Abstract
Objective:
To assess the one-year period prevalence of stimulant combination therapy and switching in children/ adolescents with attention deficit/hyperactivity disorder (ADHD) in Quebec, Canada.
Method:
Patients aged 6–17 years, with at least two ADHD diagnosis codes documented in different visits and at least 30 days’ supply of a stimulant during their most recent one-year observation period were selected from the Regie de l’assurance maladie du Quebec database (03/2007–02/2012). Combination therapy was defined as at least 30 consecutive days of concomitant use of multiple stimulants with different active moieties, or use of a stimulant and another psychotropic medication. Therapy switching was defined as a prescription claim for a new psychotropic medication less than 30 days before or after the end of supply of a stimulant. The one-year period prevalence of therapy combination and switching was calculated.
Results:
The one-year period prevalence of combination therapy and switching among 9,431 children and adolescents with ADHD treated with stimulants was 19.8% and 18.7%, respectively. The most frequent combination categories were atypical antipsychotics (AAP: 10.8%), atomoxetine (ATX: 5.5%) and clonidine (5.3%). The most frequent switched-to categories were other stimulants (7.9%), AAP (5.5%) and ATX (4.7%).
Conclusions:
Approximately one in five children/adolescents with ADHD on a stimulant experienced combination therapy or therapy switching; however, the majority of the medications used in combination or switching were not label-indicated for the treatment of ADHD in Canada. These results highlight the need for further research to evaluate the risk-benefit of stimulant combination and switching in children and adolescents with ADHD.
Keywords: combination therapy, ADHD, RAMQ, switching, stimulants
Résumé
Objectif:
Évaluer la prévalence sur une période d’un an de la traitement par combinaison et par changement de stimulants chez les enfants et les adolescents souffrant du trouble de déficit de l’attention avec hyperactivité (TDAH) au Québec, Canada.
Méthode:
Des patients de 6 à 17 ans, ayant au moins deux codes diagnostiques de TDAH documentés à différentes visites et une provision d’au moins 30 jours d’un stimulant durant leur plus récente période d’observation d’un an, ont été choisis dans la base de données de la Régie de l’assurance maladie du Québec (03/2007–02/2012). La traitement par combinaison a été définie comme étant au moins 30 jours consécutifs d’utilisation concomitante de multiples stimulants ayant différentes parties actives, ou d’utilisation d’un stimulant et d’un autre médicament psychotrope. La traitement par changement a été définie comme étant une demande de prescription d’un nouveau médicament psychotrope moins de 30 jours avant ou après la fin d’une provision d’un stimulant. La prévalence sur une période d’un an de la traitement par combinaison et par changement a été calculée.
Résultats:
La prévalence sur une période d’un an de la traitement par combinaison et par changement chez 9 431 enfants et adolescents souffrant de TDAH traités par stimulants était de 19,8% et 18,7%, respectivement. Les catégories de combinaison les plus fréquentes étaient les antipsychotiques atypiques (APA: 10,8%), l’atomoxétine (ATX: 5,5%) et la clonidine (5,3%). Les catégories pour lesquelles les changements se faisaient le plus souvent étaient d’autres stimulants (7,9%), les APA (5,5%) et l’ATX (4,7%).
Conclusions:
Environ un enfant/adolescent sur cinq qui souffrent de TDAH et prennent des stimulants ont fait l’expérience d’une thérapie par combinaison ou par changement; toutefois, la majorité des médicaments utilisés en combinaison ou pour le changement n’étaient pas indiqués sur l’étiquette pour le traitement du TDAH au Canada. Ces résultats font ressortir le besoin de plus de recherche pour évaluer les risques-avantages de la combinaison et du changement de stimulants chez les enfants et adolescents souffrant de TDAH.
Keywords: traitement par combinaison, TDAH, RAMQ, changement, stimulants
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a common chronic psychiatric disorder in children, affecting 2.6% of children in Canada (Brault & Lacourse, 2012), which normally persists into adolescence and adulthood (Adesman, 2001). According to studies using epidemiological and clinical samples, many patients with ADHD also have other psychiatric conditions, such as conduct disorders (30–50%), mood disorders (15–75%), and anxiety disorders (approximately 25%) (Spencer, 2006), with a considerable proportion (24.7%) having two or more psychiatric comorbidities (Jensen et al., 2001). Because of its chronic nature and comorbidities, ADHD poses a substantial economic burden to society. Although yet to be quantified in Canada, in the US the annual incremental societal costs among children and adolescents with ADHD are estimated at $38–$72 billion (in 2010 USD) (Doshi et al., 2012).
