Abstract
Abstract
Aim: The aim of our study was to investigate the gene and serum protein expression profiles of IL-8 in colon cancer and associated hepatic metastasis and to correlate these results with clinicopathologic variables of the patients.
Materials and methods: IL-8 was evaluated by qPCR and ELISA in a total number of 62 colon cancer patients (n=42 by qPCR and n=20 by ELISA) in normal and tumoral tissue specimens and serum samples respectively. Additionally synchronous metastasis from 5 of these patients were also collected at the time of surgery and analyzed by qPCR.
Results: IL-8 was up regulated in all analyzed tumoral samples compared with normal tissue (P-value = 0.01) and higher expressed in metastatic tissues compared with tumoral tissues (P -value= 0.03). The median expression of IL-8 in patients over 60 years old was found to be higher compared with the median expression of IL8 in patients less than 60 years old (3.89 compared with 14.69, P -value= 0.005). According to tumor grading, we found that IL-8 in tumors with well differentiated adenocarcinoma have a median mRNA expression of 9.78 compared with a median mRNA IL8 expression of 26.63 in moderate or poor differentiated adenocarcinoma.
Levels of IL-8 determined in serum were statistically significant correlated with preoperative carcinoembryonic antigen level (P -value= 0.003, R=0.57) and with distant metastasis (P-value =0.008). Serum level of IL-8 increased proportionally along with TNM tumor stage and was found to be statistically significant correlated with C-reactive protein (P -value, R=0.64). Colon cancer patients had higher IL-8 levels as determined by ELISA (median value= 29.64 pg/ml) compared with healthy controls (median value= 4.86 pg/ml).
Discussions: Our results provide additional support for the role of inflammation in colon cancer and indicate that IL-8 could be further validated in association with other already used markers for prognostic and diagnostic of evolutional disease in colon cancer patients.
Brief abstract
By investigating the gene and serum protein expression profiles of IL-8 in colon cancer and associated hepatic metastasis, we found correlations between these results and clinicopathological variables of the patients. IL-8 is involved in colon cancer progression and could be monitored in a panel with other biomarkers as an early indicator of the tumor’s evolution.
Keywords: interleukine-8, biomarkers, colon cancer, metastasis, qPCR
Introduction
It is well established that there is a profound link between colon cancer and chronic inflammation [1,2]. Infectious agents lead to chronic inflammation at the site of tumor development [3], which attributes to the inflammation of a causative part in [4] the initiation, promotion and progression of cancer.
IL-8 (CXCL8) is involved in a variety of physiopathological processes [5] and along with other members of TNF superfamily were shown to be involved in the proliferation, invasion and metastasis of several cancer [6,7] including colon cancer [8,10].
IL-8 is a member of neutrophil-specific CXC-chemokines family with ELR (Glu-Leu-Arg) motif [11,12] that belong to G-protein-coupled receptor family [13] and exert its function through biding to two receptors, CXCR1 and CXCR2. Its activation is mediated through NF-kB pathway [14].
At the tumoral level, IL-8 influences the tumor growth, survival, invasion, angiogenesis, metastasis [15], resistance and recurrence [8] and its overexpression is associated with a poor prognosis [16].
The aim of our paper was to investigate the potential links between gene and protein IL-8 expression with clinicopathologic variables of colon cancer patients and to evaluate its role in colon cancer progression.
Materials and methods
Patient selection and classification The ethics committee of Fundeni Clinical Institute approved our study according to the in force legislation. All the patients signed a written informed consent. A total number of 62 patients with sporadic colon cancer were used in our study (n=42 for transcriptomic study and n=20 for ELISA tests). Additionally, blood was drawn from 20 healthy subjects who represented the control group for ELISA tests. All the patients had no records of oncological treatment prior to surgery. Clinicopathologic data are shown in Table 1.
Table 1.
