEGF is critical in driving angiogenesis in developing, growing and healing long bones.11,12,13,14,15,16 However, paracrine VEGF-driven effects on angiogenesis represent only one aspect of the complex interplay between the chondrocyte and osteoblast lineage cells and the vascular supply—and hence the oxygen supply—to these cells. Indeed, the cellular oxygen sensing machinery has crucial roles in chondrocytes, osteoprogenitors and osteoblasts, both by regulating their proliferation, differentiation and/or survival cell-autonomously and by controlling their production of VEGF that itself can also affect these processes by acting in auto-, intra- and paracrine ways7,17,18,19,20,21 (Figure 1). The key players in coupling the sensing of a relative hypoxic state to cellular responses attempting to cope with this state or to remediate it are the transcription factor hypoxia-inducible factor (HIF) and its upstream regulatory components, particularly the prolyl 4-hydroxylases (PHDs) and the Von Hippel–Lindau (VHL) protein.22 Both of these components represent negative regulators of HIF: when sufficient oxygen is available, and the hypoxia-adaptive actions of the HIF are consequently unnecessary, the PHD enzymes mediate the oxygen-dependent hydroxylation of residues in the α subunit of the HIF-1 protein, rendering it a target for the E3 ubiquitin ligase VHL and subsequent proteasomal degradation. In hypoxic conditions––or in experimental, pathological or pharmacological circumstances of PHD or VHL inhibition––the HIF-1α protein is stabilized and can execute its comprehensive transcriptional program. This includes the activation of genes and pathways involved in angiogenesis, erythropoiesis and cellular metabolism, for instance stimulating oxygen-sparing glycolysis.22 Mice with osteoblast-specific deletion of VHL developed extremely dense, heavily vascularized long bones, associated with expression of high levels of VEGF.7 Conversely, inactivation of HIF-1α in mature osteoblasts led to reductions in the bone and blood vessel volume.7 Thus, ample previous studies identified VEGF and hypoxia-signaling components as key molecular players of angiogenic–osteogenic coupling, as viewed from the osteogenic perspective5,6,18 (Figure 1, upper and right side of the scheme depicting previously published findings in brown).
New work by the group of Ralf Adams, recently published as a Letter and an Article in Nature,23,24 for the first time studied signaling in vivo in the endothelial cell (EC) compartment of the skeleton directly, by generating EC-specific conditional knockout (cKO) mice (Figure 1). The potential problems of embryonic lethality or adverse systemic effects that could be associated with the induction of a generalized mutation in the vascular system throughout the body were circumvented in this work by using the tamoxifen-inducible Cre-ERt system in postnatal life. Specifically, a variety of iEC-cKO mice were generated by crossing mice carrying floxed alleles of the target genes with the Cdh5(PAC)-CreERT2 driver to mediate EC-specific recombination following tamoxifen administration.
In their article, the authors have analyzed the microarchitecture and properties of the bone's vascular system, using advanced fluorescent staining techniques to visualize EC markers in thick sections of murine long bones, and FACS sorting of specific cell populations followed by molecular characterization of their transcriptome.23 On the basis of these results, they propose that the vasculature of long bones can be seen as a continuum of two subtypes of interconnected blood vessels that have distinct morphological, molecular and functional properties. They term these H-type and L-type vessels, on the basis of expression levels of CD31 (PECAM-1) and Endomucin (Emcn) in the ECs that make up their walls. CD31high/Emcnhigh cells (defining ‘H-type vessels') typify the blood vessels at the endosteal surfaces and those in the metaphysis, which run as longitudinal tubes toward the chondro–osseous junction of the growth plate, where they form arch-shaped vessel loops as their distal ends reach the terminal row of hypertrophic chondrocytes. In contrast, the lining of the sinusoidal capillaries deeper in the diaphysis consists of CD31low/Emcnlow cells (constituting the ‘L-type vessels' of the bone marrow vasculature).