ADHD often requires multi-modal, including both behavioural and pharmacological, treatments. When choosing pharmacological therapy, the Canadian ADHD Resource Alliance (CADDRA) guidelines recommend stimulants as first-line treatment (CADDRA, 2011). Previous studies suggest that approximately 70% of patients respond to stimulants (Kratochvil, 2002; Olfson, 2004), and these patients continue to experience improvements in their ADHD symptoms for as long as two years (Barbaresi et al., 2006; Hechtman & Greenfield, 2003). Those unresponsive or intolerant to their initial stimulant monotherapy would require a change in treatment (Antshel et al., 2011; Cormier, 2008), e.g., combining the stimulant with or switching to a different stimulant or a non-stimulant. Two non-stimulants are currently approved by Health Canada for the treatment of ADHD: atomoxetine HCl (ATX; Strattera®), a selective norepinephrine reuptake inhibitor approved for monotherapy for children, adolescents, and adults, and guanfacine extended release (GXR; Intuniv®), a selective alpha-2a adrenergic receptor agonist approved for monotherapy for children (aged 6–12 years) and for adjunctive therapy to stimulants for the subgroup of children with a sub-optimal response to stimulants. In addition to the approved ADHD treatments, medications that are label-indicated for other psychiatric disorders are often prescribed off-label for the treatment of ADHD, including clonidine immediate release (IR), atypical antipsychotics (AAPs), typical antipsychotics (TAPs), bupropion, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs) and tricyclic anti-depressants (TCAs) (Czaja & Valuck, 2012; Kratochvil, 2002). Of these off-label medications, AAPs have received special attention for such off-label use because ADHD is among the top diagnoses associated with AAP prescriptions, which have increased five-fold from 1999 to 2008 (Alessi-Severini, Biscontri, Collins, Sareen, & Enns, 2012).
To date, there is limited understanding of overall treatment patterns among patients with ADHD in Canada. A 2012 study of Canadian children and adolescents with ADHD investigated the patterns of combination therapy and therapy switching within the class of stimulants (i.e. short-acting [SA] and long-acting [LA]) (Lachaine, Beauchemin, Sasane, & Hodgkins, 2012), observing 21.8% of patients combining and 29.1% switching between SA and LA stimulants. However, a comprehensive evaluation of treatment patterns including all medications (stimulants and non-stimulants, label-indicated and off-label) is lacking. Such real-world information about combination therapy and therapy switching is crucial, given the controversial use of therapies that are not label-indicated for ADHD (Chen, Gerhard, & Winterstein, 2009; Weiss et al., 2009; Zito et al., 2008).
In order to gain a better understanding of the real-world treatment patterns among children and adolescents with ADHD in Canada, the current study evaluated the one-year period prevalence of stimulant combination therapy and switching among children and adolescents with ADHD using a Canadian healthcare database for the province of Quebec.
Methods
Data Source
The Régie de l’assurance maladie du Québec (RAMQ) database is Quebec’s provincial health plan database, containing information on medical services for the entire population of Quebec (about 7.5 million lives covered annually) and prescription drug claims from the RAMQ prescription drug plan (about 3.3 million lives). Enrollees include recipients of last-resort financial assistance, people who are not eligible for a private insurance plan and their dependents, and people who are 65 years or older.
Sample Selection and Construction
For this study, children and adolescents with ADHD who were continuously enrolled in both medical and drug plans for at least 19 months between March 1, 2007, and February 29, 2012, and who had at least one stimulant prescription filled were selected. The most recent 19-month period was defined as the observation period, with the first six months of the observation period defined as the baseline period and the following 12 months defined as the study period (the first day of the study period defined as the index date). The 19th month was used to assess the total number of overlapping days between two treatments for patients who started one or both treatments during the last month of the study period. Patients were further required to have at least 30 consecutive days’ supply of a stimulant (see list in online Appendix A) during the study period. They were also required to have at least two documented diagnoses of ADHD (International Classification of Diseases, Version 9 [ICD-9] code: 314.0–314.9) at different visits: one prior to or on the date of the first stimulant prescription fill during the study period, and the other within 24 months of the date of the first stimulant prescription fill during the study period. To conform to the patient confidentiality guidelines, age was reported in groups with a two to three year interval in the datasets. Based on these age groups, patients were required to be in the children and adolescent age category (6–17 years of age) as of January 1st of the year of the index date.
Patients were further classified into two subgroups based on the presence of psychiatric or neurological comorbidities. Patients with a documented diagnosis for a psychiatric or neurological condition (see the list of ICD-9 codes in online Appendix B) during the baseline or study period were defined as comorbid patients. Otherwise, patients were classified as non-comorbid patients. Analyses were conducted in the overall ADHD population, and the comorbid and non-comorbid subgroups.
Study Measures
In order to better describe treatment patterns, the study classified ADHD pharmacologic treatments into nine categories: stimulants, ATX, clonidine IR, AAPs, TAPs, bupropion, SNRIs, SSRIs, and TCAs (online Appendix A). At the time of the study, GXR was not licensed in Canada and hence was not included. Combination therapy was defined as the combination of a stimulant with a psychotropic medication from any of the above categories, including combination of two stimulants with different active moieties. Both medications had to be taken concomitantly for at least 30 consecutive days, which is consistent with other studies (Balkrishnan et al., 2009; Lachaine et al., 2012). The stimulant in the combination could be initiated before, simultaneously with, or after the other medication (online Appendix C). Therapy switching was defined as a prescription fill of another psychotropic medication in one of the nine categories (including a stimulant with a different active moiety) less than 30 days before or after the end of the supply of a stimulant. The newly prescribed medication was defined as the “switched-to” medication. The fill of a new stimulant with the same active moiety but different formulation was not considered as combination therapy or therapy switching.