Clinicopathologic features of the patients
| Parameter | N ( %) |
|---|---|
| Age, years (average ± SD) | 63.94 ± 8.5 |
| Men | 39 (62.9) |
| Women | 23 (37.1) |
| TNM tumor stage | |
| I | 3 (4.8 ) |
| II | 20 (32.3) |
| III | 20 ( 32.3) |
| IV | 19 (30.6 ) |
| Lymph node status | |
| 0 | 26 (42.0) |
| 1 | 17 (27.4) |
| 2 | 19 (30.6 ) |
| pT | |
| 2 | 5 (8.1) |
| 3 | 56 (90.3) |
| 4 | 1 (1.6) |
| Distant metastasis | |
| M0 | 43 (69.4) |
| M1 | 19 (30.6 ) |
| Differentiation degree | |
| G1 | 47 (75.8 ) |
| G1-G2 | 7 (11.3) |
| G2 | 6 (9.7) |
| G3 | 2 (3.2) |
Tissue and serum sample collection
Blood samples were collected preoperatively for each patient. Blood was drawn under fasting conditions in vacutainers without anticoagulant and serum was collected after centrifugation and stored at -80°C.
During each patient’s surgery, normal and tumoral samples were collected. In the case of 5 patients, specimens from synchronous metastasis were also collected. A trained pathologist made a histological diagnostic from samples immersed in formalin and then paraffin embedded. The transcriptomic study was performed by using tissue samples that were immediately snapped frozen in liquid nitrogen.
RNA extractions
Total RNA was isolated from normal, tumoral and when the case from metastatic samples by means of tri reagent (Sigma, St. Louis, MO) according to the manufacturer`s instructions. Purification was done with RNeasy Mini Kit (QIAGEN, Valencia, CA). The quantity and quality of the total RNA were assessed by spectrophotometry (Nano Drop 1000; Thermo Scientific, Arlington, TX) and by lab-on-a-chip Agilent 2100 technology (Agilent Technology, Santa Clara, CA). All samples had a 260:280 nm ratio greater than 1.8, a 28S:18S ratio greater than 1.5, and an RNA integrity number greater than 7.
Real-time qPCR
cDNA was obtained from 2 µg of total RNA, by using High Capacity cDNA Archive Kit (ABI, Foster City, CA) in a total volume of 20 µL. Samples were diluted to 2 ng/µL, and qPCR amplification was performed in triplicate for each sample in a total volume of 25 µL under the following conditions: 95°C for 10 minutes, 95°C for 15 seconds, and 1 minute at 60°C for 40 cycles.
The 2-step relative quantification was performed with the 7300 Real-Time PCR (ABI, Foster City, CA) system with hydrolysis probes labeled with 6-carboxyfluorescein. Normal tissues were used as control and mRNA was normalized to reference genes RPLP0 (20X). Data were analyzed with SDS 1.4 software by using the comparative Ct method [2^(-delta delta Ct)]. The tested gene was IL-8 (Hs00174103_m1).
CEA testing
Carcinoembryonic antigen (CEA) was assessed for each patient by chemiluminescence in preoperative serum samples by using a commercial kit (Cobas Core, Roche Diagnostic Systems).
Enzyme-linked immunosorbent assay
Serum concentration of IL-8 was determined by using a sandwich ELISA system (Uscn Life Science, Wuhan, China) according to the manufacturer’s instructions. The color change was detected spectrophotometrically at a wavelength of 450 nm and the concentrations of the samples were interpolated on the standard curve.
C-reactive protein (CRP)
The C-reactive protein (CRP) levels were measured by means of a commercially available assay by using Abbott c8000 automated equipment according to the manufacturer protocol (Abbott, North Chicago, IL).
Statistical analysis
Calculations were performed with Graph Pad Prism 5 (Graph Pad Software Inc, San Diego, CA). Data was tested for normal distribution by using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Comparisons among the groups were made by means of the Kruskal-Wallis and Mann Whitney U tests. A P-value less than or equal to 0.05 was considered statistically significant.