Interestingly, although the CD31high/Emcnhigh ECs represent only a small fraction of the total endothelium in a long bone, particularly the H-type microvessels appear to be driving vascular growth in bone and express distinct sets of molecules including several growth factors that upon secretion (as so-called ‘angiocrine signals') could modulate osteoblast lineage cells. Moreover, perivascular osteoprogenitors, including those expressing Osterix (Osx+), were found to selectively associate with type H endothelium in the metaphysis and endosteum and were not found around diaphyseal type L vessels (Figure 1). In long bones of ageing mice, concomitant with the age-related loss of bone mass, both the type H vessels and the abundance of the osteoprogenitor populations drastically reduced.23
Functional differences between H- and L-type vessels may affect also the oxygenic and metabolic microenvironment they generate. For instance, hypoxia detection was strongest in the diaphysis, whereas it was low in the metaphysis, and the presence of the HIF-1α protein and some of its transcriptional targets followed this pattern. Yet, ECs of the CD31high/Emcnhigh signature expressed higher levels of HIF-1α compared with CD31low/Emcnlow cells, and H-type vessels were more sensitive of loss of HIF-1α compared with L-type vessels. Indeed, when Kusumbe et al.23 inactivated HIF-1α in the Cdh5(PAC)-CreERT2+ endothelium, a phenotype of severely reduced metaphyseal and endosteal type H vascularization resulted in the HIF-1α iEC-cKO mice, whereas the diaphyseal type L vessels were maintained. The vascular defects were associated with reduced prevalence of perivascular osteoprogenitors. The opposite was seen when the negative regulator of HIF signaling VHL was inactivated using the same targeting strategy: these mice had expanded metaphyseal vascularization, increased Runx2+ and Osx+ osteoprogenitors, enhanced bone formation and a higher trabecular bone volume.23
Thus, these data underscore the prime role of the hypoxia-signaling pathway in angiogenic–osteogenic coupling, acting both in the endothelial and in the osteogenic compartments. The precise mechanisms that mediate the coupling remain to be determined, but the fact that the phenotypic outcome of the genetic mutations in the VHL/HIF axis in either ECs or OBs appears to be strikingly similar may argue in favor of common (secreted) signals driving the cross talk between the two cell lineages. Although not further explored in the current work, VEGF could be a coupling factor. Altering its expression in ECs in the current studies (by targeting HIF) might have jeopardized the beneficial effects of its autocrine actions in the ECs proper,25 thereby resulting in reduced secretion of other angiocrine, osteogenic factors from the endothelium. This could perhaps explain the reduced numbers of osteoprogenitors observed in the HIF-1α iEC-cKO model. Altered levels of EC-derived VEGF secreted in the bone microenvironment (speculatively increased in the VHL and decreased in the HIF-1α iEC-cKO models) could also have contributed directly to the changes in ossification due to its paracrine stimulatory effects on osteoblast lineage cells. VEGF is known to regulate the migration, proliferation and differentiation of osteogenic cells (reviewed in Dirckx et al.10), and its temporary overexpression in postnatal long bones has previously been shown to result in a marked phenotype of aberrant angiogenesis and osteogenesis, bone marrow fibrosis and hematopoietic alterations.13
It is important to recognize though that the VHL/HIF pathway regulates several other targets, including cellular metabolic pathways, which might have been altered in the mouse models studied here. It would be of interest to learn more about the potential contribution of this aspect in the observed phenotypes. In addition, the potential role of Noggin in this model (see below) also needs further consideration. Another aspect that remains to be clarified is how to reconcile the seemingly contradictory aspect on the location of hypoxia (particularly in the L-type vascularized regions) and the expression and requirement of HIF selectively in the H-type endothelium.