The one-year period prevalence of combination therapy was defined as the number of patients who had at least one combination therapy during the study period divided by the total number of patients in the sample. The definition of prevalence also allowed combination therapy to start during the baseline period and continue in the study period (Lachaine et al., 2012; Steinberg et al., 2008). The same denominator was used to calculate the one-year period prevalence of combination therapy for each of the nine medication categories. The numerator was the number of patients who had at least one medication from that category used in combination with a stimulant during the study period. Patients were considered as having only one distinct combination therapy if they experienced combinations with multiple medications in the same category. However, patients who had combination therapies with medications from different medication categories could be classified into more than one category. The overall period prevalence of stimulant switching and the period prevalence of switching to each individual medication category were estimated using similar approaches, except that switching could only occur during the study period.
Baseline Characteristics
Baseline characteristics included demographics, comorbidities, stimulant use, psychiatric visits, and the specialty of the prescribing physicians.
Statistical Analyses
Baseline characteristics were described for the overall population, and stratified by the status of combination therapy and therapy switching. Mean, median and standard deviation were calculated for continuous variables, while frequency and percentage were calculated for categorical variables.
The one-year period prevalence of combination therapy and switching overall and for each medication category were calculated. In addition, the number of distinct combination therapies and distinct switches during the study period were also estimated. All analyses were conducted in the overall sample and in the comorbid and non-comorbid subgroups separately. As this was an observational epidemiological study of period prevalence, no statistical comparisons were conducted between the subgroups.
Results
Patient Characteristics
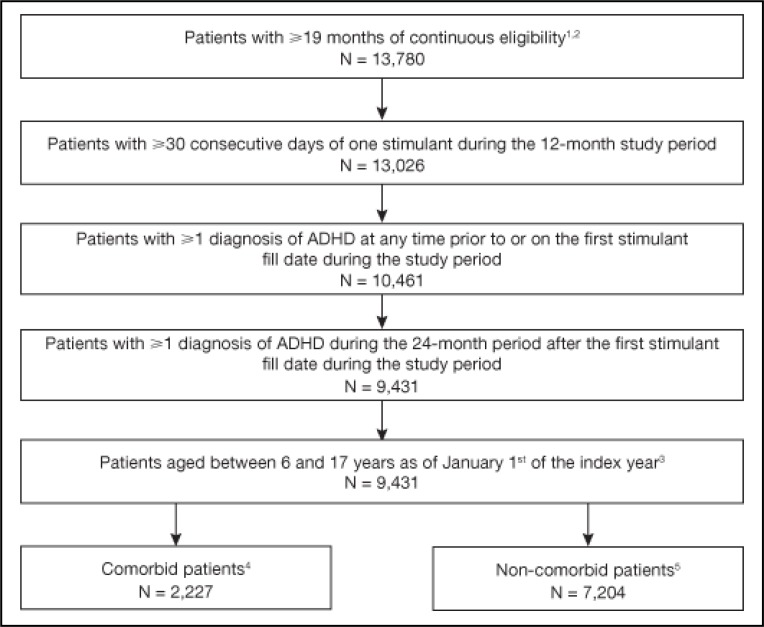
Overall, 9,431 patients met the study selection criteria (Figure 1). The sample had a mean age of 11.3 years with 64.1% children (aged 6–12 years) and 72.3% male. The majority of patients were treated with a stimulant during the six-month baseline period (53.8% with methylphenidate [MPH] LA, 27.6% with MPH SA, and 19.6% with amphetamine LA) (Table 1). In total, 20.5% had at least one documented comorbidity in the baseline period. The most common psychiatric/neurological comorbidities included learning disability (2.7%), adjustment reaction (2.6%) and anxiety disorder (2.0%); the most common physical comorbidities were accidents and injuries (8.9%), followed by asthma (2.4%). During the baseline period, 19.9% of patients had at least one psychiatric visit, with an average number of 3.9 psychiatrist visits. During the baseline period, 47.4% of patients received stimulant prescriptions from paediatricians and 11.5% from psychiatrists. A summary of baseline characteristics stratified by the status of combination therapy and therapy switching is provided in online Appendix D.
Figure 1.
Sample selection
1The following criteria were used for data extraction from the RAMQ data: patients must be continuously enrolled in both medical and drug plans for at least 18 consecutive months, have at least one diagnosis of ADHD during the continuous enrolment period, have at least one prescription of a stimulant during the continuous enrolment period and within 18 months of a diagnosis of ADHD and must be ≥6 and <18 years old as of January 1st of the year meeting all the above criteria.
2The last 19 months of continuous eligibility meeting all selection criteria were classified into baseline period (six months), followed by study period (12 months) and one month of additional evaluation period. The additional evaluation period is required to evaluate whether a therapy addition to a stimulant during the last month of the study period can be qualified as combination therapy.
3The index year is defined as the year of the index date, which is defined as the first day of the study period.
4Patients with at least one psychiatric/neurological comorbidity diagnosis during the baseline or study period were defined as comorbid. See online Appendix B for the detailed list of psychiatric/neurological comorbidity diagnoses.
5Patients with no psychiatric/neurological comorbidity diagnosis during the baseline or study period were defined as non-comorbid. See online Appendix B for the detailed list of psychiatric/neurological comorbidity diagnoses.
Table 1.
