Abstract
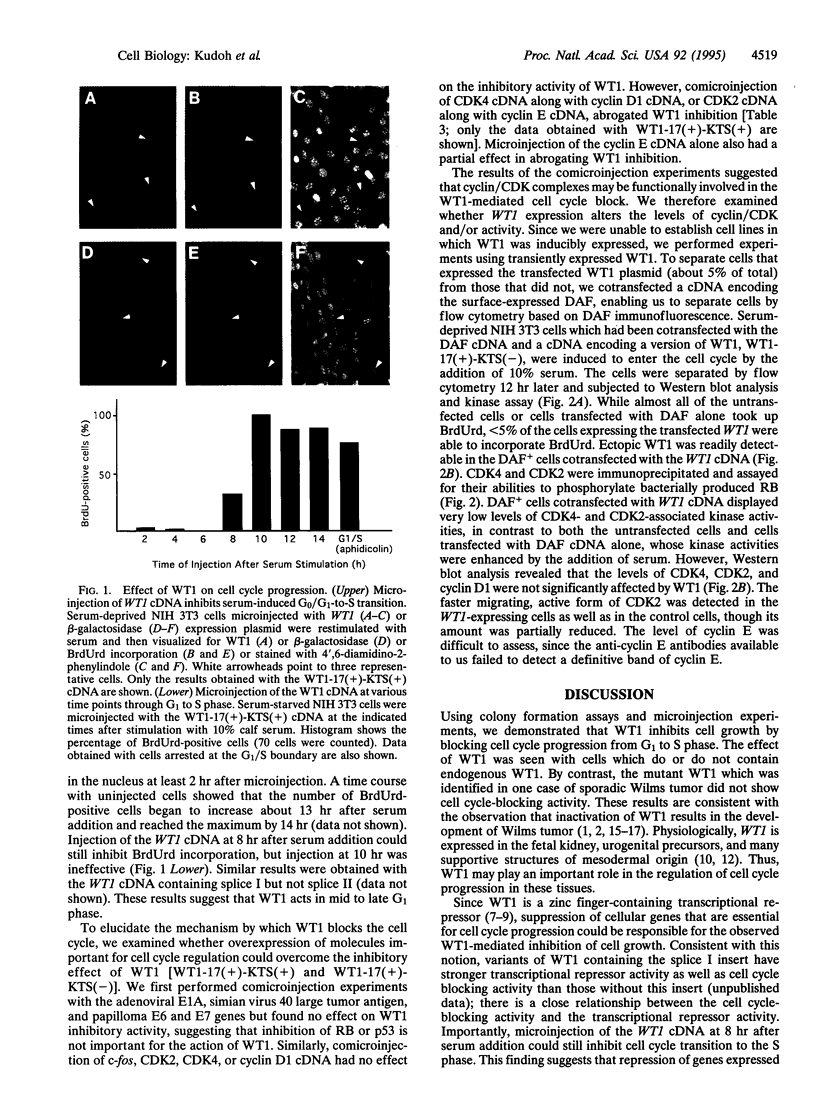
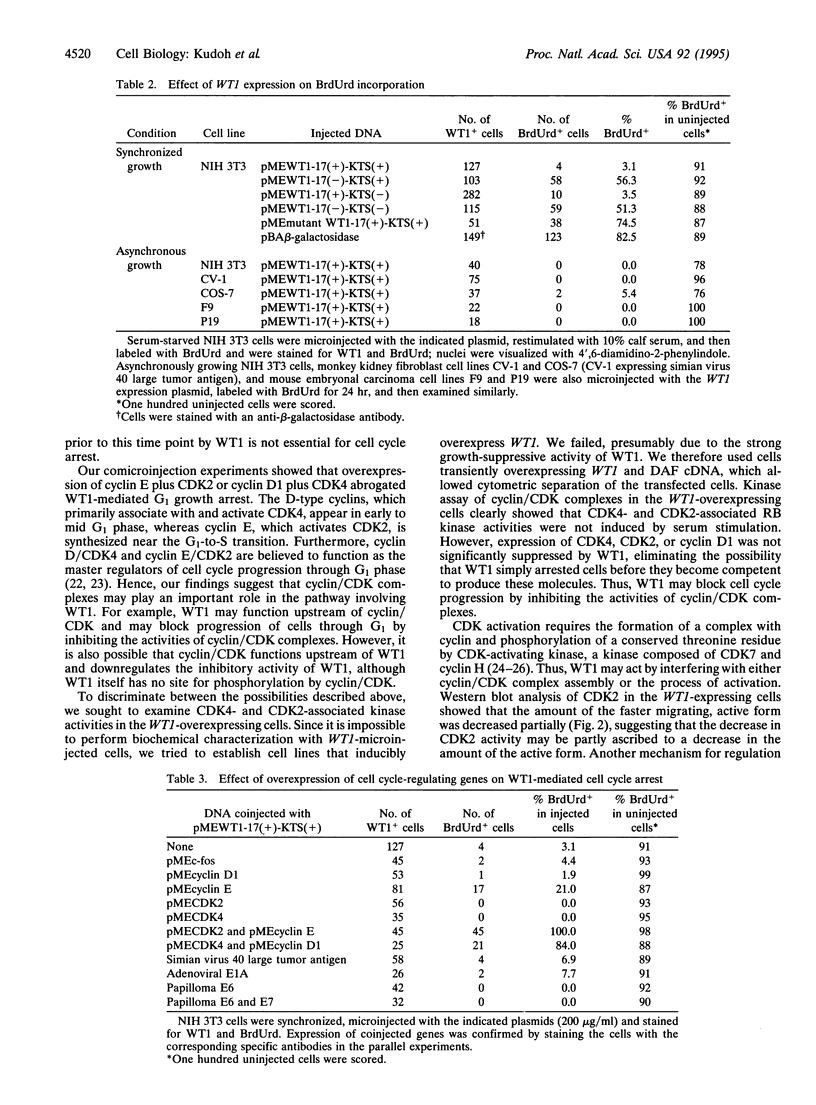
WT1, the Wilms tumor-suppressor gene, maps to the human chromosomal region 11p13 and encodes a transcriptional repressor, WT1, implicated in controlling normal urogenital development. Microinjection of the WT1 cDNA into quiescent cells or cells in early to mid G1 phase blocked serum-induced cell cycle progression into S phase. The activity of WT1 varied significantly depending on the presence or absence of an alternatively spliced region located upstream of the zinc finger domain. The inhibitory activity of WT1 was abrogated by the overexpression of cyclin E/CDK2 as well as cyclin D1/CDK4. Furthermore, both CDK4- and CDK2-associated kinase activities were downregulated in cells overexpressing WT1, whereas the levels of CDK4, CDK2, and cyclin D1 expression were unchanged. These findings suggest that inhibition of the activity of cyclin/CDK complexes may be involved in mediating the WT1-induced cell cycle block.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ohuchi T., Sumida S., Matsumoto K., Toyoshima K. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Fisher R. P., Morgan D. O. A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell. 1994 Aug 26;78(4):713–724. doi: 10.1016/0092-8674(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Turck C. W., Morgan D. O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993 Dec 16;366(6456):707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Park S., Maheswaran S., Englert C., Re G. G., Hazen-Martin D. J., Sens D. A., Garvin A. J. WT1-mediated growth suppression of Wilms tumor cells expressing a WT1 splicing variant. Science. 1993 Dec 24;262(5142):2057–2059. doi: 10.1126/science.8266105. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Sohn R. L., Buckler A. J., Pelletier J., Call K. M., Housman D. E. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Inoue K., Sugiyama H., Ogawa H., Nakagawa M., Yamagami T., Miwa H., Kita K., Hiraoka A., Masaoka T., Nasu K. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994 Nov 1;84(9):3071–3079. [PubMed] [Google Scholar]