Results
Expression of mRNA IL-8 in colon cancer tissues
mRNA IL-8 gene expression was found to be up-regulated in tumoral tissue compared with normal tissues (P -value= 0.01). In patients in whom the metastatic tissue was available, IL-8 mRNA was found to be higher expressed compared with the tumoral tissue (P -value= 0.03) (Fig. 1).
Fig. 1.
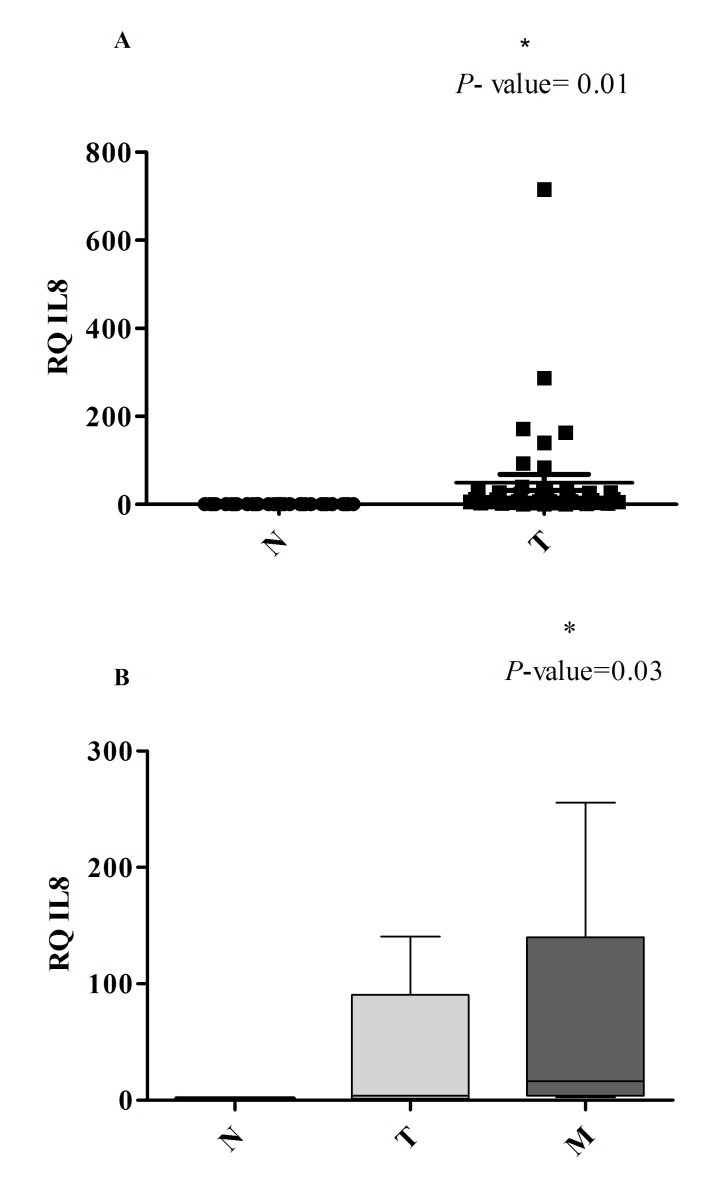
qPCR measurements of IL-8 gene expression in (A) tumoral tissue compared with normal tissue and (B) tumoral and metastatic tissue compared with normal tissue. The values are expressed as means of three independent replicates
The analysis of IL-8 gene expression in correlation with the clinicopathologic features of the patients revealed a differential expression in different patients groups (Fig. 2).
Fig. 2.