Baseline characteristics of patients with attention deficit hyperactivity disorder
| Baseline characteristics | Overall (N=9,431) |
|---|---|
| Demographic characteristics | |
| Average age (years)a, mean ± SD [median] | 11.30 ± 2.72 [11.0] |
| Children (6–12 years)b, N (%) | 6,049 (64.1) |
| Adolescents (12–17 years)b, N (%) | 3,382 (35.9) |
| Female, N (%) | 2,608 (27.7) |
| Enrolment typec, N (%) | |
| Employment assistance recipient | 2,457 (26.1) |
| Subscriber | 6,974 (73.9) |
| Number of distinct stimulantsd, mean ± SD [median] | 1.03 ± 0.54 [1.0] |
| Class of baseline stimulant(s)e, N (%) | |
| AMPH SA | 165 (1.7) |
| AMPH LA | 1,849 (19.6) |
| MPH SA | 2,604 (27.6) |
| MPH LA | 5,072 (53.8) |
| Comorbidity profile, N (%) | |
| Mental health comorbidities | |
| Adjustment reaction | 243 (2.6) |
| Anxiety disorder | 192 (2.0) |
| Learning disability | 252 (2.7) |
| Physical comorbidities | |
| Accidents and injuries | 835 (8.9) |
| Number of comorbidities | |
| Patients with 1 comorbidity | 1,657 (17.6) |
| Patients with 2 comorbidities | 230 (2.4) |
| Patients with ≥3 comorbidities | 45 (0.5) |
| Patients with ≥1 psychiatric visit, N (%) | 1,873 (19.9) |
| Mean number of psychiatric visitsf, mean ± SD [median] | 3.86 ± 7.72 [2.0] |
| Physician specialtyg | |
| Paediatrics | 4,472 (47.4) |
| Psychiatry | 1,083 (11.5) |
| Neurology | 213 (2.3) |
| Missing | 2,992 (31.7) |
AMPH, amphetamine; LA, long-acting; MPH, methylphenidate; SA, short-acting; SD, standard deviation
Age group is calculated as of January 1st of the year of the index date. The average age is calculated as the midpoint for the age group the patient belongs to.
Age groups were calculated as of January 1st of the year of the index date; 75% and 50% of the patients in the 10–13 and the 11–14 age groups, respectively, were randomly allocated to the children category, the remaining patients in these age groups were allocated to the adolescent category.
As patients may switch health plan over time, for each enrolment type, the number of days for which patients were enrolled during the six-month baseline period were added for all patients, and divided by 180 days to obtain the number of patients eligible full time.
Total number of distinct stimulant classes during baseline period (AMPH SA, AMPH LA, MPH SA, MPH LA).
Class of stimulant used during the baseline period. Classes were not mutually exclusive as some patients used more than one class during the baseline period.
Mean number of psychiatric visits for patients who had ≥1 psychiatric visit.
Physician having provided at least one medical service or having prescribed at least one psychotropic medication.
Combination Therapy and Therapy Switching
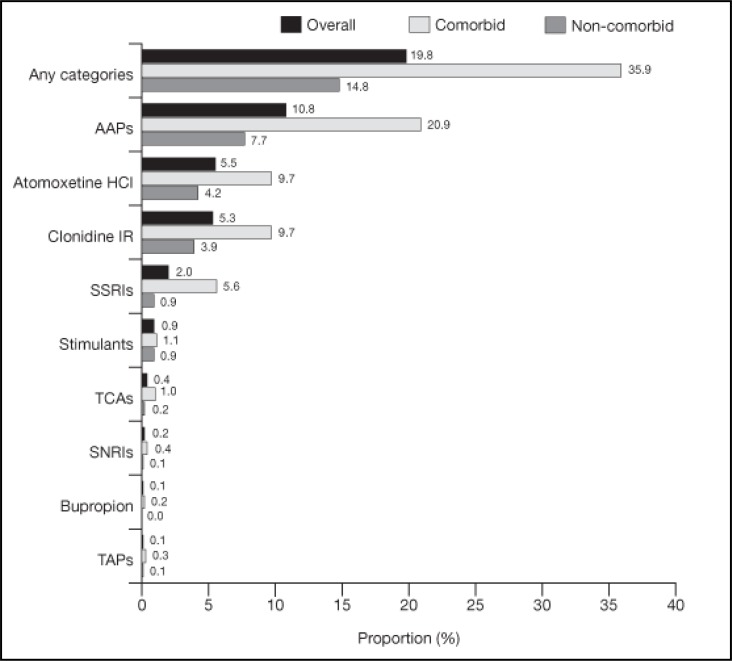
The one-year period prevalence of stimulant combination therapy was 19.8% in the overall population, 18.3% in children and 22.4% in adolescents (Figure 2). Among patients who received combination therapy, 29.5% had more than one distinct combination therapy (Table 2). The most common medications used in combination with stimulants were AAPs (10.8%), ATX (5.5%) and clonidine IR (5.3%) (Figure 2).
Figure 2.
Period prevalence of combination therapy1 among patients with ADHD (one-year study period)2–4
1Combination therapy was defined as the combination of a stimulant with a psychotropic medication as listed in online Appendix A. This included a combination of two stimulants with different active moieties. Both medications had to be taken concomitantly for at least 30 consecutive days.