In any case, the discovery of the role of endothelial VHL/HIF signaling in mediating angiogenic–osteogenic coupling is likely to boost therapeutic exploration of this pathway in low bone mass disorders such as osteoporosis. As already shown by Kusumbe et al. in this paper, the age-related decline in type H capillaries and associated Osx+ osteoprogenitors could be reversed by pharmacological treatment of aged mice with the PHD inhibitor deferoxamine mesylate (DFM), resulting in a restoration of the bone mass.23
In the second paper of the Adams group, Ramasamy et al.24 report on the use of the same recombination strategy to analyze the role of Notch signaling in the endothelium of bone. In the vascular system of most other organs, with the exception of the liver, the Notch signaling pathway is a major determinant of the process of vascular expansion through sprouting angiogenesis. In brief, an endothelial cell at the forefront of the vessel sprout is induced to extend abundant filopodia and lead the growth of the sprout toward the angiogenic stimulus, typically a high concentration of VEGF such as emitted from an hypoxic region. High VEGF levels induce migration and proliferation in these endothelial cells, and strong expression of the Notch ligand Delta like ligand 4 (Dll4). Through cell–cell contact-dependent signaling between endothelial cells, Dll4 activates Notch in the neighboring endothelial cells, which results in blunting of the cell's responsiveness to VEGF signaling (by downregulating VEGFR2 (Flk-1/KDR) and by upregulating the decoy receptor soluble VEGFR1 (sFlt-1)), limiting sprouting and mitosis and inducing quiescence; these cells will make up the lumen of the growing vasculature.26 Thus, in general, Notch negatively regulates angiogenesis.
Interestingly, analysis of long bones from Notch loss-of-function and gain-of-function iEC-cKO mice provides evidence that the mechanism through which Notch controls vascular growth in bone differs from this classic sprouting angiogenesis model active in other tissues and in tumors.24 Indeed, induction of EC-specific loss of Notch and Dll4 signaling in 2-week-old mice resulted, by 4 weeks of age, in a drastic reduction of the number of blood vessels growing toward the growth plate in the metaphysis, associated with reduced EC proliferation in these (H-type) vessels. The defective vascularization was associated with impaired bone lengthening, severely disorganized growth plates characterized by an enlargement of the zone of hypertrophic chondrocytes that, however, virtually lacked Sox9 and VEGF expression, and reduced bone formation and trabecular bone volume. Although both the number of early osteoblast precursors (Runx2+ cells) and the expression levels of mature osteoblast markers were profoundly reduced, the abundance of Osx+ osteoprogenitors in the metaphysis was increased. The opposite phenotype was observed in the gain-of-function mutants, which showed increased numbers of vessel arches running longitudinally toward the growth plate. Interestingly, Notch signaling in the endothelium of bone led to upregulation of VEGFR2 and downregulation of sFlt-1, precisely opposite to the normal consequences of endothelial Notch/Dll4 signaling. Together, these data make a convincing case that Notch signaling in the ECs of postnatal long bones promotes endothelial cell proliferation and vessel growth, and couples the vascular effects to effects on the growth plate chondrocytes and osteoblast lineage cells, affecting bone growth and ossification.24
To identify potential downstream mediators of the coupling event, the authors surveyed a number of candidate growth factors, including TGF-β, BMPs, FGFs and Wnts, in bone-derived ECs sorted from mice carrying a Notch gain-of-function mutation. The expression of the secreted BMP antagonist Noggin was most pronouncedly affected, being upregulated upon gain-of-function and downregulated upon loss-of-function of endothelial Notch signaling. This finding led the authors to test whether the administration of recombinant Noggin could rescue the phenotype of the iEC-cKO Notch pathway mutants. Notably, this treatment resulted in a normalization of the bone formation rate, the presence of the Osx+ osteoprogenitors in the vascular wall and the organization of the growth plate, and even corrected the vascular phenotype itself. Thus, Notch signaling in ECs of bone mediates the angiocrine release of Noggin into the bone environment, coupling modulations in the vascular system to the functioning of osteoprogenitors, osteoblasts and chondrocytes24 (Figure 1).
An important conceptual advance stemming from these papers is the notion that metaphyseal type H blood vessels may have a unique molecular signature. The vessels approaching the growth plate are described morphologically as endothelial columns, interconnected by arch-shaped vessel loops at their distal end, which extend budding, blind-ended, lumen-containing endothelial protrusions and filopodia toward the chondrocytes during active growth,23,24 corresponding to earlier observations made by vascular perfusion methods, corrosion casting, electron microscopy and histology.27,28 Interestingly, this description correlates very closely with that of vessels invading the cartilage of the fetal bone template during skeletal development and of the callus tissue during fracture healing.8 These neo-angiogenesis processes mediate the conversion of the avascular cartilaginous template into actual bone and bone marrow tissue, much like the process occurring at the growth plate. It will be interesting to clarify whether these vessels fit the type H definition and whether the mechanism of vascular growth in fetal life and during bone regeneration corresponds to the unique Notch-stimulated mode of angiogenic growth described here to mediate postnatal skeletal growth. We do know that in all these settings, VEGF is a prime driver of the angiogenic process;11,12,13,14,15,16 it is now to be determined in follow-up studies how VEGF functions upstream of Notch signaling in the bone ECs––according to the classical mechanism operating in ECs of other tissues, VEGF would be expected to induce Dll4 and Notch signaling.