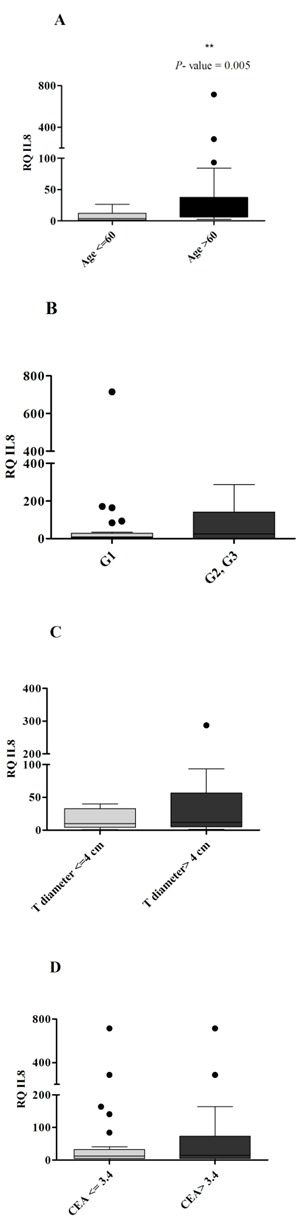
Differential IL-8 gene expression in (A) patients below and above 60 years old (B) well differentiated and moderate and poor differentiated tumors (C) tumors with the diameter less than or greater than 4 cm (D) patients with CEA below or greater than 3.4 ng/ml
Association between IL-8 assessed by ELISA and clinicopathologic parameters of the patients
Enzyme linked immunosorbent assay (ELISA) on the serum samples detected measurable levels in 90% of the tested samples for IL-8.
The levels of IL-8 determined by ELISA in the serum of the patients were found to be statistically correlated with the level of the carcinoembryonic antigen (CEA) determined in the preoperative serum of the patients (P-value =0.003, R= 0.64), with tumor stage (P -value =0.01, R= 0.57), with distant metastasis (P-value =0.008) and with C-reactive protein (P -value =0.003, R= 0.64).
IL-8 concentration was found to be statistically higher (P -value =0.004) in colon cancer patients (median value= 29.64 pg/ml) compared with the concentration of IL-8 determined in a group of 20 healthy controls (median value= 4.86 pg/ml) (Fig. 3A). The area under the curve for IL-8 was determined to be 0.765 (95%CI: 0.609-0.922).
The level of IL-8 determined by ELISA was found to increase from tumors with stage I and II TNM to stage III TNM and to stage IV TNM (P -value= 0.027). The same tendency was observed for C-reactive protein (Fig. 3 B).
Fig. 3.
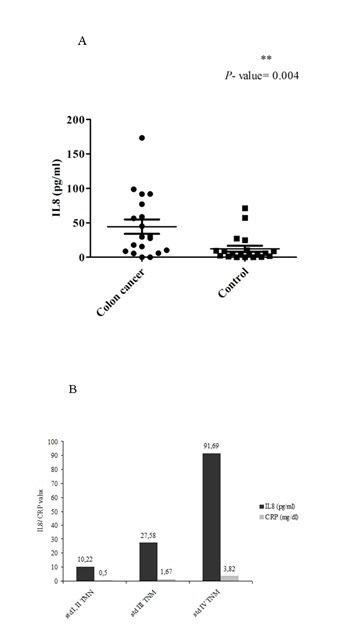
(A) IL-8 assessed by ELISA in colon cancer patients versus control subjects and (B) IL-8 assessed by ELISA and CRP in colon cancer patients with tumors stage I and II, III and IV TNM
Assessing the serum level of IL-8 depending on lymph node stage (number of negative/ positive lymph nodes) we found out that in patients without affected lymph nodes, the level of IL-8 is 14.0 pg/ml while in patients with one or more than one affected lymph nodes the level of IL-8 increases to 56.52 pg/ml.
Discussions
The goal of our study was to evaluate the involvement of IL-8 in colon cancer progression by assessing its gene and protein expression with clinicopathologic features of the patients.
IL-8 is produced by both normal as well as tumor cells and is implicated in the initiation and amplification of inflammatory processes that occur in cancer [17,18].
In normal cells, IL-8 is secreted at very low levels [14] but its production is stimulated by other cytokines [19,21], bacteria [22,23] or stress [24,26].
IL-8 favors both metastatic spread of cancer cells as well as angiogenesis and tumor growth [27,16].