2The one-year period prevalence of combination therapy was defined as the number of patients who had at least one combination therapy during the study period divided by the total number of patients in the sample. The definition of prevalence allowed combination therapy to start during the baseline period and continue in the study period or start during the study period. To calculate period prevalence for combination therapy, the numerator is the number of patients who had a combination therapy (consisting of a stimulant and a medication from the evaluated medication category) that either started during the baseline period (i.e. six months pre-index) and continued during the study period (i.e. 12 months post-index) or started during the study period. The denominator is the number of all patients included in the study.
3Patients with at least one psychiatric/neurological comorbidity diagnosis during the baseline or study period were defined as comorbid. All other patients were defined as noncomorbid. See online Appendix B for the detailed list of psychiatric/neurological comorbidity diagnoses.
4To calculate the one-year period prevalence of combination therapy for each of the nine medication categories, the numerator was the number of patients who had at least one medication from that category used in combination with a stimulant during the study period. Patients could be classified into more than one combination medication category if they had combination therapies with medications from multiple medication categories during the study period. Patients were considered as having only one distinct combination therapy if they experienced combinations with multiple medications in the same category.
Table 2.
Distribution of the number of combination therapy and therapy switching medication categories among patients with attention deficit hyperactivity disorder (one-year study period)
| Numbera | Combination therapy
|
Therapy switching
|
||||
|---|---|---|---|---|---|---|
| Non-comorbid frequency (%) (N=7,204) | Comorbid frequency (%) (N=2,227) | Overall frequency (%) (N=9,431) | Non-comorbid frequency (%) (N=7,204) | Comorbid frequency (%) (N=2,227) | Overall frequency (%) (N=9,431) | |
| 0 | 6,139 (85.2) | 1,428 (64.1) | 7,567 (80.2) | 6,141 (85.2) | 1,523 (68.4) | 7,664 (81.3) |
| 1 | 796 (11.0) | 518 (23.3) | 1,314 (13.9) | 892 (12.4) | 508 (22.8) | 1,400 (14.8) |
| 2 | 209 (2.9) | 216 (9.7) | 425 (4.5) | 115 (1.6) | 141 (6.3) | 256 (2.7) |
| 3 | 33 (0.5) | 45 (2.0) | 78 (0.8) | 43 (0.6) | 45 (2.0) | 88 (0.9) |
| 4 | 17 (0.2) | 13 (0.6) | 30 (0.3) | 4 (0.1) | 6 (0.3) | 10 (0.1) |
| 5 | 5 (0.1) | 3 (0.1) | 8 (0.1) | 6 (0.1) | 3 (0.1) | 9 (0.1) |
| 6 | 4 (0.1) | 4 (0.2) | 8 (0.1) | 0 (0.0) | 1 (0.0) | 1 (0.0) |
| 7 | 1 (0.0) | 0 (0.0) | 1 (0.0) | 3 (0.0) | 0 (0.0) | 3 (0.0) |
| ≥8 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Number refers to the number of different medication categories the patients used as combination therapy or therapy switching. Each combination therapy or therapy switching taking place in the study period was classified based on the category of the combination or switched-to medication or medication class. The medication categories include stimulant, atypical antipsychotic, typical antipsychotic, atomoxetine HCl, bupropion, clonidine IR, serotonin–norepinephrine reuptake inhibitor, selective serotonin reuptake inhibitor and tricyclic antidepressant.
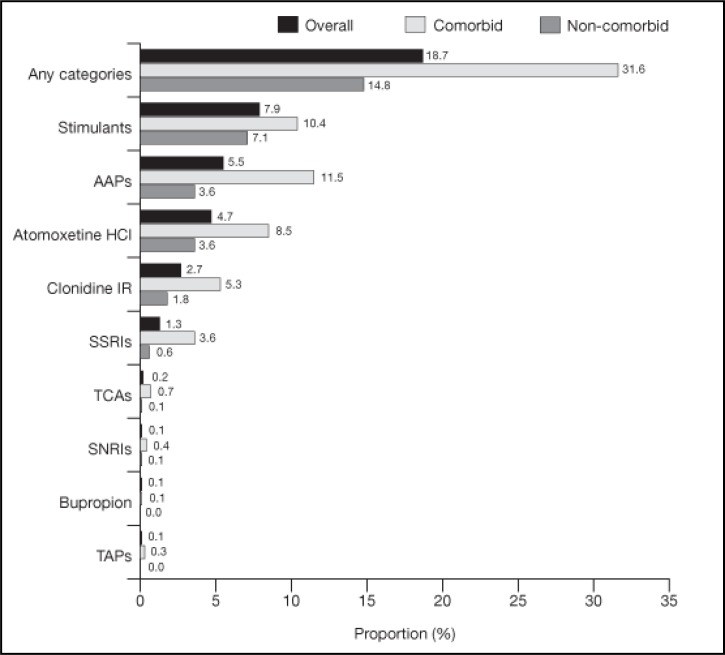
The one-year period prevalence of stimulant switching was 18.7% in the overall population, 19.5% in children and 17.4% in adolescents (Figure 3). Among patients who switched stimulant therapy, 20.8% had more than one distinct therapy switching (Table 2). The most common “switched to” medications were stimulants with a different active moiety (7.9%), AAPs (5.5%) and ATX (4.7%).
Figure 3.
Period prevalence of therapy switching1 among all patients with ADHD (one-year study period)2–4
1Therapy switching was defined as a prescription fill of another psychotropic medication in one of the nine categories (including a stimulant with a different active moiety) less than 30 days before or after the end of the supply of a stimulant.