- Inoue N., Kinoshita T., Orii T., Takeda J. Cloning of a human gene, PIG-F, a component of glycosylphosphatidylinositol anchor biosynthesis, by a novel expression cloning strategy. J Biol Chem. 1993 Apr 5;268(10):6882–6885. [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M. H., Prosser J., Condie A., Smith P. J., Van Heyningen V., Hastie N. D. Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4791–4795. doi: 10.1073/pnas.89.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Kato J. Y., Fisher R. P., Morgan D. O., Sherr C. J. Activation of cyclin-dependent kinase 4 (cdk4) by mouse MO15-associated kinase. Mol Cell Biol. 1994 Nov;14(11):7265–7275. doi: 10.1128/mcb.14.11.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994 Mar;14(3):2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H., Beran M., Saunders G. F. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992 May;6(5):405–409. [PubMed] [Google Scholar]
- Mäkelä T. P., Tassan J. P., Nigg E. A., Frutiger S., Hughes G. J., Weinberg R. A. A cyclin associated with the CDK-activating kinase MO15. Nature. 1994 Sep 15;371(6494):254–257. doi: 10.1038/371254a0. [DOI] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Park S., Schalling M., Bernard A., Maheswaran S., Shipley G. C., Roberts D., Fletcher J., Shipman R., Rheinwald J., Demetri G. The Wilms tumour gene WT1 is expressed in murine mesoderm-derived tissues and mutated in a human mesothelioma. Nat Genet. 1993 Aug;4(4):415–420. doi: 10.1038/ng0893-415. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K., Fleming S., Davidson D., Bickmore W., Porteous D., Gosden C., Bard J., Buckler A., Pelletier J., Housman D. The candidate Wilms' tumour gene is involved in genitourinary development. Nature. 1990 Jul 12;346(6280):194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]