In our study, we showed that IL-8 is unregulated in all analyzed tissues and its mRNA level increases from normal to tumoral and further to metastatic tissue. Further different gene expression level for IL-8 was obtained according to tumor grading (well differentiated versus moderate and poorly differentiated adenocarcinoma).
Our data demonstrate a statistical correlation between IL-8 determined by ELISA and clinicopathologic features of the patients.
IL-8 measured by ELISA was able to significantly distinguish between case and control groups.
Moreover, a statistical correlation between IL-8 with CRP is suggestive for the inflammatory component of colon cancer.
The results obtained in our study demonstrated that IL-8 has a higher gene and protein expression in colon cancer samples and its level increases as the disease progresses and metastasizes.
IL-8 is an important cytokine that is involved in colon cancer progression and could be monitored in panel with other biomarkers as an early indicator of the tumor’s evolution.
Footnotes
Founding source: This study was financially supported by the Romanian Program for Research, Development and Innovation project VALICC, research grant PN II, 42-165/2008.
Disclosures and Conflicts of Interest: None specified
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? The Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Monteleone G, Pallone F. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13:11071–11084. doi: 10.3390/ijms130911071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos-Nino E. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013 doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Wolf M. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Yoo KH, Park YK. DNA hypomethylation of interleukin 8 in clear cell renal cell carcinoma. Oncol Lett . 2012;1:39–42. doi: 10.3892/ol.2012.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Lee YS, Choi I. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LC, Hao CY. Alteration of gene expression in normal-appearing colon mucosa of APC(min) mice and human cancer patients. Cancer Res. 2004;64:3694–3700. doi: 10.1158/0008-5472.CAN-03-3264. [DOI] [PubMed] [Google Scholar]
- 10.Klampfer L. Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets. 2011;11:451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harada A, Sekido N. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 12.Lee YS, Choi I. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidemann J, Ogawa H. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann E, Dittrich-Breiholz O. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 15.Yuan A, Chen JJ. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 16.Ning Y, Manegold PC. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cacev T, Radosević S. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis. 2008;8:1572–1580. doi: 10.1093/carcin/bgn164. [DOI] [PubMed] [Google Scholar]
- 18.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/s1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 19.Ogami K, Yamaguchi R. Computational gene network analysis reveals TNF-induced angiogenesis. BMC Syst Biol. 2012;S12:2–12. doi: 10.1186/1752-0509-6-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasahara T, Mukaida N. IL-1 and TNF-alpha induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line Immunology. 1991;74:60–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Brasier AR, Jamaluddin M. A promoter recruitment mechanism for tumor necrosis factor-alpha-induced interleukin-8 transcription in type II pulmonary epithelial cells. Dependence on nuclear abundance of Rel A, NF-kappaB1, and c-Rel transcription factors . J. Biol. Chem. 1998;273:3551–3561. doi: 10.1074/jbc.273.6.3551. [DOI] [PubMed] [Google Scholar]
- 22.Aihara M, Tsuchimoto D. Mechanisms involved in Helicobacter pylori-induced interleukin-8 production by a gastric cancer cell line. MKN45 Inf. Immun. 1997;65:3218–3224. doi: 10.1128/iai.65.8.3218-3224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbie S, Chen LM. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 24.Shahzad MM, Arevalo JM. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeForge LE, Preston AM. Regulation of interleukin 8 gene expression by oxidant stress. J. Biol. Chem. 1993;268:25568–25576. [PubMed] [Google Scholar]
- 26.Lee LF, Haskill SJ. Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol. Cell. Biol. 1997;17:5097–5105. doi: 10.1128/mcb.17.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doll D, Keller L. Differential expression of the chemokines GRO-2, GRO-3 and interleukin-8 in colon cancer and their impact on metastasis disease and survival. Int J Colorectal Dis. 2010;25:573–581. doi: 10.1007/s00384-010-0901-1. [DOI] [PubMed] [Google Scholar]