2The one-year period prevalence of combination therapy was defined as the number of patients who had at least one combination therapy during the study period divided by the total number of patients in the sample. Switching could only occur during the study period.
3Patients with at least one psychiatric/neurological comorbidity diagnosis during the baseline or study period were defined as comorbid. All other patients were defined as noncomorbid. See online Appendix B for the detailed list of psychiatric/neurological comorbidity diagnoses.
4To calculate the one-year period prevalence of therapy switching for each of the nine medication categories, the numerator was the number of patients who had switched to at least one medication from that category from a stimulant during the study period. Patients could be classified into more than one “switched-to” medication category if they had therapy switching with medications from multiple medication categories during the study period. Patients were considered as having only one distinct therapy switching if they experienced switches with multiple medications in the same category.
Subgroup Analysis
The majority of patients (76.4%) did not have any documented psychiatric or neurological comorbidity during the baseline or study period (Table 2). The one-year period prevalence of combination therapy was 35.9% in comorbid patients and 14.8% in non-comorbid patients. The most common medications used in combination with stimulants were the same in both subgroups. Combinations with AAPs, ATX and clonidine IR accounted for 20.9%, 9.7% and 9.7% of all combinations, respectively, in comorbid patients and 7.7%, 4.2% and 3.9%, respectively, in non-comorbid patients (Figure 2).
The one-year period prevalence of therapy switching was 31.6% in comorbid patients and 14.8% in non-comorbid patients (Figure 3). The most common “switched-to” medications for comorbid patients were AAPs (11.5%), stimulants (10.4%) and ATX (8.5%). The most common “switched-to” medications for non-comorbid patients were stimulants (7.1%), AAPs (3.6%) and ATX (3.6%).
Discussion
To our knowledge, this is the first study to systematically assess the prevalence of stimulant combination therapy and switching with both label-indicated medications and off-label medications that could potentially be used in the treatment of ADHD in Canada. This study found that almost one in five (19.8%) children and adolescents with ADHD in Quebec combined a stimulant with another psychotropic medication during the one-year study period. A similar prevalence was observed for stimulant switching (18.7%). The rate of combination therapy was consistent with a recent European retrospective chart review study which observed a combination (defined in the study as concomitant use of a label-indicated and a non-indicated psychotropic medication) rate of 14.1% in six European countries, including France, Germany, Italy, the Netherlands, Spain and the UK, ranging from 4.1% (Germany) to 32.7% (Italy) (Sikirica, Fridman, Bruno, Hodgkins, & Erder, 2013). While AAPs were also observed to be the most commonly observed combination medication (5.4%) in the European study, the current study observed a much higher prevalence of combination use of stimulants and AAPs (10.8%). The difference could be due to variations in clinical practice, healthcare systems, or in availability of approved ADHD products between the countries.
The study also estimated the period prevalence of stimulant combination therapy and switching in subgroups of comorbid and non-comorbid patients. The prevalence of combination therapy was higher in the comorbid subgroup (35.9%) compared with the non-comorbid group (14.8%). This could potentially reflect that comorbid patients need another medication to control symptoms associated with their comorbidities or that their ADHD symptoms may not be well controlled by stimulant monotherapy alone. Unfortunately, the current database has limited information on diagnosis associated with prescription, but future research is needed to confirm and better understand these treatment patterns. Future prospective research should assess whether combination therapy used for the treatment of ADHD is truly more prevalent in comorbid patients. We also observed a high prevalence of switching (31.6%) among comorbid patients, which may suggest that a large proportion of patients in the comorbid group did not achieve optimal control of their ADHD symptoms with their initial stimulant monotherapy. Future studies are warranted to assess reasons for treatment changes. The most common switched-to medication category was AAPs (11.5%), which is consistent with previous studies demonstrating ADHD to be one of the top diagnoses associated with an AAP prescription in children and adolescents (Patten, Waheed, & Bresee, 2012).
Even among the non-comorbid ADHD subgroup, composed of patients with no documented diagnoses for which most psychotropic medications other than stimulants are indicated, combination therapy occurred in 14.8% of patients. The majority of these patients were prescribed medications not label-indicated for ADHD. One caveat is that the estimated proportion of comorbid patients in this study sample was 23.6%, which is lower than the ADHD comorbidity rates observed in the European medical chart review study (Sikirica et al., 2013) and in a previous US study using clinical samples (Jensen et al., 2001). This may be due to underreporting of the diagnoses in the RAMQ database, and the observed combination therapy could be used for symptoms associated with comorbidities unobserved in the data. However, this limitation does not affect the prevalence of combination therapy in the overall sample, which is quite similar across the two studies. Moreover, similar to the comorbid patients, a large proportion of non-comorbid patients also switched therapy, including 3.6% switching to AAPs. The high prevalence of combination therapy and stimulant switching observed in this study is noteworthy. Although the study cannot confirm the off-label use of a medication, the high prevalence of these events involving psychotropic medications that are not indicated for ADHD suggests that potential off-label use in this population is worth further investigation. Of the top three medications used in combination therapy, only ATX is label-indicated for ADHD, but it is only approved for monotherapy use. Of note, AAPs were the most commonly used medication category for combination therapy (10.8% overall; 20.9% in comorbid and 7.7% in non-comorbid) and for switching in comorbid patients as well (11.5%). AAPs may be used in the clinical setting to control specific ADHD symptoms e.g., impulsivity and emotional dysregulation. However, existing evidence of treatment effect of AAPs in controlling ADHD symptoms is modest at best (Armenteros, Lewis, & Davalos, 2007; Zeni, Tramontina, Ketzer, Pheula, & Rohde, 2009). On the other hand, side effects associated with AAPs, such as metabolic syndrome, type 2 diabetes, extrapyramidal symptoms and cardiac risks, are prominent and could outweigh the benefit of treatment effect (Ben Amor, 2012; Bobo et al., 2013; Bussing & Winterstein, 2012). Given the high prevalence of psychotropic medications other than stimulants used by children and adolescents with ADHD, future studies assessing the efficacy and safety of these medications, either used in combination with a stimulant or as a monotherapy, are warranted. In addition, as treatment becomes more evidence-based, the risk-benefit profiles of these off-label medications should be evaluated and compared with the label-indicated ADHD medications before they are recommended in the ADHD paediatric population.