In conclusion, the studies published by the Adams team23,24 for the first time start to shed light on the molecular specialization and functional organization of the peculiar bone endothelium, on the intracellular signaling cascades regulating the angiogenic processes that are crucial to drive long bone growth and homeostasis, and on the EC-secreted angiocrine factors that communicate essential signals to chondrocytes, perivascular osteoprogenitors and osteoblasts in vivo. These new insights into the angiogenic–osteogenic coupling paradigm will help ongoing and future research aimed at answering emerging questions such as whether these mechanisms act universally in settings of bone formation, which roles are played by the H-type and the L-type vessels in supporting hematopoiesis and in providing entry sites and niches for metastasizing tumor cells homing to bone, and whether these findings can be effectively translated to angio–osteo–anabolic therapeutic applications to battle osteoporosis and promote bone repair and regeneration.
Acknowledgments
The authors' research is supported by grant 282131 from the European Research Council under the European Union's Seventh Framework Programme (FP7/20072013) to CM, FWO G.0795.14N to CM and NIH grant AR049410 to TC. TC is also supported by a Senior Career Scientist Award from the Veterans Administration.
Footnotes
The authors declare no conflict of interest.
References
- Maes C. Role and regulation of vascularization processes in endochondral bones. Calcif Tissue Int 2013;92:307–323 [DOI] [PubMed] [Google Scholar]
- Lafage-Proust MH, Prisby R, Roche B, Vico L. Bone vascularization and remodeling. Joint Bone Spine 2010;77:521–524 [DOI] [PubMed] [Google Scholar]
- Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 2009;174:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B et al. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 1987;8:157–164 [DOI] [PubMed] [Google Scholar]
- Riddle RC, Khatri R, Schipani E, Clemens TL. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med 2009;87:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 2009;24:1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert S et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 2007;117:1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 2010;19:329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336 [DOI] [PubMed] [Google Scholar]
- Dirckx N, Van Hul M, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today 2013;99:170–191 [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 1999;5:623–628 [DOI] [PubMed] [Google Scholar]
- Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D et al. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev 2002;111:61–73 [DOI] [PubMed] [Google Scholar]
- Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J 2010;29:424–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Oh CD, Akiyama H et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development 2009;136:1263–1272 [DOI] [PubMed] [Google Scholar]
- Jacobsen KA, Al Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res 2008;23:596–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr., Ferrara N et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 2002;99:9656–9661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS et al. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci 2010;1192:322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Carmeliet G, Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol 2012;8:358–366 [DOI] [PubMed] [Google Scholar]
- Maes C, Araldi E, Haigh K, Khatri R, Van Looveren R, Giaccia AJ et al. VEGF-independent cell-autonomous functions of HIF-1alpha regulating oxygen consumption in fetal cartilage are critical for chondrocyte survival. J Bone Miner Res 2012;27:596–609 [DOI] [PubMed] [Google Scholar]
- Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N et al. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 2012;122:3101–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev 2001;15:2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 2012;148:399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014;507:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014;507:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007;130:691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146:873–887 [DOI] [PubMed] [Google Scholar]
- Wirth T, Syed Ali MM, Rauer C, Suss D, Griss P, Syed Ali S. The blood supply of the growth plate and the epiphysis: a comparative scanning electron microscopy and histological experimental study in growing sheep. Calcif Tissue Int 2002;70:312–319 [DOI] [PubMed] [Google Scholar]
- Roche B, David V, Vanden-Bossche A, Peyrin F, Malaval L, Vico L et al. Structure and quantification of microvascularisation within mouse long bones: what and how should we measure? Bone 2012;50:390–399 [DOI] [PubMed] [Google Scholar]