The present study is subject to several limitations. The current study did not include combination and switching of stimulants with the same active moiety but with different formulations. A previous Canadian study reported approximately 20–30% of patients combining or switching between SA and LA stimulants, including stimulants with the same active moiety (Lachaine et al., 2012). Therefore, if such combination or switching was considered, the prevalence may have been higher. The current definition was chosen to provide a conservative estimate from the clinical perspective. This study is subject to limitations inherent to retrospective studies using claims data, e.g. lack of documented rationale for combination therapy or switching, and identification of combination therapy and switching based on claims for prescriptions filled, which does not necessarily mean that patients actually took the medications. It is also subject to common limitations specific to the use of the RAMQ database (Lachaine et al., 2007; Lachaine, Beauchemin, & Landry, 2010). One potential limitation is that prevalence of comorbidities may be underestimated as the listing of multiple diagnoses besides the primary diagnosis is not mandatory in the physicians’ billing statement. Further, medical services paid by a fixed income package (instead of fee) may not be reported in the database, which may also lead to missing diagnostic information. Finally, the study reports its findings based on patients enrolled in the RAMQ, who may not be fully representative of the entire population of Quebec and Canada.
In conclusion, combination therapy and switching are common among children and adolescents with ADHD treated with stimulants in Quebec, Canada, and most of the occurrences of combination therapy and therapy switching involve medications that are not label-indicated for the treatment of ADHD. These results highlight the need for further research to evaluate the extent of off-label use of psychometric medications and the risk-benefit of stimulant combination and switching in the treatment of ADHD.
Acknowledgements/Conflicts of Interest
This study was funded by Shire Development, LLC. Shire develops and markets psychiatric drugs, including medications to treat ADHD. Vanja Sikirica is an employee of Shire and owns stock/stock options. Paul Hodgkins was an employee of Shire and owned stock/stock options during the time period of the study. Valerie Carter, Martin Cloutier and Annie Guerin are employees of Analysis Group Inc., which has received consultancy fees from Shire. Leila Ben Amor, Jean Lachaine and Judy van Stralen have received consultancy fees for this research. We would like to thank Ana Bozas of Analysis Group Inc. and Caudex Medical (funded by Shire AG) for editorial assistance.
List of appendices available online at:
List of appendices available online at: http://www.cacap-acpea.org/en/cacap/Volume_23_Number_3_September_2014_s5.html?ID=1322
| Appendix A: | List of medications included in the combination and switching analyses |
| Appendix B: | ICD-9 diagnosis codes for comorbidities |
| Appendix C: | Study measures |
| Appendix D: | Baseline characteristics of patients with ADHD |
References
- Adesman AR. The diagnosis and management of attention-deficit/hyperactivity disorder in pediatric patients. Primary Care Companion Journal of Clinical Psychiatry. 2001;3(2):66–77. doi: 10.4088/pcc.v03n0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi-Severini S, Biscontri RG, Collins DM, Sareen J, Enns MW. Ten years of antipsychotic prescribing to children: A Canadian population-based study. Canadian Journal of Psychiatry Revue canadienne de psychiatrie. 2012;57(1):52–58. doi: 10.1177/070674371205700109. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Hargrave TM, Simonescu M, Kaul P, Hendricks K, Faraone SV. Advances in understanding and treating ADHD. BMC Medicine. 2011;9(1):72. doi: 10.1186/1741-7015-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenteros JL, Lewis JE, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/hyperactivity disorder: A placebo-controlled pilot study. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(5):558–565. doi: 10.1097/chi.0b013e3180323354. [DOI] [PubMed] [Google Scholar]
- Balkrishnan R, Pollack M, Joish VN, Asche CV, Pawaskar MD, Cziraky MJ. An economic evaluation of therapeutic alteration in the management of insomnia. Current Medical Research and Opinion. 2009;25(3):663–669. doi: 10.1185/03007990802714713. [DOI] [PubMed] [Google Scholar]
- Barbaresi W, Katusic S, Colligan R, Weaver A, Leibson C, Jacobsen S. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: Results from a population-based study. Journal of Developmental and Behavioral Pediatrics. 2006;27(1):1–10. doi: 10.1097/00004703-200602000-00001. [DOI] [PubMed] [Google Scholar]
- Ben Amor L. Antipsychotics in pediatric and adolescent patients: A review of comparative safety data. Journal of Affective Disorders. 2012;138:S22–30. doi: 10.1016/j.jad.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Bobo W, Cooper W, Stein C, Olfson M, Graham D, Daugherty G, …Ray W. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70(10):1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- Brault M-C, Lacourse É. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994–2007. Canadian Journal of Psychiatry Revue canadienne de psychiatrie. 2012;57(2):93–101. doi: 10.1177/070674371205700206. [DOI] [PubMed] [Google Scholar]
- Bussing R, Winterstein AG. Polypharmacy in attention deficit hyperactivity disorder treatment: Current status, challenges and next steps. Current Psychiatry Reports. 2012;14(5):447–449. doi: 10.1007/s11920-012-0295-6. [DOI] [PubMed] [Google Scholar]
- CADDRA . Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA): Canadian ADHD Practice Guidelines, Third Edition. Toronto, ON: 2011. [Google Scholar]
- Chen C-Y, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2009;19(2):187–195. doi: 10.1089/cap.2008.096. [DOI] [PubMed] [Google Scholar]
- Cormier E. Attention deficit/hyperactivity disorder: A review and update. Journal of Pediatric Nursing. 2008;23(5):345–357. doi: 10.1016/j.pedn.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Czaja AS, Valuck R. Off-label antidepressant use in children and adolescents compared with young adults: extent and level of evidence. Pharmacoepidemiology and Drug Safety. 2012;21(9):997–1004. doi: 10.1002/pds.3312. [DOI] [PubMed] [Google Scholar]
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, … Neumann PJ. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(10):990–1002.e2. doi: 10.1016/j.jaac.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Hechtman L, Greenfield B. Long-term use of stimulants in children with attention deficit hyperactivity disorder: Safety, efficacy, and long-term outcome. Paediatric Drugs. 2003;5(12):787–794. doi: 10.2165/00148581-200305120-00002. [DOI] [PubMed] [Google Scholar]
- Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, … Vitiello B. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(2):147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ. New ADHD treatment options on the horizon. Advanced Studies in Medicine. 2002;2:915–918. [Google Scholar]
- Lachaine J, Beauchemin C, Landry P-A. Clinical and economic characteristics of patients with fibromyalgia syndrome. The Clinical Journal of Pain. 2010;26(4):284–290. doi: 10.1097/AJP.0b013e3181cf599f. [DOI] [PubMed] [Google Scholar]
- Lachaine J, Beauchemin C, Sasane R, Hodgkins P. Treatment patterns, adherence, and persistence in ADHD: A Canadian perspective. Postgraduate Medicine. 2012;124(3):139–148. doi: 10.3810/pgm.2012.05.2557. [DOI] [PubMed] [Google Scholar]
- Lachaine J, Gordon A, Choinière M, Collet JP, Dion D, Tarride J. Painful neuropathic disorders : An analysis of the Régie de l’Assurance Maladie du Québec database. Pain Research & Management. 2007;12(1):31–37. doi: 10.1155/2007/713835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M. New options in the pharmacological management of attention-deficit/hyperactivity disorder. The American Journal of Managed Care. 2004;10(4):S117–S124. [PubMed] [Google Scholar]
- Patten SB, Waheed W, Bresee L. A review of pharmacoepidemiologic studies of antipsychotic use in children and adolescents. Canadian Journal of Psychiatry Revue canadienne de psychiatrie. 2012;57(12):717–721. doi: 10.1177/070674371205701202. [DOI] [PubMed] [Google Scholar]
- Sikirica V, Fridman M, Bruno A, Hodgkins P, Erder MH. Concomitant pharmacotherapy of psychotropic medications in EU children and adolescents with attention-deficit/hyperactivity disorder. Drugs in R&D. 2013;13(4):271–280. doi: 10.1007/s40268-013-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ. ADHD and comorbidity in childhood. The Journal of Clinical Psychiatry. 2006;67(Suppl 8):27–31. [PubMed] [Google Scholar]
- Steinberg M, Shao H, Zandi P, Lykestos C, Welsh-Bohme K, Norton M, … Tschanz J. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. International Journal of Geriatric Psychiatry. 2008;23(2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M, Panagiotopoulos C, Giles L, Gibbins C, Kuzeljevic B, Davidson J, Harrison R. A naturalistic study of predictors and risks of atypical antipsychotic use in an attention-deficit/hyperactivity disorder clinic. Journal of Child and Adolescent Psychopharmacology. 2009;19(5):575–582. doi: 10.1089/cap.2009.0050. [DOI] [PubMed] [Google Scholar]
- Zeni CP, Tramontina S, Ketzer CR, Pheula GF, Rohde LA. Methylphenidate combined with aripiprazole in children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder: A randomized crossover trial. Journal of Child and Adolescent Psychopharmacology. 2009;19(5):553–561. doi: 10.1089/cap.2009.0037. [DOI] [PubMed] [Google Scholar]
- Zito JM, Derivan AT, Kratochvil CJ, Safer DJ, Fegert JM, Greenhill LL. Off-label psychopharmacologic prescribing for children: History supports close clinical monitoring. Child and Adolescent Psychiatry and Mental Health. 2008;2(1):24. doi: 10.1186/1753-2000-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]